Looking beyond ejection fraction: what we have in echocardiography
REVIEW
Looking beyond ejection fraction: what we have in echocardiography
Article Summary
- DOI: 10.24969/hvt.2019.165
- Page(s): 143-151
- Echocardiography
- Published: 04/11/2019
- Received: 01/11/2019
- Accepted: 03/11/2019
- Views: 28435
- Downloads: 8810
-
Citations
- Keywords: ejection fraction, left ventricular filling pressure, left atrial volume, global longitudinal strain, left ventricular mass
PDF PRINT VERSION CommentsAddress for CorrespondenceAddress for Correspondence: Oben Baysan, Guven Hospital, Ankara, Turkey, Email: obbaysan@gmail.com
Oben Baysan1, İlke Zehra Akyıldız21Guven Hospital Cardiology Clinic, Ankara, Turkey
2Private cardiology clinic, Izmir, TurkeyAbstract
Left ventricular ejection fraction (LVEF) is the most frequently used echocardiographic parameter. LVEF based heart failure classification, timely intervention in a patient with valvular disease (e.g. aortic stenosis, mitral regurgitation), deterioration of myocardial function in a patient treated with chemotherapy; all need a simple LVEF value for decision-making process. An echocardiographic examination also contains several parameters with invaluable importance. Measurement and evaluation of these parameters should be made in every patient suspected of having myocardial disease.
Keywords: ejection fraction, left ventricular filling pressure, left atrial volume, global longitudinal strain, left ventricular mass
IntroductionEjection Fraction
Left ventricular ejection fraction (LVEF), fraction of end diastolic volume (EDV) ejected with each heartbeat, has a very strong place in any decision-making process in patients with various cardiovascular diseases including heart failure, cardiomyopathy, valvular heart problems.
Every 1% decrease of baseline LVEF value points to 4% increase in incident heart failure risk (1, 2). LVEF can also be used for sudden cardiac death prediction, and hence, an implantable cardiac defibrillator (ICD) implantation decision (3). Recent valvular heart diseases guideline contains pathways for decisions based on LVEF value especially in patients with mitral regurgitation (MR) (4).
Ejection fraction is directly affected by stroke volume (SV) and end-diastolic volume (EDV). A dilated left ventricle with increased end-diastolic and end-systolic volumes (ESV) as in heart failure or athlete’s heart generates normal SV with lower LVEF values. Contrary to this, a small ventricle with decreased volumes may show normal LVEF value albeit with low SV.
Besides contractility, EDV and ESV are mainly affected by afterload and preload, respectively. Any confounder having an effect on preload, afterload and contractility also has direct relationship with calculated LVEF value (Fig. 1).
Main tools available for LVEF measurement are cardiac magnetic resonance imaging (cMR), computerized tomography, nuclear scintigraphy and echocardiography. cMR is a standard test for LVEF measurement due to its high spatial and contrast resolution. In spite of its inherent disadvantages (lower measured volumes compared to cMR, lower spatial resolution and worse test-retest reliability), 2D echocardiography is the most frequently used method for LVEF measurement due to its easy availability, usability and practicality.
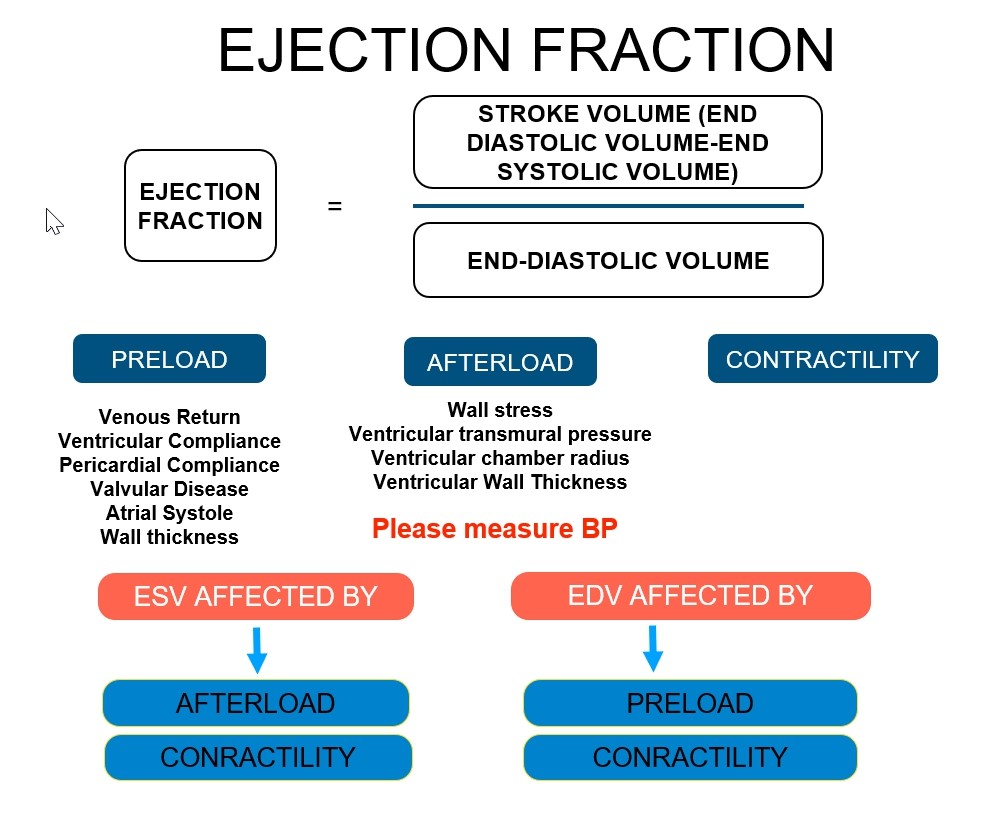 Figure 1. Factors affecting ejection fractionThe biplane method of disks (modified Simpson’s rule) is the currently recommended 2D method to assess LVEF (5). LVEF <52% for men and <54% for women should be considered abnormal (5). Another echocardiographic method, 3D echo based LVEF measurement, provides more reproducible and accurate data without any geometric assumption (5).
Figure 1. Factors affecting ejection fractionThe biplane method of disks (modified Simpson’s rule) is the currently recommended 2D method to assess LVEF (5). LVEF <52% for men and <54% for women should be considered abnormal (5). Another echocardiographic method, 3D echo based LVEF measurement, provides more reproducible and accurate data without any geometric assumption (5).Echocardiographic LVEF measurement has many pitfalls, which directly affects reliability of the measurement. A good image quality, an image without apical foreshortening and correct geometric assumptions (2D echocardiography) are prerequisites. This is not always the case. A distorted ventricular shape due to ischemic heart disease makes any geometric assumption useless. Poor imaging quality due to obesity or chronic obstructive lung disease brings a very strong obstacle to correct LVEF measurement. Moreover, it is usually not possible to obtain same echocardiographic imaging windows in a patient on repeated examinations. Very low or very high heart rate, irregular rhythms such as atrial fibrillation (AF) and conduction problems as in left bundle branch block also decrease reliability of LVEF calculation. All those factors certainly limit reliability and robustness of echocardiographic LVEF calculation (Fig. 2).
In experienced hands, 2D measurement has a standard error of 6.3%, an upper limit of confidence of 11.4%, and an inter-observer variability of 8.2% (6). The smallest LVEF change detected with 95% confidence was 0.11 (7). More than 10 percentage point change is needed for accepting meaningful LVEF difference (8).
Apart from technical difficulties, a simple LVEF value cannot be a surrogate for left ventricular systolic function. Left ventricle undergoes repetitive cycles of deformation in three directions during each heartbeat: longitudinal lengthening-shortening, circumferential lengthening-shortening and radial thinning-thickening. Rotation around its long-axis should also be added to this framework. 2D echocardiography LVEF calculation from apical views as recommended by guidelines (5) mainly contains information about longitudinal shortening and radial thickening. Longitudinal subendocardial myocardial fibers are accepted as most susceptible to myocardial damage. Stokke et al showed that major contribution to LVEF comes from circumferential shortening (9). They also determined that any loss of longitudinal myocardial function could be compensated by a change in other two parameters (circumferential and radial). Therefore, LVEF can be within normal limits in spite of a decreased longitudinal shortening.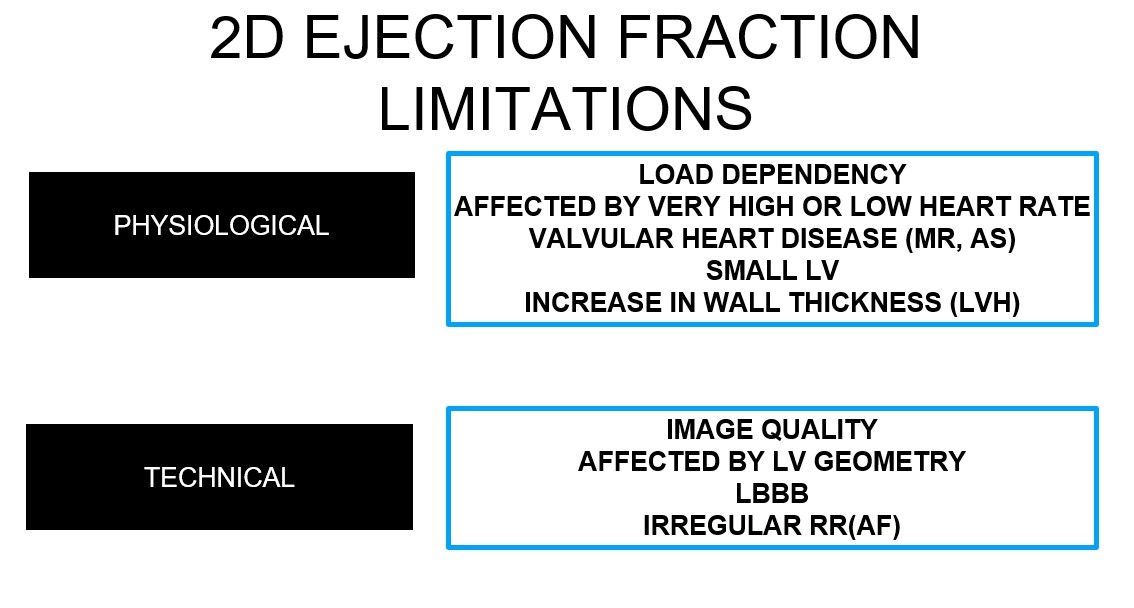
Figure 2. Physiological and technical factors affecting ejection fraction measurement
(AF-atrial fibrillation, AS-aortic stenosis, LVH–left ventricular hypertrophy, MR-mitral regurgitation)
Many echocardiographic parameters have been used for estimating left ventricular filling pressures (LVFPs) such as mitral inflow E/A ratio, difference between mitral A wave duration-pulmonary vein A duration but E/e’ ratio has emerged as most robust parameter. It has very high specificity (77-100%) but poor sensitivity (0-73%) (11) for an increased LVEDP. It has modest correlation with invasively determined LVFP (12). Despite this pitfall, high E/e’ ratio is a strong surrogate marker for cardiovascular death, heart failure hospitalization, or aborted cardiac arrest (13).Previous studies showed that LVEF has prognostic significance particularly when LVEF<40%. However, LVEF lost its relation with outcome and prognostic power when it has values more than 40% (2). Inherent insensitivity of LVEF to subtle myocardial damage particularly in longitudinal direction leads to missing a patient with subclinical left ventricular dysfunction.
In any patient with normal LVEF but suspected as having myocardial dysfunction, an echocardiographic examination should include other parameters beyond a simple LVEF value. Various patient subgroups such as heart failure with preserved ejection fraction (HFpEF), aortic stenosis (AS), MR and receiving chemotherapy certainly need more in-depth echocardiographic analysis. Non-invasive estimation of left ventricular filling pressure, left atrial (LA) volume and function analysis, left ventricular shape-wall thickness and left ventricular myocardial longitudinal deformation should be interrogated for obtaining relevant information about occult myocardial damage.
LOOKING BEYOND EF
1) High Left Ventricular Pressure: E/E’ ratio
Left ventricle has to be relaxed during diastolic period for allowing filling and providing enough SV to next systole. Myocardial relaxation, myocardial and chamber stiffness are main determinants of this period (10). Diastolic dysfunction can be defined, in strictest term, as an increase in end-diastolic pressure (LVEDP) with same amount of volume loading. LVEDP, LA or pulmonary pressures are not identical and they can show an increased value irrespective of each other. Mitral stenosis may increase LA pressure with normal LVEDP or pulmonic vein stenosis causes high pulmonary artery pressure with normal LA and ventricular filling pressure (10).
2016 update of diastolic function evaluation guideline suggested E/e’ cutoff value of 14 (14). In contrast, recent HFpEF diagnostic algorithm proposed an E/E’ ratio ≥15 as a major criterion. Intermediate values between 10
-2) Left Atrium
2a) Volume Analysis
The evaluation of left atrium shows an evolution from an anteroposterior diameter measurement to left atrial volume (LAV) and functional analysis using speckle tracking. Left atrial volume index (LAVi), LAV divided by body surface area, is measured from apical 4- and 2-chamber views using Simpsons’ or Area-Length methods. It is a powerful surrogate marker for long-standing high LVFPs (16). LAVi>34mL/m2 independently predicts death, heart failure, AF and ischemic stroke in patients without AF or valvular heart disease (16, 17). Permanent AF causes larger LAV, which usually 35% more dilated than LAV in sinus rhythm (18). Cut-off values for LAVi are provided in Figure 3.
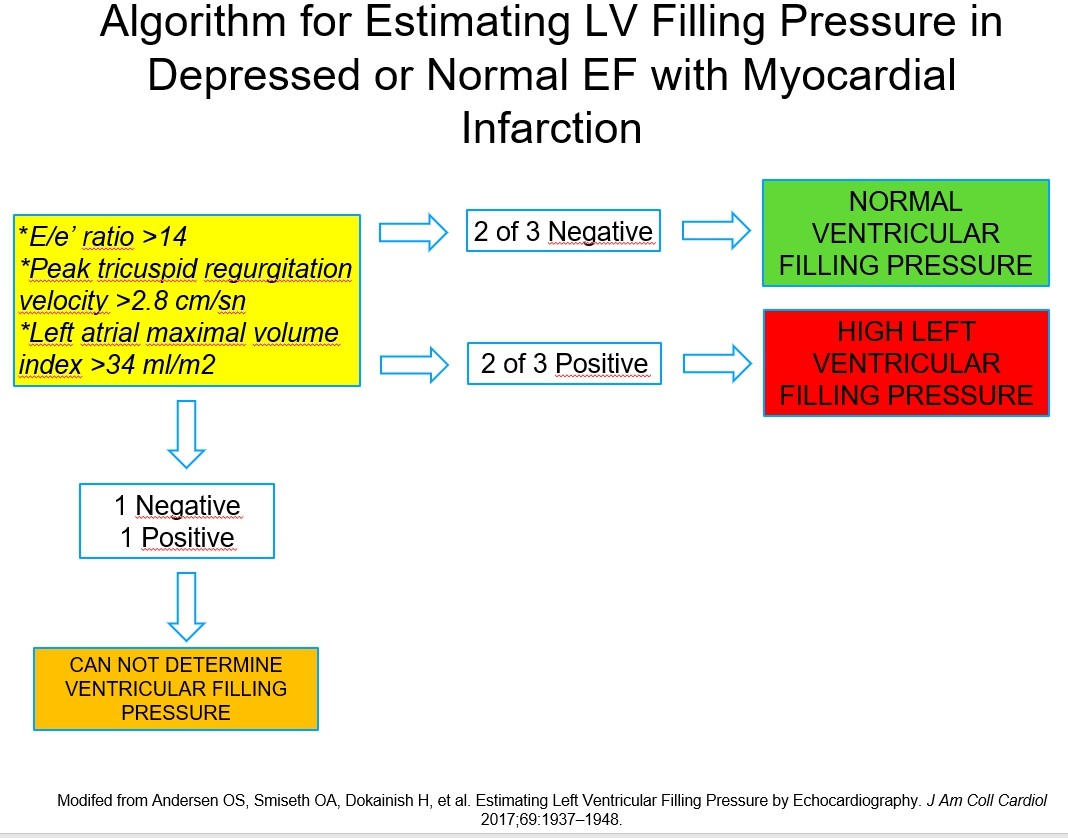
A dilated LA ((34 ml/m2) in combination of high E/e’ ratio (>14) and peak tricuspid regurgitation velocity more than 2.8 m/sec is used as a marker for high LVFP (19, 20) (Fig. 4a-b).
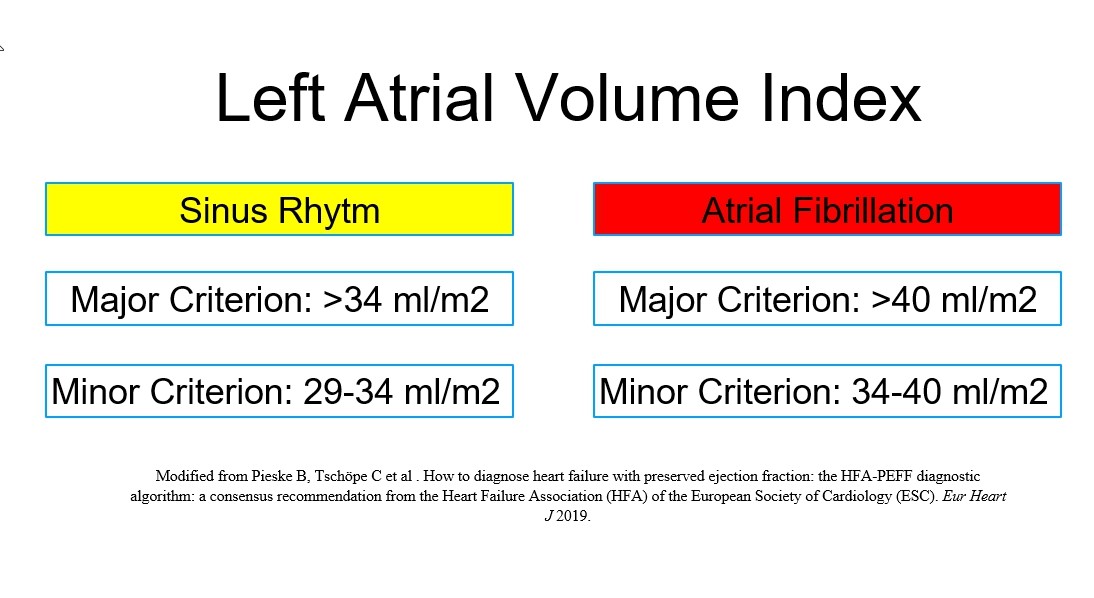
Figure 3. Cut-off values for abnormally increased LAVi
LAVi – left atrial volume index
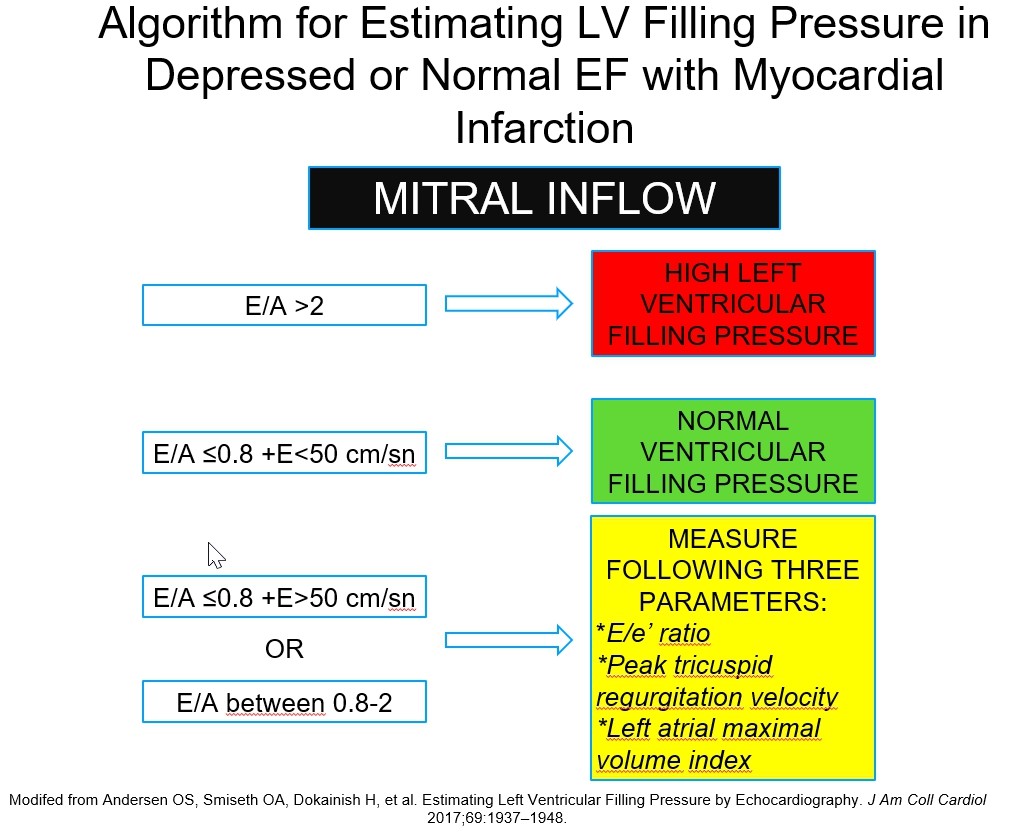
A)
B)
Figure 4. Algorithm for estimating left ventricular filling pressure in depressed or normal EF with myocardial infarction
(EF – ejection fraction, LV – left ventricle)
2b) Functional Analysis of Left Atrial Global Strain During Reservoir PhaseLeft atrium has three main functions: reservoir during ventricular systole, conduit during early diastolic phase, additional pumping of blood via atrial contraction during late phase of diastole. Volumetric analysis can be used for LA functional analysis but speckle-based deformation imaging seems to more suitable.
LA global reservoir strain shows reverse correlation with decreasing left ventricular diastolic function (21). It has higher sensitivity compared to LAV for detecting diastolic dysfunction. Morris et al. showed in 517 patients with hypertension, diabetes, and coronary artery disease with preserved LV ejection fraction that LA strain was reduced in 62%, whereas LAV was enlarged only in 34% (22).
A non-foreshortened apical 4-chamber view with end-diastolic reference point is recommended for LA global reservoir strain measurement (23) (Fig. 5). Normal LA global reservoir strain value is above 35% (21). A far posterior localization of LA and its thin wall create a difficulty for LA strain measurement, but nevertheless, LA global strain value lower than 20-23% or points to very severely reduced LA function (21, 24).
3) Left Ventricular Global Longitudinal Strain (LV-GLS)
Among myocardial deformation parameters, global longitudinal strain has a prominent role in the detection of early myocardial dysfunction. Tissue Doppler data could be used for strain analysis, but nowadays, angle independent speckle-tracking based strain imaging is the preferred route for this purpose.
Technical details about how to measure LV-GLS can be found elsewhere (25) (Fig. 6). Normal value for LV-GLS is around -20% (5). A GLS value lower than -12% roughly corresponds to a LVEF value worse than 35%. LV-GLS has better inter-observer and intra-observer variability (5% to 8% relative difference) compared to 8% to 10% for LVEF (26). It should be kept in mind that a LV-GLS measurement may show variation according to a particular software or vendor but there is ongoing effort to reduce this problem (27).
As mentioned previously, LVEF does not provide prognostic information in patients with normal or near normal LVEF values. LV-GLS has an adjunctive role in these patient groups. Identifying left ventricular dysfunction via LV-GLS calculation is a very logical target in various disease states.
In a population based prospective study, participants with low LV-GLS had a higher cardiovascular event risk compared to participants with normal LV-GLS (28). The LV-GLS prognostic value was incremental to risk factors and LVEF both in the overall population and in participants with normal LVEF. Another study supports this finding by showing that the change in LV-GLS is a stronger predictor of all-cause mortality than change in LVEF value (29).
HFpEF is a syndrome encompassing heterogeneous patient groups with several comorbidities. More than 70% of heart failure patients older than the age of 65 years have normal ejection fraction (30). Besides age and female sex, hypertension, diabetes mellitus, obesity, renal dysfunction, anemia, and chronic obstructive pulmonary disease are usually coexist in a particular HFpEF patient. These patients usually have following clinical presentations: (1) exercise intolerance with elevated LV filling pressures, (2) volume overload, or (3) right heart failure (31).
LV-GLS has been found to be decreased in patients with HFpEF compared to controls (32) and has an added useful prognostic information (32). The recent consensus recommendation about how to diagnose HFpEF put GLS (<16%) to minor functional abnormality in its diagnostic algorithm (15).
3a) LV-GLS in Chemotherapy-Related Cardiac Dysfunction
A cancer patient with symptoms of heart failure is considered to have a chemotherapy-related cardiac dysfunction (CTRCD) if their baseline LVEF value drops more than 5% points to below 53% during follow-up (33). More than 10 points reduction is required for the CTRCD diagnosis in an asymptomatic patient (33). LV-GLS has been found to be a more sensitive parameter for detecting cardiac toxicity. An 11% reduction in LV-GLS has a sensitivity of 65% and a specificity of 94% for subsequent cardiotoxicity during chemotherapy (34). In a recent report, chemotherapy-related cardiac dysfunction is defined as a LV-GLS with >15% relative reduction from baseline with preservation of LVEF (33).
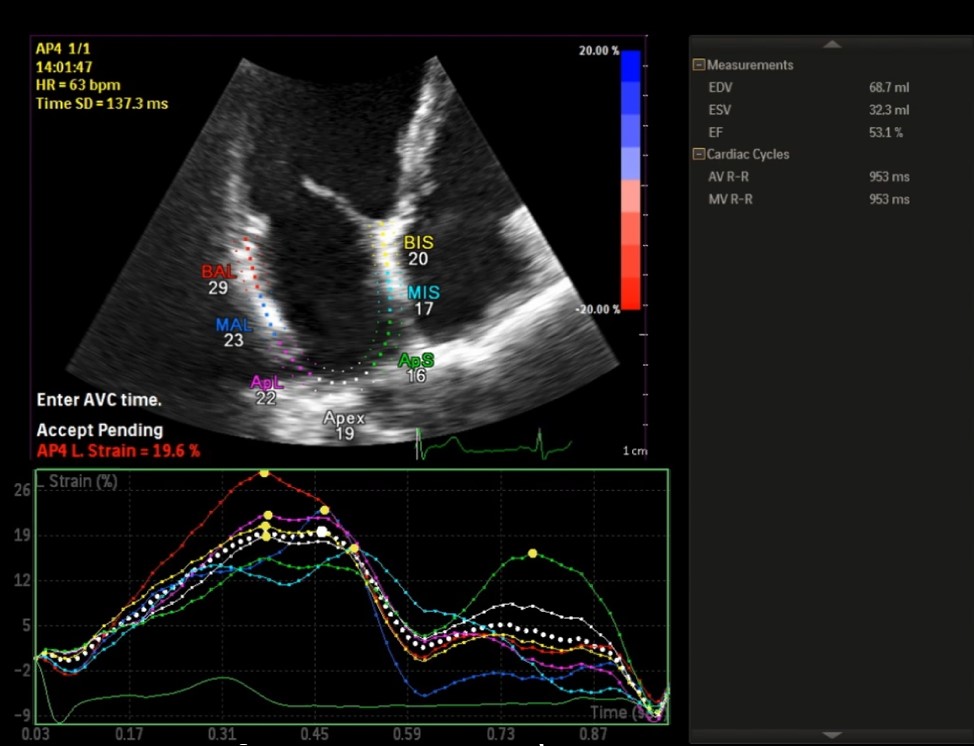 Figure 5. Speckle-based left atrial global strain in reservoir phase
Figure 5. Speckle-based left atrial global strain in reservoir phase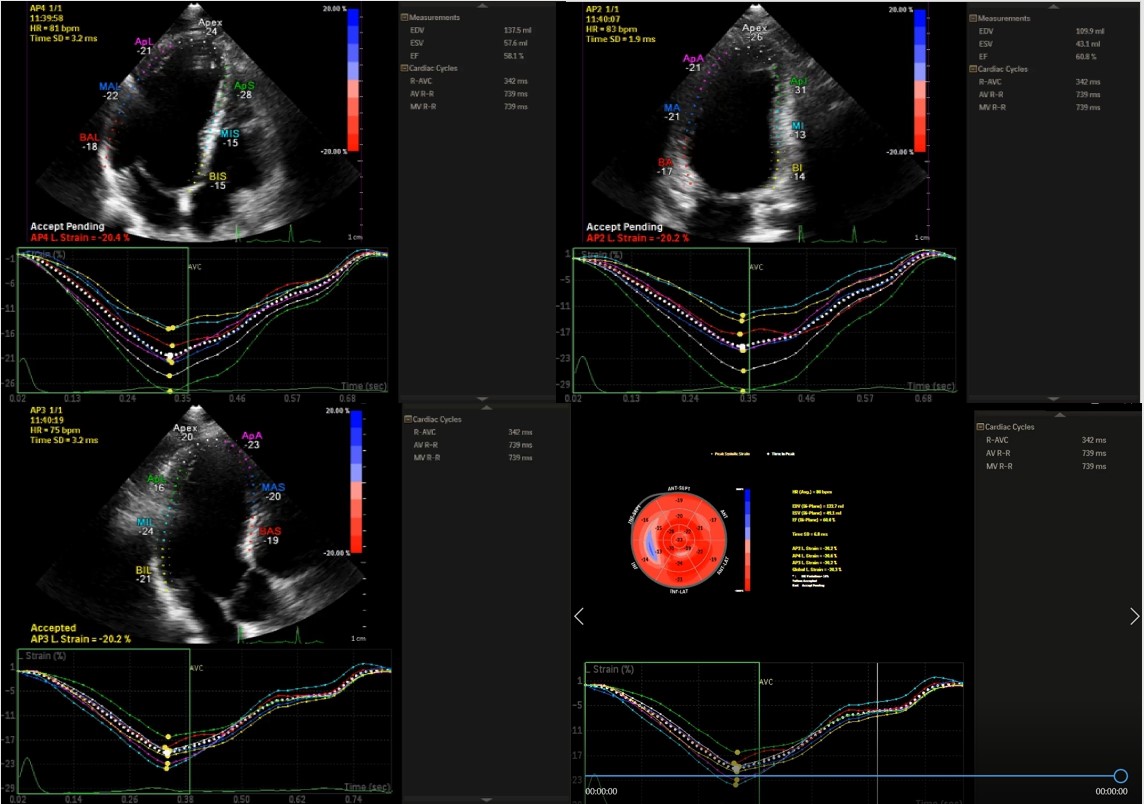
Figure 6. The measurement of global longitudinal strain
Conclusion3b) LV-GLS in Aortic Stenosis
Severe AS patients with decreased LVEF value (<50%) should undergo surgery regardless of symptomatic status (35). Unfortunately, the occurrence of low LVEF (<50%) in the absence of symptoms in severe AS is very rare (0.4%) (36). Irrespective of symptomatic status, patients with severe AS may have a subclinical left ventricular dysfunction determined by a decreased LV-GLS value (37). Recent studies suggested that even patients with LVEF value between 50% and 59% had unfavorable postoperative outcome compared to patients with LVEF value more than 60% (36). Identifying AS patients with normal LVEF value but concealed myocardial dysfunction is very important for decision-making. An asymptomatic severe AS patient with normal LVEF and normal LV-GLS (>-18%) should be followed regularly (1-2 years) but similar patient with a decreased LV-GLS (<-16.7%) should undergo further evaluation with cMR (36).
3c) LV-GLS in Mitral Regurgitation
Mitral valve surgery is recommended in severe MR patients having LVEF<60% and/or left ventricular end-systolic diameter >45mm. Early surgery in severe MR patients is associated with preserved left ventricular function and lower incident heart failure risk (38, 39). Bijvoet et al made a literature review about usefulness of LV-GLS in asymptomatic MR patients (40). They found that an impaired LV-GLS (range: –17.9 and –21.7%) is a predictor of both left ventricular dysfunction and an increased mortality (40).
4) Left Ventricular Wall Thickness, Shape and Volume
Left ventricular geometry can be described based on mass and relative wall thickness (RWT). An increase in left ventricular mass points to the presence of left ventricular hypertrophy, which is associated with heightened cardiovascular mortality (41). Simplest way of left ventricular hypertrophy determination is echocardiography (2D or M-Mode) via using Devereux formula (42). Left ventricular mass is usually indexed to body surface area. 3D echocardiographic left ventricular mass measurement has best correlation with cMR (5), but needs high quality images, which is not always possible.
RWT (2x end-diastolic posterior wall thickness divided by left ventricular end-diastolic diameter) should also be calculated. In hypertensive patients, an increased left ventricular mass index (LVMI) (≥115 g/m2 for men and ≥95 g/m2 for women) and RWT (< or > 0.42) are used for definition of four categories:
• Normal geometry (normal LVMI and RWT <0.42)
• Concentric remodeling (normal LVMI with increased RWT >0.42)
• Eccentric hypertrophy (increased LVMI and RWT <0.42)
• Concentric hypertrophy (increased LVMI and RWT >0.42)
Concentric hypertrophy in a hypertensive patient is a bad prognostic marker (41). Increased wall thickness with normal left ventricular mass, concentric remodeling, is associated with normal LVEF, reduced longitudinal deformation and a compensatory increased circumferential deformation (43).
Patients having excessive long-standing afterload (severe AS, hypertension) may show transition from a state characterized by increased wall thickness and normal left ventricular diameter to another state associated with left ventricular dilatation and decreased wall thickness (low RWT). At the beginning, myocardial thickness increase is actually very helpful for decreasing wall stress and providing enough SV. When left ventricle begins to dilate, wall stress is also increased due to Laplace’s law (wall stress: pressure x radius divided by wall thickness). Nevertheless, a dilated ventricle could provide enough SV in even a low contractile state.
Diastolic dimension of left ventricle is the denominator of RWT equation, and hence, any increase in diastolic diameter would decrease RWT. Myocardial thinning (a low RWT value) further increase wall stress and myocardial energy consumption. In a failing heart, compensatory mechanism for providing enough SV is eventually exhausted and cardiac output declines. Dini et al. showed in heart failure patients that very severe left ventricular hypertrophy (LVMI: 148 g/m2 in men and 122 g/m2 in women) concomitant with decreased RWT (<0.34) was a harbinger of poor survival (44).
Ejection fraction per se should not be used for defining the presence or absence of myocardial disease. We know that at least half of heart failure patients have normal LVEF value. Other parameters obtained from a detailed echocardiographic examination can be used for better delineation of myocardial dysfunction. The more sensitive parameters - high left ventricular filling pressure, left atrial dilatation; low left atrial global reservoir strain warn the clinician about occult myocardial dysfunction. Left ventricular mass and thickness may also provide additional information and should be taken into account.
Peer-review: Internal and external
Conflict of interest: None to declare
Authorship: O.B. and I.Z.A equally contributed to preparation of manuscript
and fulfilled authorship criteria
Acknowledgement and funding: None to declare
References
1. Lewis EF, Moye LA, Rouleau JL, Sacks FM, Arnold JMO, Warnica JW, et al. Predictors of late development of heart failure in stable survivors of myocardial infarction: the CARE study. J Am Coll Cardiol 2003; 42: 1446-53. https://doi.org/10.1016/S0735-1097(03)01057-X 2. Solomon SD, Anavekar N, Skali H, McMurray JJV, Swedberg K, Yusuf S, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005; 112: 3738-44. https://doi.org/10.1161/CIRCULATIONAHA.105.561423 PMid:16330684 3. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2018; 15: e190-252. 4. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017; 135: e1159-95. https://doi.org/10.1161/CIR.0000000000000503 PMid:28298458 5. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 2015; 28: 1-39.e14. https://doi.org/10.1016/j.echo.2014.10.003 PMid:25559473 6. Mele D, Campana M, Sclavo M, Seveso G, Aschieri D, Nesta F, et al. Impact of tissue harmonic imaging in patients with distorted left ventricles: improvement in accuracy and reproducibility of visual, manual and automated echocardiographic assessment of left ventricular ejection fraction. Eur J Echocardiogr 2003; 4: 59-67. https://doi.org/10.1053/euje.4.1.59 PMid:12565064 7. Otterstad JE, Froeland G, St John Sutton M, Holme I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J 1997; 18: 507-13. https://doi.org/10.1093/oxfordjournals.eurheartj.a015273 PMid:9076390 8. Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol 2013; 61: 77-84. https://doi.org/10.1016/j.jacc.2012.09.035 PMid:23199515 9. Stokke TM, Hasselberg NE, Smedsrud MK, Sarvari SI, Haugaa KH, Smiseth OA, et al. Geometry as a confounder when assessing ventricular systolic function: comparison between ejection fraction and strain. J Am Coll Cardiol 2017; 70: 942-54. https://doi.org/10.1016/j.jacc.2017.06.046 PMid:28818204 10. Popović ZB, Sato K, Desai MY. Is universal grading of diastolic function by echocardiography feasible? Cardiovasc Diagn Ther 2018; 8: 18-28. https://doi.org/10.21037/cdt.2017.07.02 PMid:29541608 PMCid:PMC5835651 11. Obokata M, Reddy YNV, Borlaug BA. The role of echocardiography in heart failure with preserved ejection fraction: What do we want from imaging? Heart Fail Clin 2019; 15: 241-56. https://doi.org/10.1016/j.hfc.2018.12.004 PMid:30832815 12. Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Qui-ones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 1997; 30: 1527-33. https://doi.org/10.1016/S0735-1097(97)00344-6 13. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O'Meara E, et al. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail 2014; 7: 740-51. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000887 https://doi.org/10.1161/CIRCHEARTFAILURE.114.001583 PMid:25122186 PMCid:PMC4916914 14. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277-314. https://doi.org/10.1016/j.echo.2016.01.011 PMid:27037982 15. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019; 40: 3297-317. https://doi.org/10.1093/eurheartj/ehz641 PMid:31504452 16. Leung DY, Chi C, Allman C, Boyd A, Ng AC, Kadappu KK, et al. Prognostic implications of left atrial volume index in patients in sinus rhythm. Am J Cardiol 2010; 105: 1635-9. https://doi.org/10.1016/j.amjcard.2010.01.027 PMid:20494675 17. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 2006; 47: 2357-63. https://doi.org/10.1016/j.jacc.2006.02.048 PMid:16781359 18. Lam CSP, Rienstra M, Tay WT, Liu LCY, Hummel YM, van der Meer P, et al. Atrial fibrillation in heart failure with preserved ejection fraction: association with exercise capacity, left ventricular filling pressures, natriuretic peptides, and left atrial volume. JACC Heart Fail 2017; 5: 92-8. https://doi.org/10.1016/j.jchf.2016.10.005 PMid:28017355 19. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018; 138: 861-70. https://doi.org/10.1161/CIRCULATIONAHA.118.034646 PMid:29792299 PMCid:PMC6202181 20. Andersen OS, Smiseth OA, Dokainish H, Abudiab MM, Schutt RC, Kumar A, et al. Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol 2017; 69: 1937-48. https://doi.org/10.1016/j.jacc.2017.01.058 PMid:28408024 21. Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC State-of-the-Art Review. J Am Coll Cardiol 2019; 73:1961-77. https://doi.org/10.1016/j.jacc.2019.01.059 PMid:31000000 22. Morris DA, Belyavskiy E, Aravind-Kumar R, Kropf M, Frydas A, Braunauer K, et al. Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc Imaging 2018; 11: 1405-15. https://doi.org/10.1016/j.jcmg.2017.07.029 PMid:29153567 23. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J - Cardiovasc Imaging 2018; 19: 591-600. https://doi.org/10.1093/ehjci/jey042 PMid:29596561 24. Cameli M, Lisi M, Righini FM, Massoni A, Natali BM, Focardi M, et al. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol 2013; 111: 595-601. https://doi.org/10.1016/j.amjcard.2012.10.049 PMid:23211360 25. Baysan O, Ocaklı EP, Saglam Y, Altuner TK. Advances in echocardiography: global longitudinal strain, intra-cardiac multidirectional flow imaging and automated 3d volume analysis. Heart Vessels Transplantation 2018; 2: 113-22. https://doi.org/10.24969/hvt.2018.83 26. Mirea O, Pagourelias ED, Duchenne J, Bogaert J, Thomas JD, Badano LP, et al. Variability and reproducibility of segmental longitudinal strain measurement: A report from the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc Imaging 2018; 11: 15-24. https://doi.org/10.1016/j.jcmg.2017.01.027 PMid:28528147 27. Farsalinos KE, Daraban AM, Ünlü S, Thomas JD, Badano LP, Voigt J-U. Head-to-head comparison of global longitudinal strain measurements among nine different vendors: The EACVI/ASE Inter-Vendor Comparison Study. J Am Soc Echocardiogr 2015; 28: 1171-81, e2. https://doi.org/10.1016/j.echo.2015.06.011 PMid:26209911 28. Russo C, Jin Z, Elkind MSV, Rundek T, Homma S, Sacco RL, et al. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail 2014; 16: 1301-9. https://doi.org/10.1002/ejhf.154 PMid:25211239 PMCid:PMC4672867 29. Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart Br Card Soc 2014; 100: 1673-80. https://doi.org/10.1136/heartjnl-2014-305538 PMid:24860005 30. Shah AM, Claggett B, Loehr LR, Chang PP, Matsushita K, Kitzman D, et al. Heart failure stages among older adults in the community: The Atherosclerosis Risk in Communities Study. Circulation 2017; 135: 224-40. https://doi.org/10.1161/CIRCULATIONAHA.116.024825 https://doi.org/10.1161/CIRCULATIONAHA.116.023361 PMid:27881564 PMCid:PMC5241178 31. Silverman DN, Shah SJ. Treatment of heart failure with preserved ejection fraction (HFPEF): the phenotype-guided approach. Curr Treat Options Cardiovasc Med 2019; 21: 20. https://doi.org/10.1007/s11936-019-0709-4 PMid:30982123 32. Morris DA, Ma X-X, Belyavskiy E, Kumar RA, Kropf M, Kraft R, et al. Left ventricular longitudinal systolic function analysed by 2D speckle-tracking echocardiography in heart failure with preserved ejection fraction: a meta-analysis. Open Heart 2017; 4: e000630. https://doi.org/10.1136/openhrt-2017-000630 PMid:29018535 PMCid:PMC5623331 33. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014; 27: 911-39. https://doi.org/10.1016/j.echo.2014.07.012 PMid:25172399 34. Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr 2013; 26: 493-8. 35. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38: 2739-91. https://doi.org/10.1093/eurheartj/ehx391 PMid:28886619 36. Dahl JS, Magne J, Pellikka PA, Donal E, Marwick TH. Assessment of subclinical left ventricular dysfunction in aortic stenosis. JACC Cardiovasc Imaging 2019; 12: 163-71. https://doi.org/10.1016/j.jcmg.2018.08.040 PMid:30621988 37. Vollema EM, Sugimoto T, Shen M, Tastet L, Ng ACT, Abou R, et al. Association of left ventricular global longitudinal strain with asymptomatic severe aortic stenosis: natural course and prognostic value. JAMA Cardiol 2018; 3: 839-47. https://doi.org/10.1001/jamacardio.2018.2288 PMid:30140889 PMCid:PMC6233650 38. Suri RM, Vanoverschelde J-L, Grigioni F, Schaff HV, Tribouilloy C, Avierinos J-F, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA 2013; 310: 609-16. https://doi.org/10.1001/jama.2013.8643 PMid:23942679 39. Kang D-H, Park S-J, Sun BJ, Cho EJ, Kim D-H, Yun S-C, et al. Early surgery versus conventional treatment for asymptomatic severe mitral regurgitation: a propensity analysis. J Am Coll Cardiol 2014; 63: 2398-407. https://doi.org/10.1016/j.jacc.2014.02.577 PMid:24694528 40. Bijvoet GP, Teske AJ, Chamuleau S a. J, Hart EA, Jansen R, Schaap J. Global longitudinal strain to predict left ventricular dysfunction in asymptomatic patients with severe mitral valve regurgitation: literature review. Neth Heart J 2019 Aug 13; doi: 10.1007/s12471-019-01318-8. https://doi.org/10.1007/s12471-019-01318-8 PMid:31410717 41. de Simone G, Izzo R, Aurigemma GP, De Marco M, Rozza F, Trimarco V, et al. Cardiovascular risk in relation to a new classification of hypertensive left ventricular geometric abnormalities. J Hypertens 2015; 33: 745-54. https://doi.org/10.1097/HJH.0000000000000477 PMid:25915879 42. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57: 450-8. https://doi.org/10.1016/0002-9149(86)90771-X 43. Kuznetsova T, Herbots L, Richart T, D'hooge J, Thijs L, Fagard RH, et al. Left ventricular strain and strain rate in a general population. Eur Heart J 2008; 29: 2014-23. https://doi.org/10.1093/eurheartj/ehn280 PMid:18583396 44. Dini FL, Capozza P, Donati F, Simioniuc A, Corciu AI, Fontanive P, et al. Patterns of left ventricular remodeling in chronic heart failure: Prevalence and prognostic implications. Am Heart J 2011; 161: 1088-95. https://doi.org/10.1016/j.ahj.2011.03.027 PMid:21641355 Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.Archive of Issues
AUTHOR'S CORNER
 Authors having problems with submissions please notify editor: editor@hvt-journal.com
Authors having problems with submissions please notify editor: editor@hvt-journal.com
