Results of surgical correction of gigantic left atrium
CASE REPORT
Results of surgical correction of gigantic left atrium
Article Summary
- DOI: 10.24969/hvt.2020.180
- Page(s): 67-75
- Published: 14/04/2020
- Received: 20/12/2019
- Revised: 12/03/2020
- Accepted: 14/04/2020
- Views: 11189
- Downloads: 7504
- Keywords: giant left atrium, mitral regurgitation, atrial fibrillation, atrioplasty, mitral valve replacement
Address for Correspondence: Kuat Abzaliyev, SRI of Cardiology and Internal Medicine, Kazakh Medical University of Continued Education, Email: kuat_abzaliev@mail.ru
Rustem M.Tuleutayev1,2, Daurenbek O. Urazbekov1,3, Kuat B. Abzaliyev1,2,4, Baurjan A. Rakishev3, Nazym A. Nurollaeva3, Dinara Z. Baiguisova3, Aigerim N. Muhamedjanova3
1SRI of Cardiology and Internal Medicine
2Kazakh Medical University of Continued Education
3National Center of Surgery by AN Syzganov
4Al-Farabi Kazakh National University, Almaty, Kazakhstan
Abstract
We presented a clinical case of surgical treatment of gigantic left atrium in longstanding mitral regurgitation due to mitral valve disease diagnosed 23 years ago (patient refused surgery and was on medical treatment) and complicated by atrial fibrillation. The patient was referred for surgery with complaints on severe dyspnea on minimal exertion, weakness, fatigue, palpitations and massive leg edema. Diagnosis was established using electrocardiography, chest X-Ray, transthoracic and transesophageal echocardiography, and computed tomography. The patient underwent mitral valve replacement, tricuspid valve annuloplasty and left atrial reduction surgery (atrioplasty by Kawazoe). After surgery, left atrial volume decreased from 813 ml to 294 ml and antero-posterior size from 11.2 to 6.2 cm. The patient was discharged on 8th day after surgery. Control examinations after 6 months and 1 year showed reduction of left atrial volume (319 ml and 294 ml); patient feels well and has no complaints.
Thus, our case demonstrated reduction of left atrium early in postoperative period and its slow reduction after surgery during 1 year. It is also showed human reserve capacity and possibility of left atrial dilatation to such sizes. Late diagnosis of such changes in heart is possibly related to the fact that patient was afraid to see doctors and undergo surgery. The left atrial cavity size determination can be done intraoperatively using method of surgical glove we suggested.
Key words: giant left atrium, mitral regurgitation, atrial fibrillation, atrioplasty, mitral valve replacement
Introduction
Giant left atrium (LA) was first described by Hewitt in 1849 (1). It is a rare disease with frequency of 0.3-10%, which usually develops secondary to rheumatic mitral valve disease. Piccoli et al. (2) defined giant left atrium as a cardiothoracic ratio >0.7 in combination with transthoracic echocardiographic antero-posterior size of left atrium of >8cm. Oh et al. (3) recommended a arbitrarily defined cut-off value: more than 6.5 cm. Hurst (4) defined gigantic left atrium as ``that touches right lateral wall of chest``.
Giant LA or atriomegaly is a result of longstanding pressure or volume overload of cardiac chamber, and most frequently is due to mitral regurgitation (5). For the diagnosis of a ``giant left atrium`` we accept antero-posterior size of LA >8cm measured from parasternal long-axis view on transthoracic echocardiography. This condition has significant hemodynamic effects and requires specific treatment.
Many authors proved, that correction of atriomegaly positively influences early and late postoperative periods, and prognosis and survival of such patients are improved (7, 8). Accordingly, during last years, surgical treatment of patients with mitral valve disease, atriomegaly and atrial fibrillation (AF) has received new development and foresees complex approach directed not only at removal of actual valve pathology, but also treatment of complications caused by mitral valve disease. For the first time in 1967, Johnson et al. (9) reported use of LA wall plication for reduction of its size. Further, different modifications of plication stitching and application of redundant atrial wall resection have been suggested (10, 11).
We present a clinical case of giant left atrium associated with longstanding mitral valve regurgitation, discuss etiology, clinical manifestations, methods of diagnostics, surgical treatment and dynamics of LA after operation.
Case report
A 60-year-old female patient was admitted to National Center of Surgery by AN Syzganov for surgical treatment with complaints on severe dyspnea on minimal exertion, palpitations, weakness, fatigue and leg edema. She has had history of mitral valve disease and permanent AF for 23 years. Earlier she refused recommended surgical treatment and received medical treatment. She had no history of diabetes, hypertension, overweight or obesity (she had normal body mass index), family history of coronary heart disease or alcoholism. She was a non-smoker.
On physical examination, she had weakened first heart sound (S1), apical pansystolic murmur radiating to the left axillary area and under left scapula. The second heart sound was accentuated over pulmonary artery. The liver edge was palpated 5 cm below costal margin. She had edema of lower extremities up to knee level.
Her electrocardiogram (ECG) displayed AF, heart rate - 98 beats/min, ventricular premature beats in form of bigeminy and signs of left ventricular hypertrophy.
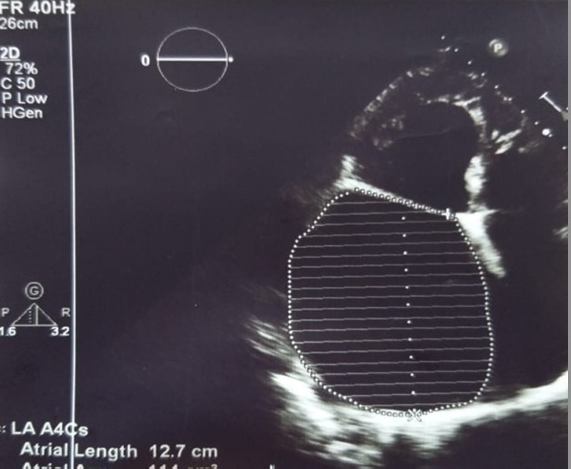
Figure 1. Echocardiographic view of a severely dilated left atrium: size - 8.3x12x10 cm and volume – 813 ml
Transthoracic echocardiogram revealed severe LA dilatation – anteroposterior size of 11.2 cm, 8.3x12x10 cm and volume – 813 ml (Fig. 1). There was dilatation of mitral annulus – 4.6 cm. Mitral valve leaflets were fibrotically changed, coaptation of leaflets - absent, chordae tendinae were thickened, fused and shortened, accompanied by severe mitral regurgitation (MR). Vena contracta - 1.1 cm. Ascending aorta was 3.3 cm. She had dilatation [(LV end-diastolic dimension (LVEDD) – 6.9 cm, end-systolic dimension (LVESD) – 4.7 cm, LV end-diastolic volume (LVEDV) -250 ml, end-systolic volume (LVESV) – 105 ml)] and hypertrophy [(interventricular septum thickness (IVS) – 1.2 cm, left ventricular posterior wall thickness (PWT) – 1.1 cm)] of left ventricle (LV), and its dysfunction: stroke volume (SV) – 146 ml, and LV ejection fraction (LVEF) – 58%. There was pulmonary hypertension – right ventricular systolic pressure (RVSP) – 55 mm Hg. There was no sign of right ventricular dysfunction - tricuspid annular plane systolic excursion (TAPSE) was 1.9 cm. Right atrial area was 41см2. Patient had severe tricuspid regurgitation.
Transesophageal echocardiography revealed giant LA with signs of spontaneous echocardiographic contrast resembling ``heavy smoke`` and ``blizzard, ice storm`, but without definite thrombi.
On chest X-ray (Fig. 2) anteroposterior view, heart was significantly enlarged in diameter. Left contour of cardiac shadow merged with thoracic wall (cor bovinum), waist was absent, 2nd and 3rd arcs moderately bulged out (3>2). On the 1st oblique lateral view there was the 3rd degree of retrocardiac space narrowing, esophagus (barium esophagram) inclined over big radius arc (>8 cm), conus pulmonalis bulged out. On the 2nd oblique lateral view there was enlargement of left and right cardiac chambers. Aorta was without changes, pulsation was preserved. Cardiothoracic index was 0.77.
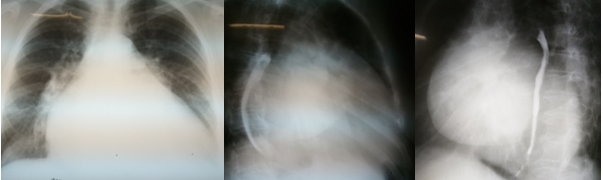
Figure 2. Cardiac silhouette size in 3 views.
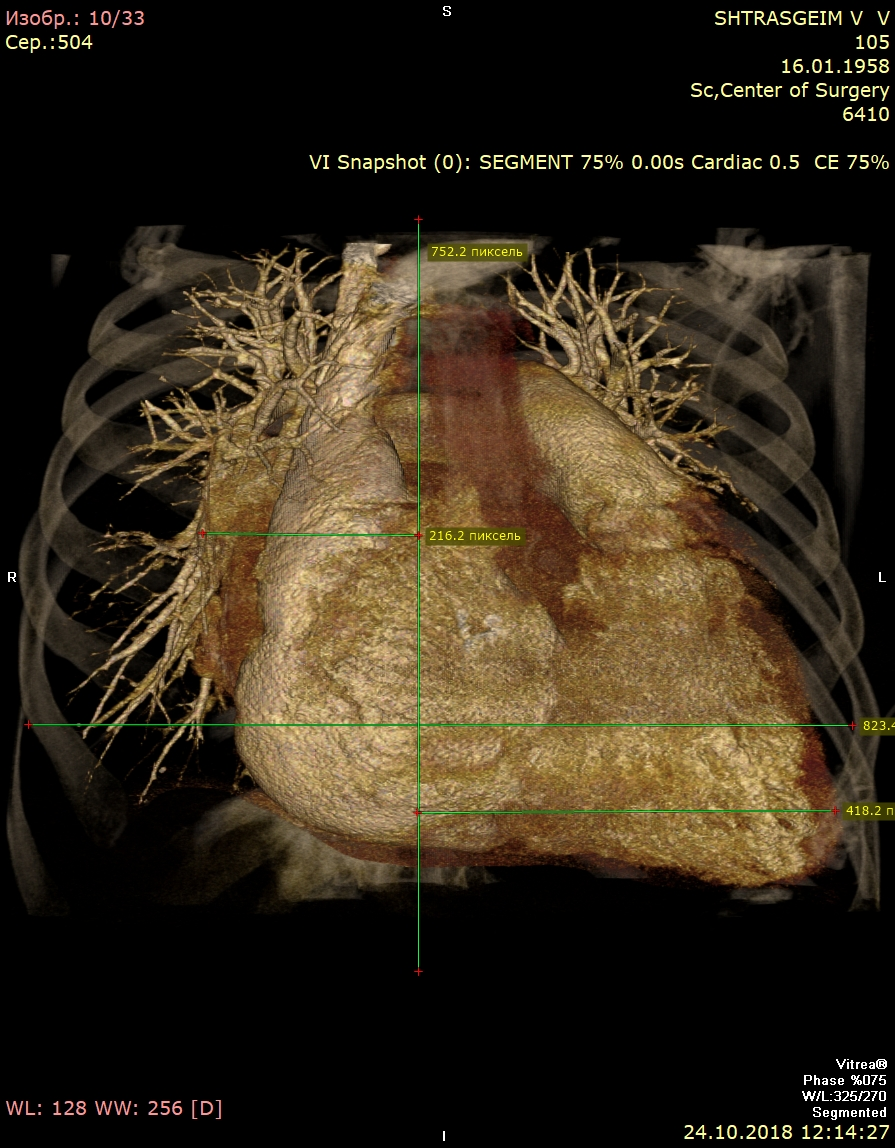
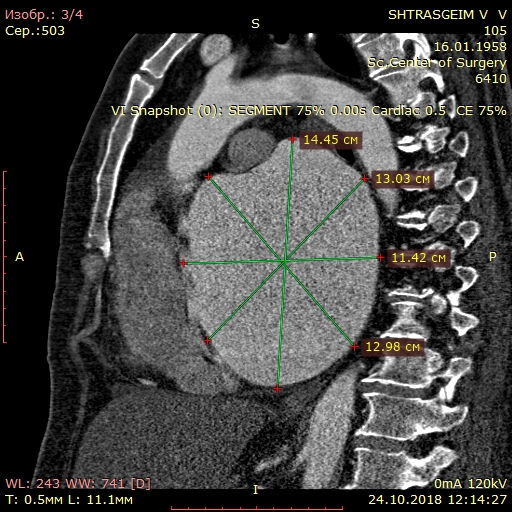
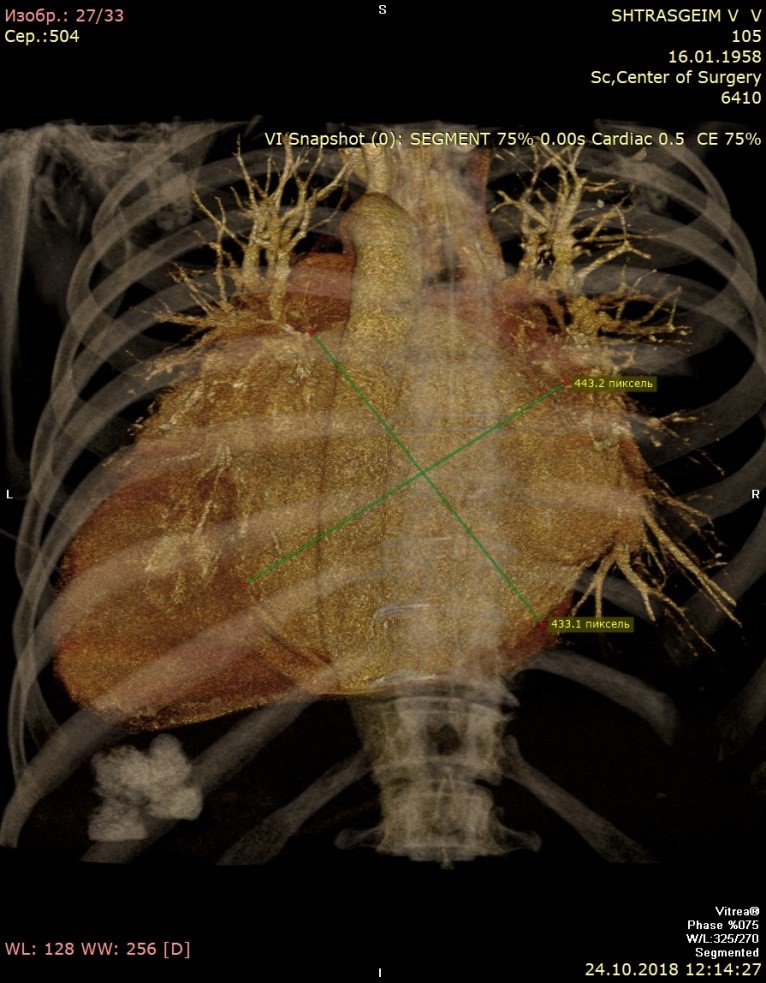
Computed tomography (Fig. 3) showed giant LA - 14.6x.11.4x14.1 cm in size. Three-dimensional volume rendering demonstrated significant dilatation of LA, occupying almost all mediastinum. Patient underwent coronary angiography, which demonstrated 40% stenosis in right coronary artery.
The patient received following medications: diuretics (weight reduced from 97 to 88 kg, peripheral edema disappeared), beta-blocker bisoprolol for rate control of AF, anticoagulants, digitalis, angiotensin-converting enzyme inhibitor and gastroprotectors.
Thus based on evaluation, the patient had class I indications for mitral valve surgery, as giant LA was associated with mitral valve disease with signs of severe mitral regurgitation (12). She was symptomatic and had left ventricular dysfunction.
Patient underwent successful mitral valve replacement (MVR) by mechanical prosthesis St.Jude No 33 with preservation of posterior leaflet, atrioplasty by Kawazoe (Fig. 4) and tricuspid valve annuloplasty (Fig. 5) under cardiopulmonary bypass and pharmaco-cold cardioplegia.
 A)
A)
 B)
B)
 С)
С)
Figure 3. Giant left atrium – anterior view (A), lateral view (B), and posterior view (C)
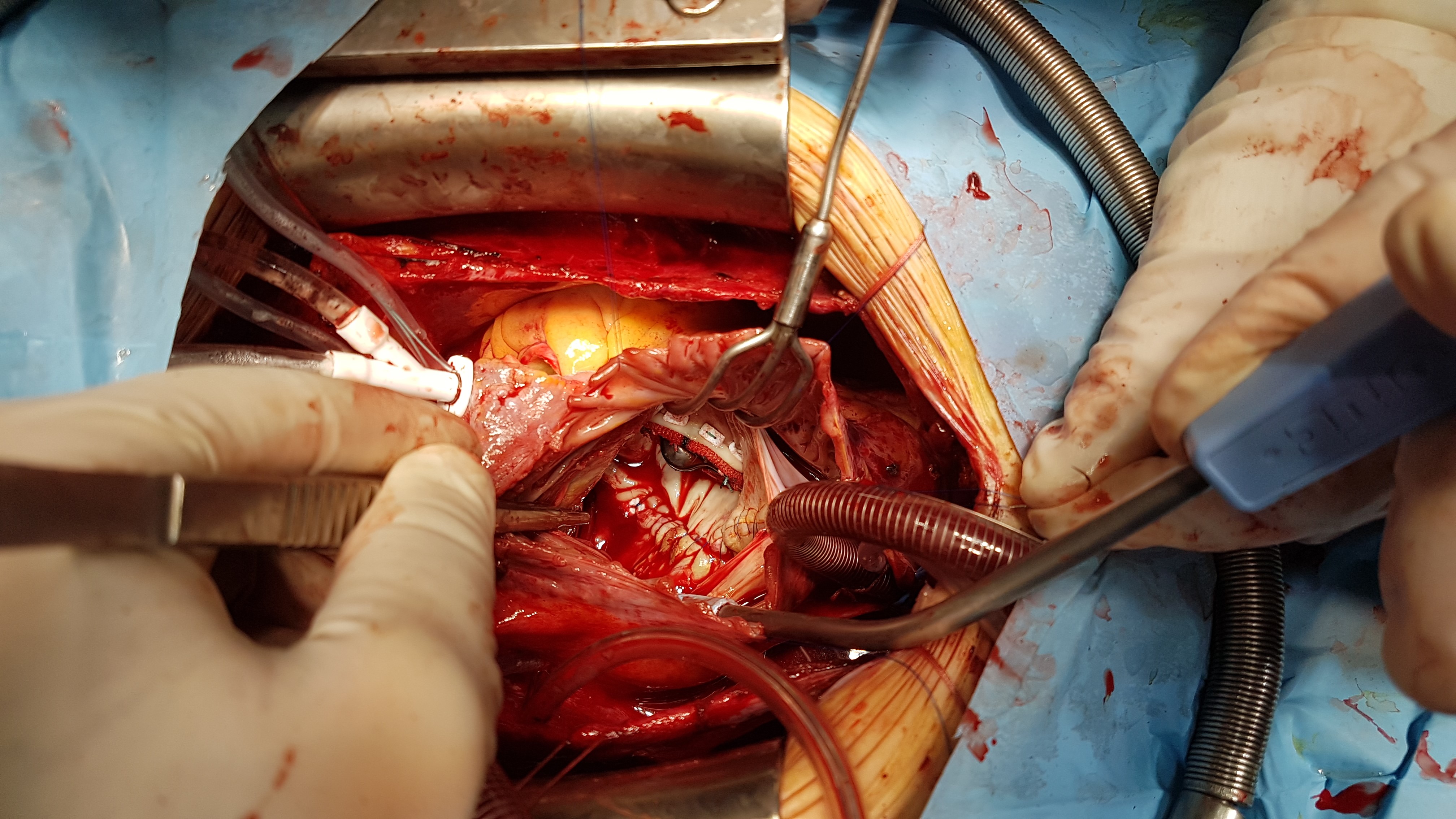
Figure 4. Replacement of mitral valve by mechanical prosthesis St.Jude N33 with preservation of posterior leaflet and atrioplasty by Kawazoe
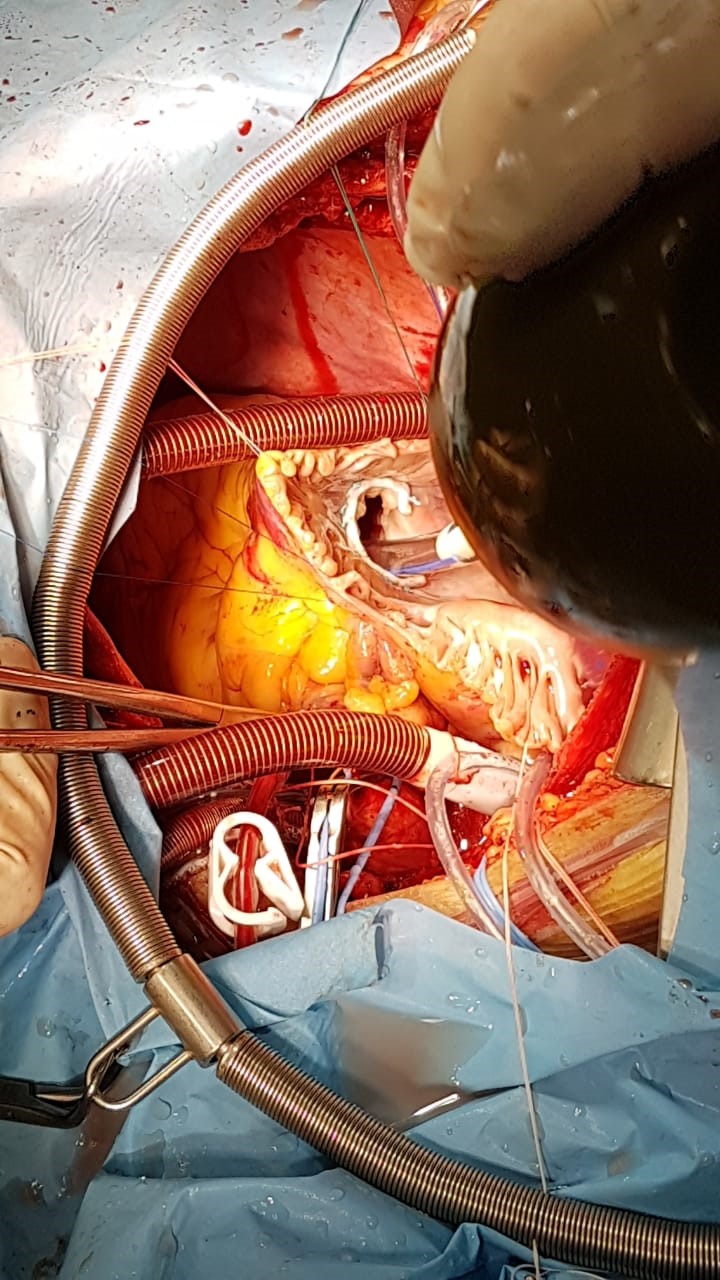
Figure 5. Annuloplasty of tricuspid valve using bearing half-ring Sorin N36
Then we used a new method of intraoperative LA volume measurement (developed by authors - Patent 19776 20.02.2007) and determined that before atrioplasty LA cavity could accommodate 990 ml of saline and after atrioplasty – 400 ml. Method is used as following: measurement of LA volume is performed before and after correction of mitral valve disease (Fig. 7). With this aim, rubber container prepared from rubber surgical glove (2) is introduced in LA (1) through incision in IVS (3). Sterile surgical glove (2) is connected to polyvinyl tubes (4) and is fixated by ligature. Saline is administered through one tube using 100 ml syringe (5) and the air is removed from glove through another tube. When the liquid will flow from the second tube, it is necessary to block it and continue administration of saline up to maximal size. Maximal quantity of liquid in the rubber container located in LA will determine the LA volume.
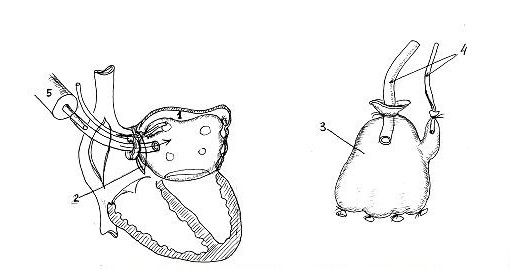
Figure 6. Determination of left atrial volume using surgical glove (Patent 19776, 20.02.2007)
Patient`s postoperative period was without complications. She was extubated in early postoperative period. Postoperative echocardiography demonstrated normally functioning mitral prosthesis, reduction in size and volume of cardiac chambers (antero-posterior size of LA of 6.2 cm and left atrial volume of 312 ml,) and reduction of RVSP – 27 mm Hg. The patient was discharged on 8th day after surgery.
Control transthoracic echocardiogram 6 months after surgery (10.06.2019) (Fig. 7) revealed no dysfunction of mitral prosthesis, minimal tricuspid regurgitation, reduction of LA size to 6.8x6.5x7.5 cm, and volume - 319 ml; reduction in LV size and volume (EDD – 6.1 cm, ESD 4.2 cm, EDV – 190 ml, ESV – 79 ml). Her LV SV was 111 ml, LVEF – 57%, IVS - 1.2 cm, and PWT-0.8 cm, and RVSP - 30 mm Hg.
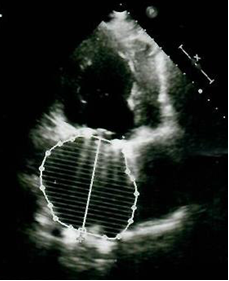
Figure 7. Left atrium volume of 319 ml on 6-month follow-up echocardiogram
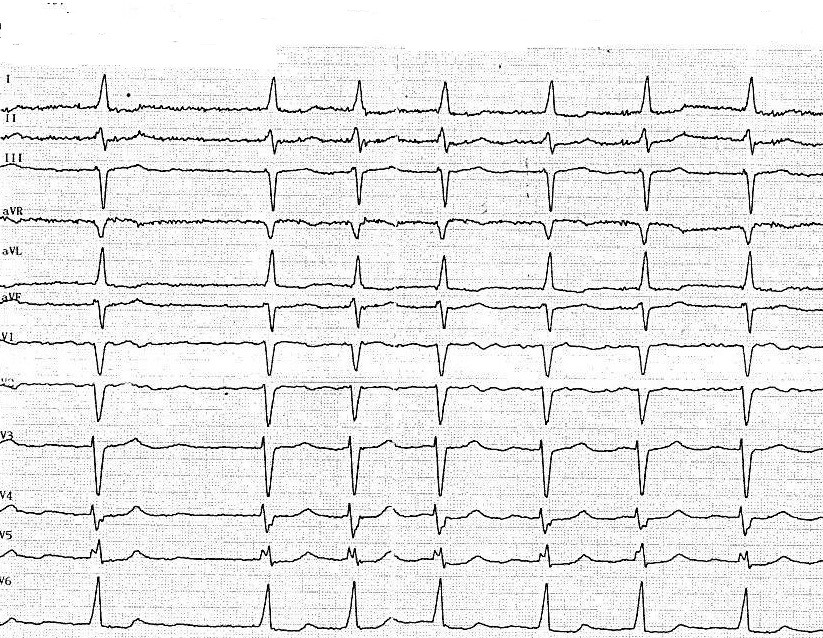
Figure 8. Electrocardiogram 6 months after surgery
Control electrocardiogram 6 months after surgery (10.06.2019) (Fig. 8) demonstrated HR – 103 beats/min, left axis deviation, high-ventricular rate AF and left ventricular hypertrophy (QRS – 0.08 sec, QRST -0.36 sec, RR – 0.46-0.78 sec, ˂α -30).
Her control ECG one year after surgery (7.11.2019) (Fig. 9) showed left axis deviation, AF, partial right bundle branch block and left ventricular hypertrophy (QRS-0.07 sec, QRST- 0.36 sec, RR-0.52-1.03 sec, ˂ α -40. HR – 86 beats/min). Control transthoracic echocardiogram 1 year after operation (7.11.2019) depicted absence of mitral prosthesis dysfunction, minimal tricuspid regurgitation, LA size of 7.1x8.4x7.8 cm and volume of 294 ml; sustained reduction in LV size and volume (EDD – 6.7 cm, ESD – 4.6 cm, EDV – 231 ml, ESV- 97 ml), SV- 134 ml, EF-57%, IVS - 1.3cm, PWT – 0.9 cm, and normal right ventricular function (TAPSE-1.9 cm). RVSP further reduced to 20 mm Hg.
Thus, there were the further reduction of LA volume, reduction of LV size and marked reduction of RVSP on control echocardiography 6 months and 1 year after surgery. As a result, left atrial volume reduced by 2.8 times from 813 ml to 294 ml. Rhythm is AF. The patient feels well, without any complaints.
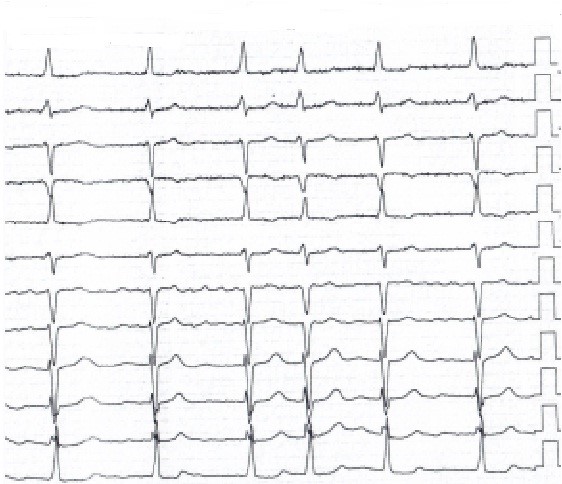
Figure 9. Electrocardiogram 1 year after surgery
Discussion
We presented as a rare case of longstanding mitral valve disease with MR and gigantic LA (LA size -8.3cm, volume -813 ml), accompanied by AF and successfully treated by LA reduction surgery, MVR and tricuspid valve repair.
Currently, giant LA is a rare entity. It is present in 19% of patients requiring surgical correction of mitral regurgitation (6). Among the factors, contributing to development of giant left atrium is the long-standing severe MR without surgical correction. Our case was remarkable by 23-year history of mitral valve disease and severe MR. She was offered mitral surgery before but refused it. It is important to remember that mitral valve surgery is indicated in symptomatic cases with severe MR and asymptomatic cases with severe MR in presence of LV dysfunction LVEF<60%, and LV ESD>4 cm (12). Our patient was symptomatic and had LV dysfunction (LVEF=58% and LVESD =4.7 cm) on presentation.
The clinical manifestation of giant LA includes the symptoms of compression of LV posterior wall and compression of left bronchi and lower lobe of right lung (7, 10, 13). However, these symptoms were absent in our patient.
The diagnosis is usually established using echocardiography (2, 3), and increased cardiothoracic ratio on chest X-ray (>0.7) (2). It is sometimes impossible to make a correct diagnosis of LA enlargement using only chest X-Ray, as it may be misdiagnosed as massive tumor or pleural effusion (13). The diagnosis of gigantic LA in our patient was established using echocardiography (LA size 11.2 cm) and computed tomography, she had also increased cardiothoracic ratio (0.77) on chest X-ray.
The aim of surgical operation in our patient was correction of mitral valve disease and reduction of giant left atrium to prevent thromboemboli and progression of heart failure.
There are several methods in arsenal of surgeons (13- 15), aimed at correction of atriomegaly. Some methods are complex enough, other are not efficient in reducing of markedly enlarged atrial volume. Some surgeons do not see the need in correction of atriomegaly and prefer not to perform additional manipulation considering this prolongs aortic cross-clumping time and cardiopulmonary bypass time. At the same time, as many authors showed (2, 8), removal of only valvular pathology in patients with atriomegaly does not lead to significant reduction of LA size and volume.
Method of left atrioplasty surgery is simple; it practically little prolongs cardiopulmonary bypass time or hypothermia. Left atrioplasty leads to a distinct reduction of LA size, disappearance of signs of compression of postero-basal segment of LV (hemodynamic instability), reduction of bifurcation angle of trachea, disappearance of compression of left main bronchi and lower lobes of right lung and reduces mortality (7, 10). This explains decrease in frequency and duration of acute heart failure in early postoperative period; due to elimination of compression of left main bronchi and lower lobes of right lung, the duration of postoperative artificial lung ventilation reduces, the lung straightening improves contributing to reduced frequency of postoperative lung atelectasis, pneumonia and trachea-bronchitis. It might shorten the stay of patient in reanimation department, facilitate rehabilitation of patients and shorten the hospitalization time.
Evaluating direct result of operative treatment, all surgeons give importance to restoration of hemodynamics, and absence of compression of surrounding tissues by enlarged LA. Moreover, reversibility of complications developed before, during and after operation is taken in account.
Studies regarding atrial reduction surgery in patients with gigantic left atrium (>65 mm) demonstrated that LA reduces early after surgery for valvular heart disease with absence of further reduction in late period after surgery (16, 17). In our case, the LA volume reduced from 813 to 312 ml early after operation and further reduced to 294 ml one year after surgery.
In patients with giant LA and AF, atrial reduction surgery with concomitant surgical ablation of AF were shown to successfully restore sinus rhythm (13, 16, 18). We did not consider rhythm control of AF in our patient, due risk of perforation of markedly enlarged left atrium, taking in account opinion of Kosakai et al. (18) on 100% efficacy of surgical ablation of AF in LA diameter <45 mm and its absolute inefficacy when LA diameter is more than 85 mm. We should also mention that our patient had 23-year history of permanent AF.
Conclusion: Thus, our case demonstrated reduction of giant left atrium early in postoperative period and its slow reduction after surgery during 1 year in longstanding mitral valve disease and MR. It is also showed human reserve capacity and possibility of left atrial dilatation to such sizes. Late diagnosis of such changes in heart is possibly related to the fact that patient was afraid to see doctors and undergo surgery. The left atrial cavity size determination can be done intraoperatively using method of surgical glove we suggested.
Peer-review: Internal and external
Conflict of interest: None to declare
Authorship: R.M.T., D.O. U., K.B.A., B.A.R., N.A.N., D.Z. B., A.N.M. equally contributed to preparation of article and fulfilled authorship criteria
Acknowledgement and funding: None to declare
References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

