Athletic heart adaptation, pathological hypertrophy and sudden cardiac death
REVIEW
Athletic heart adaptation, pathological hypertrophy and sudden cardiac death
Article Summary
- DOI: 10.24969/hvt.2020.199
- Page(s): 55-66
- CARDIOVASCULAR DISEASES
- Published: 27/05/2020
- Received: 30/01/2020
- Revised: 10/05/2020
- Accepted: 14/05/2020
- Views: 22492
- Downloads: 8017
-
Citations
- Keywords: athlete’s heart, pathological hypertrophy, maladaptive hypertrophy, sudden cardiac death, cardiovascular imaging, overtraining
PDF PRINT VERSION Аннотация (Рус.) Корутунду (Кырг.) CommentsAddress for CorrespondenceAddress for Correspondence: Damirbek Abibillaev, Scientific Research Institute of Heart Surgery and Organ Transplantation, Bishkek, Kyrgyzstan Email: kg.damir.da@gmail.com
Damirbek Abibillaev1, Fuat Kocyigit2
1 Scientific Research Institute of Heart Surgery and Organ Transplantation, Bishkek, Kyrgyzstan
2 Private consultant, Izmir, Turkey
Abstract
Cardiac hypertrophy has been continuing as the subject of the ‘hottest’ topic in research field for a long time since it creates pathophysiologic and clinical issues by structural and functional alterations of heart. Probably, it is explained by the prevalence of cardiovascular disorders in all-cause mortality. It seems that, on the basis of a contemporary data, the perception of ‘benign’ nature of the cardiac hypertrophy in athletic population is blurred. The improvement of imaging modalities and assessment tools largely contributed to comprehensive integration of scientific and clinical standpoints of athletic hypertrophy. Conventionally pathologic hypertrophy believed to be developed in a case of cardiovascular diseases, while the athlete’s heart resulted by long-standing physical training. According to recent evidence, a gray zone of hypertrophy became emerged between physiological and pathological entities, which require further extensive investigations. The emerging huge challenges in sports cardiology are overtraining and doping abuse of elite athletes by the development of an excessive cardiac hypertrophy with the increased risk of cardiovascular adverse outcomes. Additionally, the higher prevalence of sudden cardiac death in sportsmen compared to sedentary matches, especially in case of athletes with known or suspected cardiovascular diseases necessitated scrupulous investigation of athletic heart syndrome. The development, variety and severity of cardiac hypertrophy fluctuate by sportive branch and training mode. The significance of problem is more emphasized when cohort studies represented survival differences by means of cardiovascular parameters between athletes and sedentary subjects and cardiac patients.
In this review, we aimed to represent recent physiological and clinical standpoints regarding to athletic heart syndrome as well as its difference from sedentary heart changes and pathological hypertrophy, its association with sudden cardiac death.
Key words: athlete’s heart, pathological hypertrophy, maladaptive hypertrophy, sudden cardiac death, cardiovascular imaging, overtraining
IntroductionIn sports medicine, hypertrophied myocardium with or without chamber dilation, predominantly of the left ventricle has been believed as a compensatory mechanism for maintaining the exercise-induced chronic overload. Otherwise termed as an athlete's heart, this entity necessitated thorough investigations due to development of structural, functional and electrical cardiac alterations parallel to athlete’s career years (1, 2). Mainly, the aforementioned surveys were concentrated on resolution of several issues. The first one is the difference between the pathological hypertrophy developed in sedentary patients due to underlying cardiovascular conditions and adaptive hypertrophy in the highly active sportive individuals. The detection of mild and subclinical forms of cardiac diseases, particularly inherited cardiomyopathies, generates a great challenge in both pre-participation and follow-up examinations of athletic population.
The third question is the defining of controversial nature of the athletic heart syndrome whether adaptive hypertrophy shares or not the pathologic features of disease-based hypertrophy. Doping abuse and overtraining are other huge problems of sports cardiology with known and unknown deleterious outcomes. The fluctuating prevalence of sudden cardiac death (SCD) due presumably to underlying structural and electrical remodeling of athlete’s heart presents the unresolved issues.
Early investigations revealed the different pathogenesis of athlete's hypertrophy in contrast to pathological one and defined as "physiological hypertrophy", thus structural and functional changes were proportional and hypertrophy regressed by detraining for a given period (3,4).
Furthermore, studies in sports cardiology presented the different features of left ventricular hypertrophy (LVH) depending on sportive branches. Despite the controversial nature of Morganroth hypothesis, two phenotypes of athletic heart have not been fully rejected, rather modified according to static and dynamic effects of exercise on cardiac structures. According to that, eccentric hypertrophy (EccH) develops mainly due to endurance-training or exercises with high dynamic and low static exertions (long-distance runners, cross-country skiers, etc.) while concentric hypertrophy (ConH) progresses in response to long-standing power training or exercises with high static demand (weightlifting, wrestling, etc.) (5-7, 10]. Objectively, most of sportive disciplines consist of both endurance and resistance activity, so these branches are classified as mixed-training (boxing, football etc.). Another adaptive mechanism is seen in skill-training subjects (golfers, table tennis players, etc.) where functional and electrical alterations in cardiac activity observed despite the absence of structural cardiac remodeling in contrast to healthy sedentary individuals (9, 10].
For simplicity of understanding of cardiac adaptation in athletes, sedentary subjects and patients we proposed a diagram (Fig. 1).
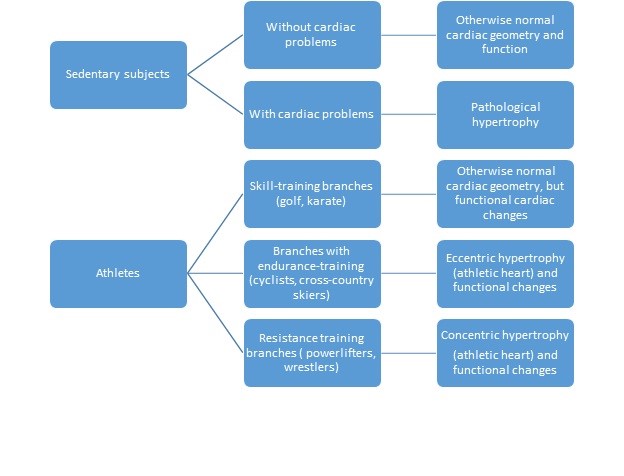
Figure 1. Different profiles of cardiac alteration in sedentary subjects, patients and athletes
In this review, we aimed to share the current standpoints in differential diagnosis of the athletic heart from pathological hypertrophy and the emergence of a maladaptive hypertrophy, as well as the nature of SCD in athletic population.For data source we used PubMed, Science Direct libraries and Google Scholar with key words “cardiac hypertrophy”, “left ventricular hypertrophy”, “athlete’s heart”, “athletic heart syndrome”, “pathological hypertrophy”, “hypertrophic cardiomyopathy”, “hypertensive heart” “physiological hypertrophy”, “maladaptive hypertrophy”, “assessment of athlete”, “sudden cardiac death”, “mortality in athletic population”, “training modes in sports”. Both abstracts and full-text articles in English language included in our review with the preference of studies published within last decade.
Assessment and differential diagnosis of athlete’s heart
Despite the latest controversies regarding to efficacy, meticulous cardiovascular pre-participation assessment of athletes remains crucial in defining the ‘whole destiny’ of sportsmen, though, establishment of false-diagnosis causes the unnecessary restriction from competition, while underassessment of comorbid pathology may lead to dramatic outcomes including SCD or development of heart failure (12, 13].
In a clinical sense, athletic heart stands for resting bradycardia and dilated chambers (1, 8]. Namely, decreased sympathetic tone, predominance of the parasympathetic system, augmented stroke volume, decreased intrinsic heart rate and atrioventricular vagal conduction have been accepted as adaptive functional changes in professional athletes.
In contrast to physiological hypertrophy of professional athletes, pathological hypertrophy emerges due to increased afterload (e.g. essential hypertension) and myocardial diseases (e.g. hypertrophic cardiomyopathy) (3, 12]. An accurate delineation of underlying cardiac conditions is essential in competitive athletes, though intense physical training increases the risk of adverse cardiovascular events, including irreversible cardiac changes. Figure 2 depicts the basic differences between athletic heart and pathological hypertrophy.
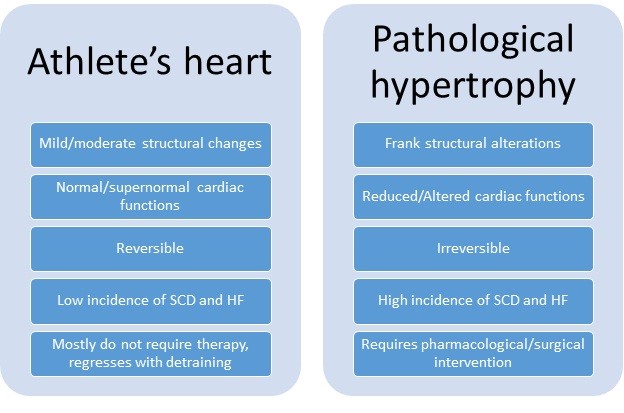
Figure 2. Characteristics of athlete`s heart and pathological hypertrophy
HF – heart failure, SCD – sudden cardiac death
Hypertrophic cardiomyopathy (HCM) is the most encountered condition in young elite athletes, which plays a leading role in the eligibility and disqualification criteria for competitions (3, 12, 13]. Beside the HCM, number of inherited, acquired, ischemic and inflammatory cardiac diseases must be ruled out in athletes with cardiac hypertrophy.
Beside the 14-point standardized history and physical examination, current guidelines strongly recommend 12-lead electrocardiography (ECG) and transthoracic echocardiography (TTE) in competitive athletes, particularly younger subjects (11-14].
Electrocardiography
A 12-lead surface ECG frequently shows increased vagal tone and nonspecific electrical axis deviations and repolarization abnormalities, along with normal patterns as evidence of physiological electrical remodeling in athletes (10, 15]. Table 1 highlights the normal and abnormal patterns of surface ECG according to recent evidence (15-17]. When the suspicious ‘red signs’ are reflected on first-line ECG, it necessitates further investigations of second-line options including Holter monitoring and exercise testing.
Table 1. Electrocardiographic features of athletic heart (modified from ref. 15-17)
Normal ECG patterns
Abnormal ECG patterns
1
↑ vagal tone: SB, 1st dg AVb in elite athletes
Advanced AVb(II dg II type, 2:1 and complete AVb, SA and SP (≥3000mc)
2
ERP predominantly in V5-V6 leads with concave STE commonly in males, blacks and those with LVH
Pathological Q-wave (˃25% of R), except AvL,
Any ST depression
3
Isolated SLC of LVH in asymptomatic athletes
Upright T-wave of AvR+TWI in V5-V6 shows apex pathology
4
TWI in V1-V3 for athletes˂16years
Any TWI with preceding depression
5
TWI preceded by concave STE or J-point elevation in blacks
TWI in Caucasians and athletes˃16 years old
6
RAD or LAD along with group 1 features (sinus bradycardia etc.)
TWI in two contiguous lateral and inferior leads
7
Voltage criteria for LAE, RAE, RVH along with group 1 features
LBBB (always)
RBBB+RA and QRSd≥140mc
8
QTc˂470 ms for males and ˂480 ms for females without Tnotch. or PPEx
QTc≥500 ms is obvious LQTS,
460-490 ms+syncope, family history of SCD, LQTS in 1st line relatives, Tnotch. and PPEx
9
Type 2 Brugada (saddle back) is non-specific
Type 1 Brugada (coved type) is always abnormal
AVb-AV block, ERP-early repolarization pattern, LAD-left axis deviation, LAE-left atrial enlargement, LBBB-left bundle branch block, LQTS-long QT syndrome, LVH-left ventricular hypertrophy, PPEx-paradoxical prolongation with exercise, RA-repolarization abnormalities, RAD-right axis deviation, RAE-right atrial enlargement, RBBB-right bundle branch block, RVH-right ventricular hypertrophy, SA-sinus arrest, SB-sinus bradycardia, SCD – sudden cardiac death, SLC-Sokolow-Lyon criterion, SP-sinus pause, STE-ST segment elevation, Tnotch.-T notching, TWI-T-wave inversion
Echocardiography
Echocardiography has a fundamental role in accurate evaluation of structural and functional status of athletic heart, as well as in diagnosis of underlying structural heart disease. Non-invasiveness, cost-effectiveness and widely availability favor the TTE being a first-line imaging modality. Measurement of
wall thickness and chamber dimensions and evaluation of left ventricular mass (LVM) identifies the heart geometry by one of the following phenotypes: normal geometry, EccH, ConH and concentric remodeling (ConR) (Table 2) (18).
As known, EccH ensues a total increase in left ventricular mass LVM and chamber dilatation despite the normal relative wall thickness (RWT), whereas, ConH is characterized by increased both LVM and RWT (8, 18]. According to recent recommendations RWT can be calculated by formula 2xPWD/LV EDD (where PWD stands for diastolic posterior left ventricular dimension and LV EDD is left ventricular end-diastolic dimension) and LVM indexed to body surface area (LVMI) obtained by ASE/Penn methods in linear measurements or by direct 2D options (area-length). RWT of 0.42 and LVMI of 95 and 115 gr/m2 respectively for female and male subjects are established as the cut-off values. Currently there are no strictly recommended linear or 2D options for LVM evaluation in athletes. Despite the 3D echocardiography derived results well correlated by cardiac magnetic resonance (CMR), no cut-off values have been established (18].
Table 2. Types of left ventricular hypertrophy and remodeling*
Cardiac geometry
RWT
LVM (gr/m2) (F and M)
Normal geometry
≤0.42
≤95 and ≤115* (≤88 and ≤102˦)
Concentric remodeling
˃0.42
≤95 and ≤115 (≤88 and ≤102)
Concentric hypertrophy
˃0.42
˃95 and ˃115 (˃88 and ˃102)
Eccentric hypertrophy
≤0.42
˃95 and ˃115 (˃88 and ˃102)
*modified from reference 18. under Creative Common license, F – female, M-male
Beside the measurement of structural parameters, echocardiography is a valuable tool for assessment of systolic and diastolic functions. Most studies revealed normal systolic indices and normal/supernormal diastolic parameters in elite athletes (18, 19]. According to Finocchiaro et al. tissue Doppler imaging (TDI) diastolic function was supernormal in 30% of athletes, reduced TDI lateral E` velocity was found only in 0.5% out of 1510 athletes, particularly in athletes ˃25 years old and with dilated chambers (20].
Nevertheless, suboptimal acoustic window, incomplete assumption of cardiac geometry especially of right chambers, inadequate assessment of cardiac functions necessitated the emergence of new techniques such as speckle tracking (STE) and three-dimensional echocardiography (3DE).
Strain and strain rate imaging modalities significantly improved the evaluation of athletic heart beside the subclinical myocardial dysfunction, HCM, restrictive diseases of myocardium and adverse effects of cancer chemotherapy, conditions that require thorough assessment of cardiac function, especially in early diagnosis (21-23]. According to recent guidelines, reference value of global longitudinal strain (GLS), the most reliable and robust marker of left ventricular systolic function fluctuates around -20% in accordance with inter-vendor variability (18, 24]. Most of conducted investigations in athletic population revealed normal GLS values in contrast to healthy sedentary controls (21, 23-26]. In a study of Zebrowska et al, basal circumferential strain (BCS) and basal rotation (BR) were significantly altered (p˂0.05) in endurance athletes with LVH in contrast to athletes and sedentary controls without LVH, and considered as a predictive marker of cardiovascular morbidity (26]. According to some researchers, the left atrial strain parameters observed as independent factors of LVH, left atrial size and filling pressures in athletes with enlarged atria (27].
Despite the diagnostic difficulties, echocardiography enables the differentiation of athlete’s heart from inherited myocardial diseases by structural and functional features (Table 3) (28). According to that, modest increase of walls and normal or proportional dilatation of chambers along with normal/augmented functions favor the diagnosis of athletic heart. Asymmetric hypertrophy with reduced left ventricular cavity and decreased diastolic function confirms HCM, whereas reduced GLS during effort indicates non-compaction cardiomyopathy. Arrhythmogenic right ventricular dysplasia (ARVD) is mainly presented by asymmetric right ventricular dilatation and wall motion abnormalities.
Table 3. Echocardiographic differences between athletic heart and cardiomyopathies*
Athlete’s heart
HCM
LV EDD
˃54mm
≤45mm
LV volume/mass
normal or ↑
reduced
Mitral E’ lateral
normal or ↑
reduced
LV RS and CS
normal or ↑
decreased
Athlete’s heart
LVNC
Trabeculation location
apical
mid-cavity
Mitral E’ lateral
normal or ↑
normal or ↓
LV GLS at rest
LV GLS at effort
normal/mild↓ normal or ↑
normal or↓ reduced
Athlete’s heart
ARVD/ARVC
RV enlargement
global
early RVOT dilation
Motion abnormalities
no
yes
Volume ratio RV /LV
˂1
≥1
*modified from reference 28 under Creative Common license
ARVD/ARVC-arrhythmogenic right ventricular dysplasia/cardiomyopathy, CS-circumferential strain, GLS-global longitudinal strain, HCM – hypertrophic cardiomyopathy, IVS-interventricular septum, LV-left ventricle, LV EDD-left ventricular end-diastolic diameter, LVNC-left ventricular non-compaction, RS-radial strain, RV-right ventricle, RVOT-right ventricular outflow tract
Beside the sophisticated assessment of cardiac structures, 3DE revealed augmented systolic indices owing to subendocardial and mid-wall contractions in top-level endurance athletes (29]. Lakatos et al. published the considerable gender difference in left atrial adaption to exercise and revealed that pronounced morphological and functional remodeling of left atrium were independent predictors of exercise capacity (30].
Cardiac magnetic resonance imaging
Cardiac magnetic resonance imaging (CMRI) substantially improved the diagnostic workup of cardiac hypertrophy. A cut-off value of diastolic wall to volume ratio of ˂0.15mm x m2/ml differentiates the athletic heart from all forms of pathological hypertrophy with 99% specificity (31]. Late gadolinium enhancement option of CMRI is valuable tool for the detection of myocardial fibrosis, a morphological hallmark of pathological hypertrophy (18, 24].
Maladaptive athletic heart and pathological hypertrophy
Several studies reported the existence of difference in indices of hypertrophy and functional parameters of athlete’s heart and pathological hypertrophy (3, 8, 16]. These differences are the evidence of limitations of upper boundaries of physiological athletic hypertrophy.
While according to Pelliccia et al., the reference ranges of LV EDD are established as 55-63 mm and left ventricular wall thickness (LVWT) - ≤13 mm, some studies on Caucasian athletes recorded the upper value for LVWT up to 14mm to 16mm, and some authors recommended LV EDD and LVWT in elite male athletes as 14mm and 65mm, for females 11mm and 60mm, respectively (32, 33].
In fact, hypertrophy parameters largely depended on the sportive branch, training mode and gender of athletes. This condition has been supported by long-term longitudinal and cross-sectional investigations. One study in which 3,500 athletes were screened, revealed the TWI on ECG and LVH with non-dilated heart chambers on TTE consistent with HCM in 0.08% of athletes (3 out of 53 with LVH). It is noteworthy that none of them had HCM in their family (34]. A longitudinal study conducted on 114 Olympic athletes on long-term uninterrupted intensive training, represented significant enlargement of left atrium and mild enlargement of aortic root while no pathologic alterations were observed in systolic function, regional wall motion, LVMI, and filling pressures of left ventricle (35].
However, the obtained results have not fully advocated the innocence of excessive cardiac hypertrophy in elite athletes. Recently, Henshen et al. reported the enlarged cardiac chambers of cross-country skiers (36]. According to current results, hypertrophic maladaptation in athletes exceeding normal limits has been included into gray zone between physiological and pathological entities. As the supporting criteria of grey zone hypertrophy, pathological Q waves on ECG, VO2max <50 ml/kg/min by cardiopulmonary exercise testing, LVWT>16 mm, diastolic dysfunction (septal E’˂9 cm/s, E/E’˃15 cm/s), LVEDD <48mm by TTE and presence of late gadolinium enhancement by CMRI were proposed (37, 38]. In Figure 3, we represented the HCM-approached mega-hypertrophy in a gray zone due to increased risk of adverse cardiovascular outcomes.
Accordingly, disproportionate mass increase in the heart, cardiomyocyte deformation, inflammation, myocardial fibrosis, diastolic dysfunction and decreased cardiac output have been considered as markers of excessive cardiac adaptation. However, as previously mentioned the hypertrophy values of physiological athletic heart and cardiomyopathy clearly differed. While wall thickness in an athlete's heart is <13mm, it is indicated as > 15mm for the maladaptive hypertrophy. While the LVEDD assessed as <60 mm in an athlete, which is 20% larger than that of healthy sedentary person, it exceeded 70mm in maladaptive athletic heart. Despite the increased myocardial mass and volume, a HCM-approached athletic heart shows lower functional capacity (32, 33, 39].
On the other hand, an athletic heart within the gray zone is characterized by diverse phenotypes of mass increase and chamber dilatation. It is explained by variety of factors that carry athlete's heart to maladaptation. For example, in those who practice intensive endurance training for a long time, the situation shows mega-dilatation especially of left chambers; and excessive myocardial wall thickening develops in athletes of extreme resistance training. Some cases are presented by ARVD (arrhythmogenic right ventricular dysplasia) features and are predisposed to life-threatening ventricular arrhythmias (40, 41].
An experimental study conducted on mice models stated that the animals exposed to intermittent excessive exercise showed the stress-induced activation of pathogenic signaling pathways, a molecular trigger of heart dysfunction rather than physiological hypertrophic growth (42]. Beside the HCM-approaching phenotype, excessive cardiac hypertrophy leads to additional adverse cardiovascular outcomes, such as arterial hypertension and atrial fibrillation (43]. Furthermore, not only exercise type, but also factors such as age, ethnicity, gender, genetic predisposition have impact on maladaptation emergence (33, 43].
To date, due to ethical impossibility of conducting of experimental studies on athletes regarding the gray zone status of cardiac hypertrophy, general standpoint of maladaptation relies solely on results of experiments on animal models. The accumulation of more analogous findings in this regard can be expected to strengthen our views on excessive cardiac hypertrophy.
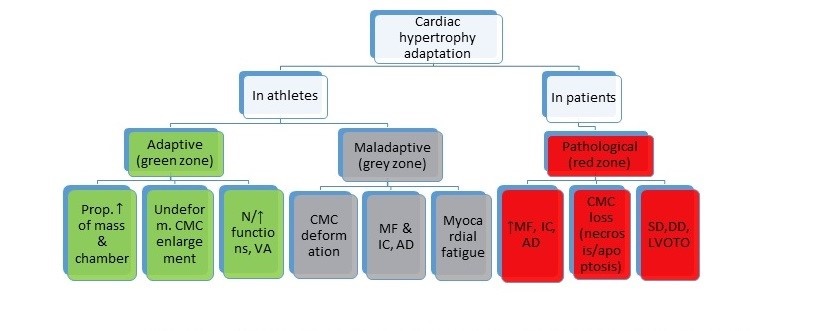
Figure 3. Cardiac adaptation profiles in athletes and patients
AD-arrhythmogenic dysplasia, N-normal, CMC-cardiomyocyte, DD-diastolic dysfunction, IC-inflammatory changes, LVOTO-left ventricular outflow tract obstruction, MF-myocardial fibrosis, SD-systolic dysfunction, VA-vagotonic adaptation, “Myocardial fatigue” refers to moderately expressed systolic and/or diastolic dysfunction
Sudden cardiac death associated with cardiac hypertrophy, exercises and other factors
Sports-related SCD is usually defined as sudden and unexpected death occurred during, or shortly after, exercise (with varying time intervals up to 24 hours) (13, 44, 45]. In fact, SCD is not a newly discovered condition in sports cardiology. Beside the creating a challenge for sports physicians and mass media, SCD influences the career of competitor and whole sports politics.
According to recent data, SCD incidence fluctuates from 0.75 in 100 000 to 1 in 40 000 and 1 in 80 000 athletic populations (46, 47]. It can be explained by variety of sports discipline, and overall health status of an athlete. Regarding to sports branch, SCD prevails in basketball players in US and in footballers in Europe (48, 49].
Furthermore, sex and ethnicity-based studies on SCD prevalence revealed the male preponderance over females and racial predilectics in basketball players, though blacks were observed to be at three-time higher risk of SCD than Caucasians in US (48-51]. In young athletes, SCD incidence has been reported between 1:9000 and 1:300 000 (48].
Nowadays, SCD is listed in the most investigated research topics in spite of its infrequent incidence in general population. Although the factors revealed by the studies on SCD are quite widespread, full consensus has not been reached yet. It is probably explained by the limited possibilities of studies on the observed cases, though, a large body of the data is based on autopsy findings (49, 52]. Another important limitation is the insidious course of SCD that most of the cases occur without preceding symptoms (49, 53).
Even so controversial nature of SCD, some approaches and consensus agreements have been obtained. Some studies stated the occurrence of SCD in athletes depending on age aspects: younger athletes under 35 years suffered mainly from HCM or other hereditary conditions, whereas athletes over 35 years of age suffered from ventricular arrhythmias due to coronary artery disease or ARVD (45, 51].
Yet another important point is that HCM-approaching excessive hypertrophy existed in grey zone, which has been mentioned in previous section may be a primary underlying factor of SCD in overtraining athletes (37, 54].
The availability of examining the cardiac conduction system by electrophysiological studies (EPS) strengthened our predictions about the SCD phenomenon (55]. One of the propositions related to the subject is that study of genetic ion channelopathies has been found as important diagnostic tool as genetic-molecular studies (56]. On the other side, excessive hypertrophy increases the risk of SCD by causing repolarization abnormalities and rhythm disturbances.
Recent retrospective studies revealed the relationships of vast majority of factors in the development of SCD (45, 52). According to literature review, we classified several factors considered to be underlying mechanisms for SCD occurrence (Fig. 4). Hereditary/genetic factors included HCM, dilated cardiomyopathy (DCM), ARVD/ARVC and other genetic cardiovascular diseases. Post-infarction scar formation, amyloidosis, antiphospholipid syndrome are listed as subsequent (acquired) factors. Issues related to gender, ethnicity and body constitution comprised as separate factors. Inadequate recovery, mode of training and sports branch are categorized in exercise-related factors. As can be seen from diagram, a single factor can be attributed to several groups, for instance HCM belongs to both hereditary (genotype+phenotype- condition), and sportive factors (high-intensity exercise worsens compensated systolic function).
In this section, we additionally concentrated on two factors: SCD linked to overtraining and doping/performance enhancer abuse. Per se, moderate exercise considered as beneficial in the management of many cardiovascular diseases. However, excessively intense and long-lasting exercise increased the likelihood of SCD in subjects with a genetic predisposition. More often long-standing overtraining, particularly endurance exercise is accompanied by development of myocardial fibrosis and coronary calcification contrary to what is expected in an athlete’s heart (57). On the other hand, doping abuse and performance enhancing drinks are known to have fatal effects on the heart (58). It is stated that substances such as growth and thyroid hormone, anabolic steroids are related to excessive hypertrophy. Additionally, excessive use of caffeine, cocaine, beside its performance-enhancing effect, leads to risky consequences such as arrhythmia and hypertension (60, 61).
To sum up, according to recent evidence, frequent high-intensity exercise without adequate warm-up and cooling intervals, overtraining, inadequate regeneration, substance abuse together with underlying hereditary, genetic, subsequent and other factors gradually increases the occurrence of SCD.
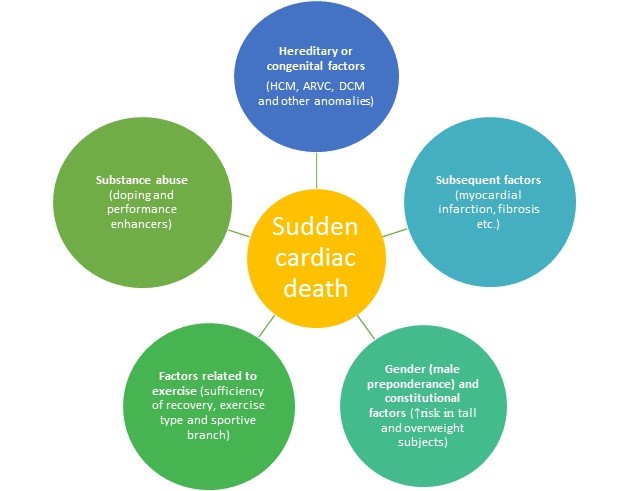
Figure 4. Underlying mechanisms of sudden cardiac death
Conclusion
In contrast to athletes, sedentary patients with cardiac issues develop pathological hypertrophy. Different phenotypes of cardiac hypertrophy and numerous functional changes without structural remodeling may occur depending on exercise type. As well as, any cardiac hypertrophy in sportsmen should not be considered as normal or benign, unless maladaptive changes and underlying genetic, congenital conditions are ruled out. Excessive cardiac adaptation falls into grey zone, which is thought to have high tendency of transformation into pathologic hypertrophy. SCD is not only associated with vigorous physical activity in maladaptation, but develops on the background of relationships of several factors including genetic, congenital and acquired ones. SCD is not only associated with vigorous physical activity in maladaptation, but also develops on the background of relationships of several factors including genetic, congenital and acquired ones.
Peer-review: Internal
Conflict of interest: None to declare
Authorship: D.A. and F.K. equally contributed to preparation of article
Acknowledgement and funding: None to declare
References
1. Maron BJ. Structural features of the athlete heart as defined by echocardiography. J Am Coll Cardiol 1986; 7: 190-203. https://doi.org/10.1016/S0735-1097(86)80282-0 2. Dores H, Freitas A, Malhhotra A, Mendes M, Sharma S. The hearts of competitive athletes: An up-to-date overview of exercise induced cardiac adaptations. Revista Portuguesa de Cardiologia 2015; 34: 51-64. https://doi.org/10.1016/j.repc.2014.07.010 PMid:25575633 3. Shimuzu I, Minamino T. Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol 2016; 97: 245-67. https://doi.org/10.1016/j.yjmcc.2016.06.001 PMid:27262674 4. Pedlar C, Brown M, Otto J, Drane A, Finch JM, Contursi M, et al. Temporal sequence of athlete's heart regression during prescribed exercise detraining: diagnostic implications. J Am Coll Cardiol 2017; 69: DOI: 10.1016/S0735-1097(17)34803-9 https://doi.org/10.1016/S0735-1097(17)34803-9 5. Rawlins J, Bhan A, Sharma S. Left ventricular hypertrophy in athletes. Eu J Echo 2009; 10: 350-6. https://doi.org/10.1093/ejechocard/jep017 PMid:19246500 6. Morganroth J, Maron BJ, Henry WL, Epstein SE. Comparative left ventricular dimensions in trained athletes. Ann Intern Med 1975; 82: 521-4. https://doi.org/10.7326/0003-4819-82-4-521 PMid:1119766 7. Haykowsky MJ, Samuel TJ, Nelson MD, La Gerche A. Athlete's heart: Is the Morganroth hypothesis obsolete? Heart, Lung Circ 2018; 27: 1037-41. https://doi.org/10.1016/j.hlc.2018.04.289 PMid:29773412 8. Galderisi M, Cardim N, D'Andrea A, Bruder O, Cosyns B, Davin L, et al. The multi-modality cardiac imaging approach to the athlete's heart: an Expert Consensus of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 353. https://doi.org/10.1093/ehjci/jeu323 PMid:25681828 9. Pelliccia A, Caselli S, Sharma S, Basso C, Bax JJ, Corrado D, et al. Internal reviewers for EAPC and EACVI. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete's heart. Eur Heart J 2018; 39: 1949-69. https://doi.org/10.1093/eurheartj/ehx532 PMid:29029207 10. Fagard R. Athlete's heart. Heart 2003; 89: 1455-61. https://doi.org/10.1136/heart.89.12.1455 PMid:14617564 PMCid:PMC1767992 11. Maron BJ, Friedman RA, Kligfield P, Levine BD, Viskin S, Chaitman BR, et al. Assessment of the 12-Lead ECG as a screening test for detection of cardiovascular disease in healthy general populations of young people (12-25 years of age): A Scientific Statement from the American Heart Association and the American College of Cardiology. Circulation 2014; 130: 1303-4. https://doi.org/10.1161/CIR.0000000000000025 PMid:25223981 12. Corrado D, Pellicia A, Bjornstad HH, Biffi A, Vanhees L, Borjesson M, et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol: Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and. Eur Heart J 2005; 26: 516-24. https://doi.org/10.1093/eurheartj/ehi108 https://doi.org/10.1093/eurheartj/ehi380 PMid:15689345 13. Mont L, Pelliccia A, Sharma S, Biffi A, Borjesson M, Terradellas JB, et al. Pre-participation cardiovascular evaluation for athletic participants to prevent sudden death: Position paper from the EHRA and the EACPR, branches of the ESC. Endorsed by APHRS, HRS, and SOLAECE. EP Europace 2017: 19: 139-63. https://doi.org/10.1093/europace/euw243 PMid:27815371 14. Borjesson N, Urhausen A, Kouidi E, Dugmore D, Sharma S, Halle M, et al. Cardiovascular evaluation of middle-aged/senior individuals engaged in leisure-time sport activities: position stand from the sections of exercise physiology and sports cardiology of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardio Prev & Rehab 2017; 18: 446-58. https://doi.org/10.1097/HJR.0b013e32833bo969 PMid:21450560 15. Drezner AJ, Fischbach P, Frolicher V, Marek J, Pellicia A, Prutkin JM, et al. Normal electrocardiographic findings: recognizing physiological adaptations in athletes. Br J Sports Med 2013; doi: 10.1136/bjsports-2012-092068. https://doi.org/10.1136/bjsports-2012-092068 PMid:23303759 16. Corrado D, Biffi A, Basso C, Pelliccia A, Thiene G. 12-lead ECG in the athlete: physiological versus pathological abnormalities. Br J Sports Med 2009; 43: 669-76. https://doi.org/10.1136/bjsm.2008.054759 PMid:19734501 17. Gavalas M. Athlete's ECGs: how to interpret and know when and how to investigate further. Care of the Athletic Heart 2019 (Internet). July 17, 2019. Available at URL: https://www.acc.org/latest-in-cardiology/articles/2019/07/17/07/03/athlete-ecgs. 18. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1-39. https://doi.org/10.1016/j.echo.2014.10.003 PMid:25559473 19. Forsythe L, MacIver DH, Johnson C, George K, Somauroo J, Papadakis M, et al. The relationship between left ventricular structure and function in the elite rugby football league athlete as determined by conventional echocardiography and myocardial strain imaging. Int J Cardiol 2018; 261: 211-7. https://doi.org/10.1016/j.ijcard.2018.01.140 PMid:29657045 20. Finocchiaro G, Dhutia H, D'Silva A, Malhotra A, Sheikh N, Narain R, et al. Role of Doppler diastolic parameters in differentiating physiological left ventricular hypertrophy from hypertrophic cardiomyopathy. JACE 2018; 31: 606-13. https://doi.org/10.1016/j.echo.2017.11.022 PMid:29482976 21. Afonso L, Condur A, Smegn M, Niraj A, Hari P, Kaur R, et al. Two-dimensional strain profiles in patients with physiological and pathological hypertrophy and preserved left ventricular systolic function: a comparative analyses. BMJ Open 2012; 2: e001390. https://doi.org/10.1136/bmjopen-2012-001390 PMid:22904333 PMCid:PMC3425901 22. Mirea O, Duchenne J, Voigt JU. Recent advances in echocardiography: strain and strain rate imaging. F1000Res 2016; 29: pii: F1000 Faculty Rev-787. https://doi.org/10.12688/f1000research.7228.1 PMid:27158476 PMCid:PMC4856110 23. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. JASE 2014; 27: 911‐39. https://doi.org/10.1016/j.echo.2014.07.012 PMid:25172399 24. Caselli S, Montesanti D, Autore C, Di Paolo FM, Pisicchio C, Squeo MR, et al. Patterns of left ventricular longitudinal strain and strain rate in Olympic athletes. Clin Invest 2015; 28: 245-53. https://doi.org/10.1016/j.echo.2014.10.010 PMid:25455545 25. Eijsvogels TMH, Oxborough DL, O'Hanlon R, Sharma S, Sharma P, White G, et al. Global and regional cardiac function in lifelong endurance athletes with and without myocardial fibrosis. European Journal of Sport Science, 2017; 17: 1297-303. https://doi.org/10.1080/17461391.2017.1373864 PMid:28910586 26. Zebrowska A, Mikołajczyk R, Was'kiewicz Z, Gasior Z, Mizia-Stec K, Kawecki D, et al. Left ventricular systolic function assessed by speckle tracking echocardiography in athletes with and without left ventricle hypertrophy. J Clin Med 2019; 8: 687. doi:10.3390/jcm8050687. https://doi.org/10.3390/jcm8050687 PMid:31096682 PMCid:PMC6571655 27. Król W, Jędrzejewska I, Konopka M, Burkhard-Jagodzińska K, Klusiewicz A, Pokrywka A et al. Left atrial enlargement in young high‐level endurance athletes - another sign of athlete's heart? J Hum Kinet 2016; 53: 81-90. DOI: 10.1515hukin-2016-0012. https://doi.org/10.1515/hukin-2016-0012 PMid:28149413 PMCid:PMC5260578 28. Grazioli G, Sanz M, Montserrat S, Vidal B, Sitges M. Echocardiography in the evaluation of athletes. F1000Research 2015, 4: 151. https://doi.org/10.12688/f1000research.6595.1 PMid:26236468 PMCid:PMC4516021 29. Lo Iudice F, Petitto M, Ferrone M, Esposito R, Vaccaro A, Buonauro A, et al. Determinants of myocardial mechanics in top-level endurance athletes: three-dimensional speckle tracking evaluation. Eur Heart J - Cardiovasc Imag 2017; 8: 549-55. Doi: 10.1093/ehjci/jew122 https://doi.org/10.1093/ehjci/jew122 PMid:27325809 30. Lakatos BK, Molnar AA, Kiss O, Sydo N, Tokodi M, Solymossi B, et al. Relationship between cardiac remodeling and exercise capacity in elite athletes: incremental value of left atrial morphology and function assessed by three-dimensional echocardiography. JASE 2020; 33: 101-9. https://doi.org/10.1016/j.echo.2019.07.017 PMid:31585830 31. Peterson SE, Selvenayagam JB, Francis JM, Myerson SJ, Wiesmann F, Robson MD, et al. Differentiation of athlete's heart from pathological forms of cardiac hypertrophy by means of geometric indices derived from cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2005; 7: 551-8. DOI: 10.1081/JCMR-60631. https://doi.org/10.1081/JCMR-200060631 PMid:15959967 32. Pellica A, Maron BJ, Spataro A, Proschan MA, Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med 1991; 324: 295-301. https://doi.org/10.1056/NEJM199101313240504 PMid:1824720 33. Whyte GP, George K, Sharma S, Firoozi S, Stephens N, Senior R, et al. The upper limit of physiological cardiac hypertrophy in elite male and female athletes: the British experience. Eur J Appl Physiol 2004: 92: 592-7. DOI: 10.1007/s00421-004-1052-2. https://doi.org/10.1007/s00421-004-1052-2 PMid:15054661 34. Basavarajaiah S, Wilson M, Whyte G, Shah A, McKenna W, Sharma S. Prevalence of hypertrophic cardiomyopathy in highly trained athletes: relevance to pre-participation screening. J Am Coll Cardiol 2008; 51: 1033-1039. DOI:10.1016/j.jacc.2007.10.055. https://doi.org/10.1016/j.jacc.2007.10.055 PMid:18325444 35. Pellicia A, Kinoshita N, Pisicchio C, Quatrini F, DiPaolo FM, Ciardo R, et al. Long-Term clinical consequences of intense, uninterrupted endurance training in Olympic athletes. J Am Coll Cardiol 2010: 55: 1619-25. DOI: 10.16/j.Jacc.'009.10.068. https://doi.org/10.1016/j.jacc.2009.10.068 PMid:20378081 36. Henshen S. Skidlauf und skidwettlauf: eine medizinische sportstudie. Mitt Med Klin Upsala 1899; 2: 15. 37. Martinez MW, Nair SU. The Athlete grey zone: distinguishing pathologic from physiologic left ventricular hypertrophy. American College of Cardiology October 29, 2014. Available at: URL: https://www.acc.org/latest-in-cardiology 38. Czimbalmos C, Csecs I, Toth A, Kiss O, Suhai FI, Sydo N, et al. The demanding grey zone: sport indices by cardiac magnetic resonance imaging differentiate hypertrophic cardiomyopathy from athlete's heart. PLoS ONE 2019; 14: e0211624. doi: 10.1371/journal.pone.0211624 https://doi.org/10.1371/journal.pone.0211624 PMid:30763323 PMCid:PMC6375568 39. Baggish AL, Battle RW, Beckerman JG, Bove AA, Lampert RJ, Levine BD, et al. Sports cardiology: core curriculum for providing cardiovascular care to competitive athletes and highly active people. J Am Coll Cardiol 2017 https://doi.org/10.1016/j.jacc.2017.08.055 PMid:28982505 70: 1902‐18. doi:10.1016/j.jacc.2017.08.055. https://doi.org/10.1016/j.jacc.2017.08.055 PMid:28982505 40. Heidbüchel, H., La Gerche, A. The right heart in athletes. Herzschr Elektrophys 2012; 23: 82-6. Doi: 10.1007/s00399-012-0180-3. https://doi.org/10.1007/s00399-012-0180-3 PMid:22782727 41. Zaidi A, Sharma S. Arrhythmogenic right ventricular remodeling in endurance athletes: Pandora's box or Achilles's heel? Eur Heart J 2015: 36: 1955-7. doi:10.1093/eurheartj/ehv199 https://doi.org/10.1093/eurheartj/ehv199 PMid:26038591 42. Perrino C, Naga Prasad SV, Mao L, Noma T, Yan Z, Kim HS, et al. İntermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular refraction. J Clin İnvest 2006; 116: 1547-60. https://doi.org/10.1172/JCI25397 PMid:16741575 PMCid:PMC1464895 43. Leischik R, Spelsberg N, Niggemann H, Dworrak B, Tiroch K. Exercise-induced arterial hypertension - an independent factor for hypertrophy and a ticking clock for cardiac fatigue or atrial fibrillation in athletes? F1000Res. 2014: 3: 105. doi:10.12688/f1000research.4001.1 https://doi.org/10.12688/f1000research.4001.1 PMid:25132960 PMCid:PMC4118759 44. Predel HG. Marathon run: cardiovascular adaptation and cardiovascular risk. Eur Heart J 2014; 35: 3091-3098, https://doi.org/10.1093/eurheartj/eht502. https://doi.org/10.1093/eurheartj/eht502 PMid:24408890 45. Fanous Y, Dorian P. The prevention and management of sudden cardiac arrest in athletes. CMAJ 2019; 191: E787-91. DOI: 10.1503/cmaj.190166. https://doi.org/10.1503/cmaj.190166 PMid:31308007 46. Landry CH, Allan KS, Connelly KA, Cunningham K, Morrison LJ, Dorian P. Rescu Investigators. Sudden cardiac arrest during participation in competitive sports. N Engl J Med 2017; 377: 1943-53. https://doi.org/10.1056/NEJMoa1615710 PMid:29141175 PMCid:PMC5726886 47. Harmon KG, Drezner JA, Wilson MG, Sharma S. Incidence of sudden cardiac death in athletes: a state-of-the-art review. Br J Sports Med 2014; 48: 1185‐92. doi:10.1136/bjsports-2014-093872. https://doi.org/10.1136/bjsports-2014-093872 PMid:24963027 48. Luwong MB, Morrison BN, Lithwick DJ, Isserow S, Heilborn B, Krahn AD. Sudden cardiac death in young competitive athletes. BCMJ 2016: 58: 138-44. 49 Morentin B, Suárez-Mier MP, Monzó A, Molina P, Lucena JS. Sports-related sudden cardiac death due to myocardial diseases on a population from 1-35 years: a multicentre forensic study in Spain. Forensic Sci Res 2019; 4: 257‐66. doi:10.1080/20961790.2019.1633729. https://doi.org/10.1080/20961790.2019.1633729 PMid:31489391 PMCid:PMC6713174 50. Maron BJ, Haas TS, Murphy CJ, Ahluwalia A, Rutten-Ramos S. Incidence and causes of sudden death in US college athletes. J Am Coll Cardiol 2014; 63: 1636 - 43. https://doi.org/10.1016/j.jacc.2014.01.041 PMid:24583295 51. Maron BJ, Epstein SE, Roberts WC. Causes of sudden death in competitive athletes. J Am Coll Cardiol 1986; 7: 204‐14. doi: 10.1016/s0735-1097(86)80283-2. https://doi.org/10.1016/S0735-1097(86)80283-2 52. Cann F, Corbett M, O'Sullivan D, Tennant S, Hailey H, Grieve JH, et al. Phenotype-driven molecular autopsy for sudden cardiac death. Clin Genet 2017: 91: 22-9. doi:10.1111/cge.12778. https://doi.org/10.1111/cge.12778 PMid:27000522 53. Calkins H, Corrado D, Marcus F. Risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation 2017: 136: 2068-82. doi.10.116/circulationaha,117.030792. https://doi.org/10.1161/CIRCULATIONAHA.117.030792 PMid:29158215 PMCid:PMC5777304 54. Maron BJ, Pellicia A. The heart of trained athletes, cardiac remodelling and the risk of sports, including sudden death. Circulation 2006: 114; 1633-44. Doi: 1.1161/Circulationaha, 106, 613562. https://doi.org/10.1161/CIRCULATIONAHA.106.613562 PMid:17030703 55. Hilfiker G, Schoenenberger AW, Erne P, Kobza R. Utility of electrophysiological studies to predict arrhythmic events. World J Cardiol 2015; 7: 344-50. doi: 10.4330/wjcv7.i6.344. https://doi.org/10.4330/wjc.v7.i6.344 PMid:26131339 PMCid:PMC4478569 56. Brito D, Magalhães A, Cortez-Dias N, Miltenberger-Miltenyi G. Rare association of two genetic causes of sudden death in a young survivor. Arq Bras Cardiol 2017: 108: 184-6. https://doi.org/10.5935/abc.20170016 PMid:28327871 PMCid:PMC5344665 57. Eijsvogels TMH, Thompson PD, Franklin BA. The "Extreme Exercise Hypothesis": recent findings and cardiovascular health implications. Curr Treat Options Cardiovasc Med 2018: 20: 84. doi: 10.1007/s11936-018-0674-3. https://doi.org/10.1007/s11936-018-0674-3 PMid:30155804 PMCid:PMC6132728 58. Angell PJ, Chester N, Sculthorpe N, Whyte G, George K, Somauro J. Performance enhancing drug abuse and cardiovascular risk in athletes: implications for the clinician. Br J Sports Med 2012; 46: i78-i84. https://doi.org/10.1136/bjsports-2012-091186 PMid:23097484 59. Palmiero CR, Anand R, Dardi IK, Balasubramaniyam N, Schwarcz MD, Weiss IA. Growth hormone and the cardiovascular system. Cardiol Rev 2012; 20: 197-207. Doi: 10.1097/CRD.0b013e318248a3e1. https://doi.org/10.1097/CRD.0b013e318248a3e1 PMid:22314142 60. Reardon CL, Credo S. Drug abuse in athletes. Substance abuse and rehabilitation 2014: 5: 95-105. https://doi.org/10.2147/SAR.S53784 PMid:25187752 PMCid:PMC4140700 Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.Archive of Issues
AUTHOR'S CORNER
 Authors having problems with submissions please notify editor: editor@hvt-journal.com
Authors having problems with submissions please notify editor: editor@hvt-journal.com
