Severe bradyarrhythmia requiring temporary pacemaker in a COVID-19 patient receiving lopinavir/ritonavir treatment: a case report
CASE REPORT
Severe bradyarrhythmia requiring temporary pacemaker in a COVID-19 patient receiving lopinavir/ritonavir treatment: a case report
Article Summary
- DOI: 10.24969/hvt.2020.198
- Page(s): 79-84
- CARDIOVASCULAR DISEASES
- Published: 05/06/2020
- Received: 25/05/2020
- Revised: 05/06/2020
- Accepted: 05/06/2020
- Views: 9114
- Downloads: 7238
-
Citations1. 10.1007/s40278-020-86719-1
2. 10.24969/hvt.2020.203
3. 10.24969/hvt.2020.211
4. 10.24969/hvt.2022.329- Keywords: COVID-19, bradyarrhythmia, sinus arrest, junctional rhythm, lopinavir, ritonavir, complication, pacemaker
PDF PRINT VERSION CommentsAddress for CorrespondenceAddress for Correspondence: Zhenisgul Tlegenova, West Kazakhstan Marat Ospanov Medical University, Email: Tlegenova_G@mail.ru
Zhenisgul Sh. Tlegenova1, Bekbolat Zholdin1, Meirambek S. Kurmangazin1, Bulat K. Khamidulla2, Zhambul E. Zhailybaev21West Kazakhstan Marat Ospanov Medical University, Aktobe, Kazakhstan
2Aktobe Medical Centre, Aktobe, Kazakhstan
Abstract
A 74-year-old Asian female with a history of hypertension and chronic obstructive pulmonary disease was admitted to hospital for coughing, shortness of breath and fatigue; her nasopharyngeal swab was positive for COVID-19.
Lopinavir/ritonavir 800mg/200 mg, daily was started. On the third day of antiviral therapy, the patient complained of dizziness, nausea, she was disorientated, and electrocardiogram showed sinus arrest, junctional escape rhythm 36 beats per min. Patient was successfully treated by temporary pacemaker, normal sinus rhythm was reverted on a 3rd day after stopping the lopinavir/ritonavir treatment. She was discharged home in stable condition.
Bradyarrhythmia in form of sinus arrest can develop during treatment with lopinavir/ritonavir. The temporal nature of the observed changes and the ECG finding suggests the use of L/R contributed to the changes.
This case highlights off-label prescribing lopinavir/ritonavir outside of a clinical trial setting should be avoided until the data have proven that treatment benefit over placebo.
Key words: COVID-19, bradyarrhythmia, sinus arrest, junctional rhythm, lopinavir, ritonavir, complication, pacemaker
Introduction
The coronavirus disease-2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) and now represents the global pandemic (1). COVID-19 is most frequently presents with respiratory symptoms that can progress to pneumonia and in severe cases acute respiratory distress syndrome and shock (2). Currently, there is no available treatment proven to be effective against COVID-19, but multiple agents, including lopinavir and ritonavir (L/R), remdesivir, chloroquine, hydroxychloroquine, ribavirin are used off label (3). The results of studies do not allow us to draw an indisputable conclusion about their effectiveness and safety. It is important to report some of these antiviral agents have cardiac toxicities (4).
We report and discuss the case a 74-year old Asian female who developed severe bradyarrhythmia requiring temporary pacemaker after the L/R treatment.
Case report
A 74-year-old Asian female with a history of hypertension and chronic obstructive pulmonary disease was admitted to hospital with complaints of dyspnea, cough and fatigue. Her polymerase chain reaction (PCR) test for SARS -CoV-2 RNA was found to be positive. Medications prior to admission included perindopril 10 mg and zopiclon 7.5 mg.
On examination, her blood pressure was 140/90 mm Hg, heart rate was 84 bpm, respiratory rate of 24 breaths per minute and a room air oxygen saturation of 95%. Her physical examination revealed a scattered bilateral crackles and no peripheral edema. A 12-lead electrocardiogram (ECG) recording at admission showed normal sinus rhythm without clinical abnormalities. She had no personal or family history of arrhythmia.
The patient was treated with L/R 800mg/200 mg daily, according to COVID-19 clinical guide (5). Patient provided written informed consent prior the treatment with L/R was started. Additionally she received ceftriaxone, lidocainum, loratadinum, ambroxol and perindopril 10 mg, while zopiclon was canceled. On the third day of antiviral therapy, the patient suddenly complained of dizziness, nausea, and general malaise. Patient was disorientated; her heart rate was slow. Brain injury was classified as moderate, the Glasgow Coma Scale (GCS) was 12 points. The ECG showed sinus arrest with junctional escape rhythm 36 bpm (Fig. 1). The high sensitivity troponin, serum electrolytes, glomerular filtration rate, PH, PCO2, PO2, SaО2 were normal (Table 1).
Bedside 2-dimensional transthoracic echocardiogram demonstrated left ventricular hypertrophy, a preserved left ventricular ejection fraction of 60% by Simpson rule, without any regional wall motion abnormalities and estimated pulmonary artery systolic pressure was 45 mm Hg.
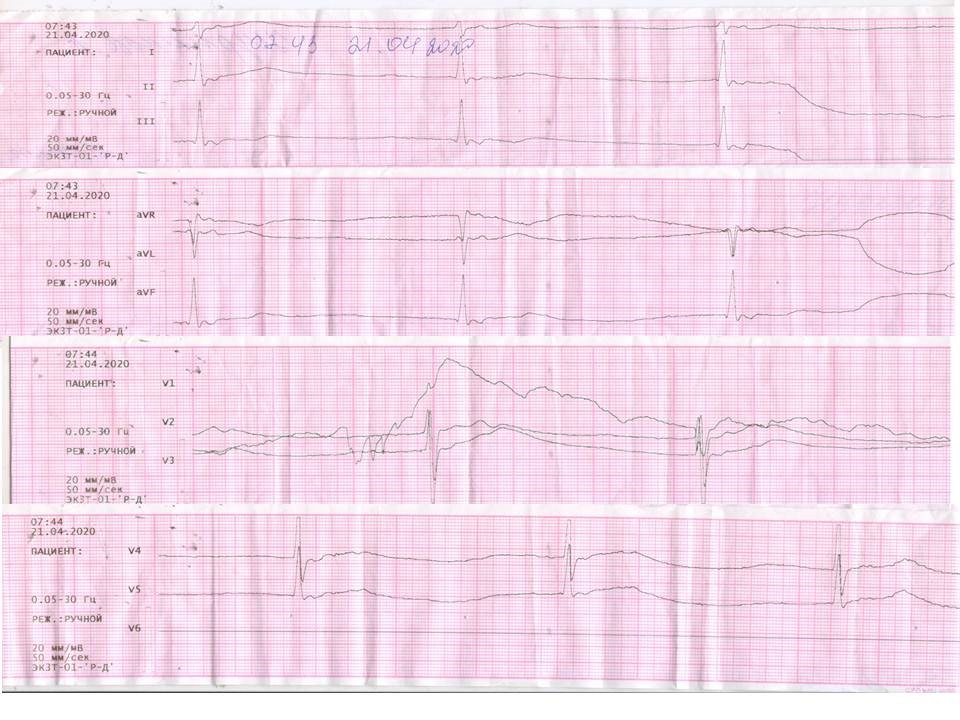
Figure 1. sinus arrest, junctional escape rhythm 36 bpm; RR =1640 msec, QTc (Framingham) = 507 msec
Table 1. Laboratory investigations
Tests
Time of admission
Time of developing bradyarrhythmia
Hemoglobin, g/l
125
128
Serum creatinine, mmol/l/
73.2
101.1
Glomerular filtration rate (Cockroft formula), ml/min/1.73 m2
63
46
Glucose, mmol/l
4.0
5.8
Serum potassium, mmol/l
4.8
4.0
High –sensitivity C-reactive protein, mg/l
8
8
High-sensitivity troponin I, ng/ml
-
<0.010
Procalcitonin, ng/ml
-
0
PH
-
7.378
PCO2, mmHg
-
35.1
PO2, mmHg
-
35.0
SaО2, %
95%
94%
Atropine sulphate and dopamine 5-6 mkg/kg/min iv were administered, but no effect on heart rate had been seen. Antiviral therapy was stopped, the patient received insertion of a temporary pacemaker, and a normal sinus rhythm was restored in 72 hours. She was discharged home in stable condition 14 days later. Her ECG after one month demonstrated normal sinus rhythm (Fig. 2).
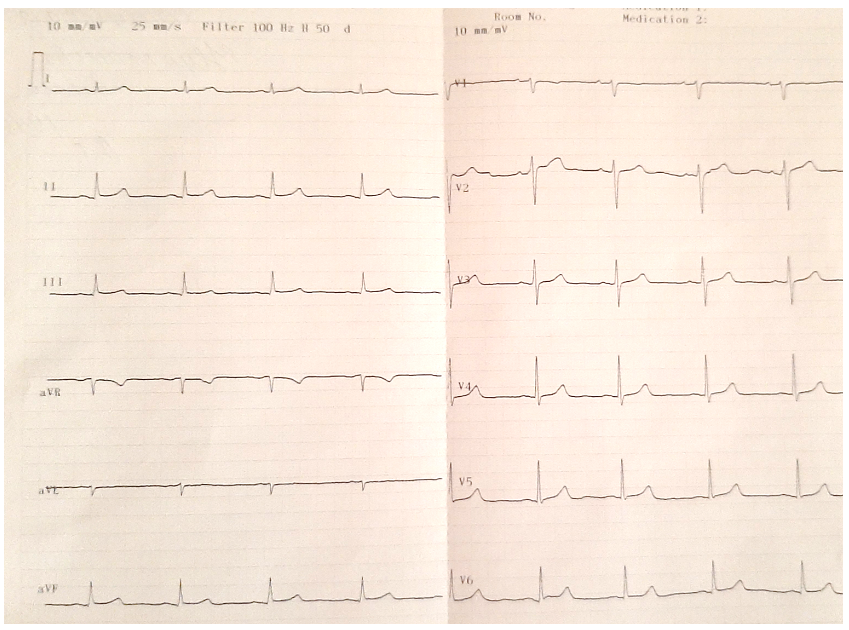
Figure 2. sinus rhythm after 1 month of discharge from hospital
DiscussionOur patient was admitted to the hospital and started the L/R treatment because her SARS -CoV-2 RNA test was positive, her condition was evaluated as moderate disease severity and she had risk factors such as advanced age, hypertension and chronic obstructive pulmonary disease those are predictors of poor clinical outcomes. On a 3rd day after L/R 400mg/100 mg twice a day was started, patient become disorientated, her ECG showed sinus arrest with junctional rhythm 36 per min.
COVID-19 patients are likely to be at risk of arrhythmia due to underlying comorbidities, the myocardial injury, polypharmacy and toxicities of the potential medications used for COVID-19 treatment (6, 7). In the study from Wuhan, China, malignant arrhythmias were reported to be present in 16.7% of COVID-19 hospitalized patients and 44.4% of intensive care patients with COVID-19 (8).
Myocardial injury in COVID-19 is associated with impairment of cardiac function, inflammation, and an increased high sensitivity troponin. The underlying mechanisms of these abnormalities in the severe stage of COVID-19 may be resulted from inflammatory damage incurred by the virus, hypoxia, metabolic derangements, acidosis, intravascular volume imbalances, neurohormonal, and catecholaminergic stress (9). In our patient markers of myocardial injury such as left ventriclular ejection fraction and high sensitive troponin were not pathological.
Our patient had severe bradyarrhythmia of 36 bpm occurred after three days of L/R treatment. Patient was disorientated that was evaluated as GCS 12 points. However, the patient did not have severe hypoxia as a room air oxygen saturation SaO2 was 94%, PCO2- 35.1 mmHg, PO2- 35.0 mmHg. The brain injury (disorientation) may be due to the toxic effect of L/R also.
L/R is the HIV protease inhibitor class of antiretroviral drugs cause dose-dependent block of human ether-a-go-go-related gene (HERG) potassium channels and predispose individuals to QT prolongation and torsade de pointes (10), especially in the setting of various co-medications that are metabolized by CYP3A4. Some cardiovascular drugs such as atorvastatin, clopidogrel, rivaroxaban are major substrates of CYP3A4 (11). In our case, L/R was co-administered with ceftriaxone/lidocaine, loratadine, ambroxol, and perindopril. We do not found major interactions between these drugs when checked their co-administration in drug interaction checker. Our patient has other risk factors for QT prolongation such as female sex, hypertension, and advanced age (12). Her QTc was 507 msec that is prolonged. A prolonged QTc interval is the most famous surrogate marker of cardiotoxicity that can increase malignant arrhythmias (13).
Concurrent atazanavir and L/R administration was associated with PR and QRS interval changes in small study population (14). Bradycardia-tachycardia syndrome, first and second degree atrioventricular blocks induced by lopinavir-ritonavir have been documented in patients with acquired immunodeficiency syndrome (15-17). Our report also documented that L/R have potential to alter sinus node.
In study from China in hospitalized adult patients with severe COVID-19 no benefit was observed with L/R treatment beyond standard care and L/R treatment was stopped early in 13.8% patients because they had adverse events (18).
Experts recommended, if it was decided the patient has an indication for the use of drugs for the treatment of COVID 19, a clear administration protocols should be in every hospital and clinic using these drugs (19). Baseline checklist that is followed includes: baseline evaluation of serum electrolytes, drug-drug interaction, interruption of other nonessential drugs that can also prolong the QT, PQ intervals and ECG parameters PQ, QRS, QTc should be measured (20, 21).
Conclusion
Bradyarrhythmia in form of sinus arrest can develop during treatment with lopinavir/ritonavir. The temporal nature of the observed changes and the ECG findings suggest the use of L/R contributed to the changes.
This case highlights it is necessary to investigate balance between efficacy and safety of lopinavir/ritonavir for COVID-19 therapy. Off –label prescribing lopinavir/ritonavir outside of a clinical trial setting should be avoided until the data have proven that treatment benefit over placebo.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: Zh. Sh. T., B. Zh. M.S.K., B. K. Kh., Zh. E. Zh. equally contributed to preparation of manuscript and fulfilled authorship criteria
Acknowledgments and funding: None to declare
References
1. WHO. Global research on coronavirus disease. Available at: URL: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov 2. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar -Pena R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 2020; 34: 101623. doi:10.1016/j.tmaid.2020.101623 https://doi.org/10.1016/j.tmaid.2020.101623 PMid:32179124 PMCid:PMC7102608 3. McCreary EK, Pogue JM. Coronavirus Disease 2019 treatment: a review of early and emerging options. Open Forum Infect Dis 2020; 7: ofaa105. Published 2020 Mar 23. doi:10.1093/ofid/ofaa105 https://doi.org/10.1093/ofid/ofaa105 PMid:32284951 PMCid:PMC7144823 4. Akhmerov A, Marbán E. COVID-19 and the heart. Circ Res 2020; 126: 1443‐55. doi:10.1161/CIRCRESAHA.120.317055 https://doi.org/10.1161/CIRCRESAHA.120.317055 PMid:32252591 5. Coronavirus infection - Covid-19. RCPHE (Republic Center of Public Health Development). Version: Clinical protocols. Available at URL: https://diseases.medelement.com/disease/коронавирусная-инфекция-2019-ncov-кп-мз-рк/16390 6. Carpenter A, Chambers OJ, El Harchi A, Bond R, Hanington O, Harmer SC, et al. COVID-19 management and arrhythmia: risks and challenges for clinicians treating patients affected by SARS-CoV-2. Front Cardiovasc Med 2020; 7: 85. doi:10.3389/fcvm.2020.00085 https://doi.org/10.3389/fcvm.2020.00085 PMid:32432127 PMCid:PMC7214683 7. Han H, Xie L, Liu R, Yang J, Liu F, Wu K, et al. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol 2020; doi: 10.1002/jmv.25809. doi:10.1002/jmv.25809 https://doi.org/10.1002/jmv.25809 8. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020; e200950. doi:10.1001/jamacardio.2020.0950 https://doi.org/10.1001/jamacardio.2020.0950 PMid:32211816 PMCid:PMC7097841 9. He J, Wu B, Chen Y, Tang J, Liu Q, Zhou S, et al. Characteristic electrocardiographic manifestations in patients with COVID-19. Can J Cardiol 2020; S0828-282X(20)30301-9. doi:10.1016/j.cjca.2020.03.028 https://doi.org/10.1016/j.cjca.2020.03.028 PMid:32299751 PMCid:PMC7156155 10. Anson BD, Weaver JG, Ackerman MJ, Akinsete O, Henry K, January CT, et al. Blockade of HERG channels by HIV protease inhibitors. Lancet 2005; 365: 682‐6. doi:10.1016/S0140-6736(05)17950-1 https://doi.org/10.1016/S0140-6736(05)17950-1 11. Drugs interactions checker. Available at: URL: https://www.drugs.com/interactions-check. 12. Vandael E, Vandenberk B, Vandenberghe J, Willems R, Foulon V. Risk factors for QTc-prolongation: systematic review of the evidence. Int J Clin Pharm 2017; 39: 16‐25. doi:10.1007/s11096-016-0414-2 https://doi.org/10.1007/s11096-016-0414-2 PMid:28012118 13. Ritter JM. Cardiac safety, drug-induced QT prolongation and torsade de pointes (TdP). Br J Clin Pharmacol 2012; 73: 331‐4. doi:10.1111/j.1365-2125.2012.04193.x https://doi.org/10.1111/j.1365-2125.2012.04193.x PMid:22329611 PMCid:PMC3370337 14. Rathbun CR, Liedtke MD, Blevins SM, Harrison D, Lockhart SM, Salvaggio M, et al. Electrocardiogram abnormalities with atazanavir and lopinavir/ritonavir. HIV Clin Trials 2009; 10: 328‐36. doi:10.1310/hct1005-328. https://doi.org/10.1310/hct1005-328 PMid:19906626 15. Chaubey SK, Sinha AK, Phillips E, Russell DB, Falhammar H. Transient cardiac arrhythmias related to lopinavir/ritonavir in two patients with HIV infection. Sex Health 2009; 6: 254‐7. doi:10.1071/SH09005 https://doi.org/10.1071/SH09005 PMid:19653965 16. Kikuchi Y, Genka I, Ishizaki A, Sunagawa K, Yasuoka A, Oka S. Serious bradyarrhythmia that was possibly induced by lopinavir-ritonavir in 2 patients with acquired immunodeficiency syndrome. Clin Infect Dis 2002; 35: 488‐90. doi:10.1086/341975 https://doi.org/10.1086/341975 PMid:12145735 17. Yotsumoto M, Kitano K, Saito H. Bradycardia-tachycardia syndrome induced by lopinavir-ritonavir in a patient with AIDS. AIDS 2005; 19: 1547‐8. doi:10.1097/01.aids.0000183942.05849.1b https://doi.org/10.1097/01.aids.0000183942.05849.1b PMid:16135911 18. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G,et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020 382: 1787‐99. doi:10.1056/NEJMoa2001282 https://doi.org/10.1056/NEJMoa2001282 PMid:32187464 PMCid:PMC7121492 19. Naksuk N, Lazar S, Peeraphatdit TB. Cardiac safety of off-label COVID-19 drug therapy: a review and proposed monitoring protocol. Eur Heart J Acute Cardiovasc Care 2020; 9: 215‐21. doi:10.1177/2048872620922784 https://doi.org/10.1177/2048872620922784 PMid:32372695 PMCid:PMC7235441 20. Asensio E, Acunzo R, Uribe W, Saad EB, Saenz LC. Recommendations for the measurement of the QT interval during the use of drugs for COVID-19 infection treatment. Updatable in accordance with the availability of new evidence. J Interv Card Electrophysiol 2020; 1‐6. doi:10.1007/s10840-020-00765-3 https://doi.org/10.1007/s10840-020-00765-3 PMid:32418181 PMCid:PMC7229438 21. Sapp JL, Alqarawi W, MacIntyre CJ, et al. Guidance on minimizing risk of drug-induced ventricular arrhythmia during treatment of COVID-19: A Statement from the Canadian Heart Rhythm Society. Can J Cardiol 2020; S0828-282X(20)30325-1. doi:10.1016/j.cjca.2020.04.003 https://doi.org/10.1016/j.cjca.2020.04.003 PMCid:PMC7195336

Flowers. Alicja Stanczyk – 4.5 years, Warsaw, Poland.
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.Archive of Issues
AUTHOR'S CORNER
 Authors having problems with submissions please notify editor: editor@hvt-journal.com
Authors having problems with submissions please notify editor: editor@hvt-journal.com
