His Bundle Pacing – Stand-alone or adjunctive physiological pacing: a systematic review
SYSTEMATIC REVIEW
His Bundle Pacing – Stand-alone or adjunctive physiological pacing: a systematic review
Article Summary
- DOI: 10.24969/hvt.2021.248
- Page(s): 51-65
- CARDIOVASCULAR DISEASES
- Published: 15/03/2021
- Received: 04/01/2021
- Revised: 28/02/2021
- Accepted: 02/03/2021
- Views: 9974
- Downloads: 6485
-
Citations
- Keywords: His bundle pacing, physiologic pacing, upgrade pacing
PDF PRINT VERSION CommentsAddress for CorrespondenceAddress for Correspondence: Narendra Kumar, Lancashire Cardiac Centre, Blackpool Victoria Hospital, Whinney Heys Rd, Blackpool FY3 8NR, UK
Email: naren.cep@gmail.com
Review
His Bundle Pacing – Stand-alone or adjunctive physiological pacing: a systematic review
Jacky Kit Chan1, Shaimaa Mostafa2, Narendra Kumar3
1Pro-Care Heart Clinic, Hong Kong
2Benha University, Faculty of Medicine, Egypt
3Lancashire Cardiac Centre, Blackpool Victoria Hospital, Whinney Heys Rd, Blackpool, UK
Abstract
His-bundle pacing (HBP) appears to be a viable stand-alone or adjunctive physiological pacing therapy in pacemaker dependent patients. It could also serve as an effective adjunct or alternative pacing therapy for heart failure patients who require cardiac resynchronization therapy or pacemaker upgrade. His-bundle pacing has demonstrated improvement of His-Purkinje conduction, left ventricular electrical / mechanical synchronization, and left ventricular ejection fraction (LVEF) compared with right ventricle pacing. Patients who have high pacing dependence and/or LVEF impairment would benefit most from HBP in terms of heart failure hospitalization and LVEF improvement. Mortality benefit has not been consistently demonstrated in latest meta-analysis. The long-term clinical benefit and safety profile of HBP remains to be explored in future studies.
Key words: His bundle pacing, physiologic pacing, upgrade pacing
IntroductionHis Bundle Pacing / Physiologic pacing
In conventional right ventricular pacing (RVP), the pacing lead is generally positioned at the right ventricular apex or right ventricular septum (1). However, right ventricular apical (RVA) or right ventricular septal pacing is associated with non-physiological electrical activation and electro-mechanical dyssynchrony. Right ventricular pacing >20-40% is associated with increased risk of heart failure (HF) hospitalization, pacing-induced cardiomyopathy (PICM), atrial fibrillation (AF) and mortality (2-6). Various physiological pacing techniques including cardiac resynchronization therapy (CRT) and His-bundle pacing (HBP) have been developed to minimize the adverse cardiovascular effect of RVP. In CRT, apart from the conventional right ventricular lead, an additional left ventricular lead is positioned in the coronary sinus to pace the left ventricle simultaneously, in order to achieve biventricular electro-mechanical synchronization in heart failure patients with electro-mechanical dyssynchrony. However, among CRT recipients, the clinical non-responder rate remains as high as 30% (7). Besides, CRT has not demonstrated consistent cardiovascular benefit in patients with narrow QRS, right bundle branch block (8), or preserved left ventricular ejection fraction (LVEF) (9, 10).
His bundle pacing is a physiological pacing technique aiming to preserve the electrical conduction of His-Purkinje system and ventricular mechanical synchrony by selectively or non-selectively pacing the His bundle area. It was first reported by Deshmukh et al (11) in 2000. Among patients with chronic AF and dilated cardiomyopathy with narrow QRS complexes, HBP was associated with left ventricular reverse remodelling and improvement of LVEF from 20±9% to 31±11% (p=0.01) (11). Over the past few years, HBP has evolved into both a stand-alone physiological pacing therapy or as an adjunct to CRT.
The objective of the review is to evaluate the implant success rate, long-term safety and clinical benefit of HBP in HF patients requiring CRT and non-heart failure patient with pacing indications.
Methods
Search strategy (Fig. 1)
A comprehensive literature search was conducted in PubMed (including Medline) online database.
Literature search was performed using the key word “His bundle pacing” in PubMed (including Medline). Journal articles published between 1st January 2000 to 8th March 2021 were included.
Exclusion criteria include review articles, systematic reviews, case reports, non-human studies, abstracts, studies involving patients under the age of 18 and other studies not fulfilling the inclusion criteria. Inclusion criteria include clinical trials, observational studies, multicenter studies, randomized control trials and meta-analysis. Articles involving left bundle branch pacing, electrophysiology study, and observational HBP studies in non-heart failure population with sample size less than 100 patients were further excluded.
Results
A total of 967 articles were screened (Fig. 1). Nine hundred and one articles were excluded. A total of 72 studies fulfilled the inclusion criteria. Thirty-five studies were further excluded based. Finally, 37 studies were included in the systematic review.
HBP as an adjunct therapy to CRT in heart failure patients
We reviewed 4 studies (72 patients) (Table 1) which explored the effect of HBP as an adjunct to CRT in heart failure patients. In patients with HF and bundle branch block (BBB), HBP with or without left ventricular pacing (LVP) has been shown to improve invasive blood pressure (12). His-optimized CRT (HOT-CRT) improved LVEF and hemodynamic parameters measured by pressure-conductance volume catheter (13). Among CRT eligible candidates, both HBP and CRT resulted in QRS narrowing, improvement of quality of life (QoL), New York Heart Association (NYHA) functional class, 6-minute walk test (6MWT) and LVEF (1). Lustgarten et al (14) demonstrated that in 10 patients with CRT indications, HBP resulted in more significant QRS narrowing compared with biventricular pacing, with satisfactory pacing threshold. However, they did not report long-term clinical outcome data. Boczar et al. (15) showed that in 14 CRT eligible patients with permanent AF, heart failure, BBB, widened QRS >130ms and impaired LVEF, HBP as an adjunct to CRT resulted in improvement of LVEF, NYHA functional class and reduction of left ventricular end-diastolic dimension at 14.4 months follow-up.
Figure1. Evidence search strategy
Table 1. Studies of HBP as an adjunct tor CRT in heart failure patients
Study
Year
Pts
n=72
Age,
yrs
Patient selection
Study design
FU,
mo
Success Rate, n(%)
Pacing threshold
QRS width, ms
Long-term outcome
Lustgarten (14)
2010
10
NR
All pts with CRT indications
(HBP + CRT)
Prospective cohort
NR
10/10 (100)
HBP
3.1 ±1.1 V
at 0.5ms
BiVP
1.3 ± 0.9 V at 0.5ms
Intrinsic:
171±13
HBP:
148 ±11
BiVP: 158±21
p<0.0001
NR
Boczar (15)
2019
14
67.35±10
CRT eligible patients with permanent AF, CHF, BBB, QRS >130ms, impaired LVEF
Prospective cohort
Multicenter
14.4
14/14 (100)
NR
Intrinsic:
159 ± 29
HBP/BiVP:
128
1 of 13 patients died of CHF.
LVEF, NYHA improved, LVEDD decreased
Vijayaraman
(HOT-CRT)
(16)
2019
27
72±15
LBBB, IVCD, RVP with CRT indication
Prospective cohort
Multicenter
14±
10
25/27 (93)
At implant:
HBP 1.7±0.9 V at 1.0 ms
LVP
1.5±0.5 V at 0.6 ms at implant
At FU
HBP
1.8±1.1 V at 1 ms
LVP
1.6±0.8 V at 0.6
ms
Intrinsic
183±27
BiVP
162±17 p=0.003
HBP 151±24
p<0.0001
Improved LVEF, NYHA & CRT clinical response rate
Deshmukh (17)
2020
21
70.7± 9.9
CRT candidates
(sequential HBP & LV pacing when HBP did not correct QRS)
Prospective cohort
32
21/21 (100)
At implant:
HBP
1.7 ± 0.7V at 0.8 ± 0.4 ms
At FU:
3.0 ± 2.3 V at 0.8 ± 0.4 ms
Intrinsic 157±16
HBP+LV 110±14
p<0.0005
Improved
LVEF and NYHA functional class
AF - atrial fibrillation, BBB - bundle branch block, BiVP - biventricular pacing, CHF - congestive heart failure, CRT - cardiac resynchronization therapy, FU – follow-up, HBP - His bundle pacing, IVCD - interventricular conduction delay, LBBB - left bundle branch block, LVEF - left ventricular ejection fraction, LV P- left ventricular pacing, mo –months, NR - not reported, NYHA - New York Heart Association, Pts – patients, RVP - right ventricular pacing, yrs-years
Vijayaraman et al (16) performed HOT-CRT in 27 CRT candidates with a high success rate of 93%. His-optimized CRT resulted in significant QRS narrowing (120±16 ms) compared with baseline (183±27ms) and CRT alone (162±17 ms), (p<0.0001). The LVEF improved from 24±7% to 38±10% (P<0.0001) at 14±10 months follow-up. The clinical response rate (84%) and echocardiographic response rate (92%) were higher compared with conventional CRT. Deshmukh et al. (17) studied 21 CRT eligible patients who received HBP as an adjunct to biventricular pacing. His bundle pacing plus LVP resulted in significant QRS narrowing, improvement in LVEF and NYHA at 32 months follow-up.
HBP as an alternative therapy to CRT in patients with CRT indications (De novo HBP implant or HBP upgrade)(Table 2)
We reviewed 13 studies (1, 11, 18-28) (651 patients) (Table 2) which explored the effect of HBP as an alternative to CRT in patients with CRT indications.
The largest study was reported by Sharma et al (28). They studied 106 patients with CRT indications. His-bundle pacing was successful in 95 patients. Thirty patients had failed previous CRT attempt while 65 adopted de novo HBP as an alternative to CRT. Patients were followed-up for 14 months. His bundle pacing resulted in significant narrowing of QRS from 157 ± 33 ms to 117 ± 18 ms (p=0.0001). The LVEF increased from 30%±10% to 43%±13% (p=0.0001). The NYHA functional class improved from 2.8±0.5 to 1.8±0.6 (p=0.0001). Lead-related complications occurred in 7% of patients. Huang et al. (23) performed HBP in 74 potential CRT candidates with HF and left bundle branch block (LBBB). The acute LBBB correction rate was 97.3%. Permanent HBP was successful in 75.7% of patients. Rest of the patients received CRT due to failed LBBB correction, high LBBB correction threshold or failed HBP lead fixation. Among the 56 patients who had successful permanent HBP, 54% completed 3 years follow-up. His-bundle pacing improved LVEF (from 32.4±8.9% to 55.9±10.7%, p<0.001), left ventricular end-systolic volume (from 137.9±64.1 mL to 52.4±32.6 mL, p<0.001) and NYHA functional class (from 2.73±0.58 to 1.03±0.18, p<0.001). The LBBB acute correction threshold was 2.13±1.19 V @0.5 ms and remained stable at 2.29±0.92 V@0.5 ms at 3 years follow-up (p>0.05). Vijayaraman et al. (25) conducted a multicenter cohort study involving 85 CRT eligible patients with atrioventricular block (AVB), chronic RVP and/or PICM. At 25±24 months following, HBP resulted in improvement of LVEF and narrowing of QRS (123±32 ms at baseline vs 177+/17ms with RVP vs 115±20ms with HBP, p<0.001). Pacing threshold was 1.47 ± 0.9 V @1 ms at implant and 1.9 ±1.3 V @ 1 ms at 25±24 months follow-up. Among the 60 patients with PICM, LVEF improved from 34.3±9.6% to 48.2±9.8% (p<0.001) after HBP. Su et al. (26) studied 94 AF patients with HF and narrow QRS who received atrioventricular node (AVN) ablation and HBP. Acute HBP success was 94.7%. The LVEF improved from 44.9 ± 14.9% to 57.6 ± 12.5% at median follow-up of 3 years (p<0.001). The HBP capture threshold was 1.0±0.7V at 0.5ms at implant and remained stable at follow-up. Heart failure hospitalization or all-cause mortality occurred in 35.9% of patients.
There were 2 randomized control trials (RCT) studying the effect of HBP as an alternative to CRT. The first single-blinded RCT was conducted by Lustgarten et al. (1). They studied 29 CRT eligible candidates (97% had LBBB). Patients were randomized to either HBP or biventricular pacing (BiVP). Patients were crossed over to the other pacing modality after 6 months. The HBP implant success rate was 96.6%.
Table 2. Studies of HBP as an alternative therapy to CRT in patients with CRT indications (De novo HBP implant or HBP upgrade)
Study
Year
Pts
n= 651
Age, yrs
Patient
selection
Study design
FU, mo
Success
Rate, n (%)
Pacing
threshold
QRS duration, ms
Long-term outcome
Deshmukh (11)
2000
18
69±
10
Chronic AF, dilated CMP, QRS<=120 ms
± AVN ablation
De novo HBP
Observational
23.4±8.3
12/14 (86)
2.4±1.0 V at 0.5 ms
Intrinsic
95±13
HBP
92.8±11
P = NS
Reduced:
LVEDD, LVESD
Improved LVEF
20±9% to 31±11%, p<0. 01
1 lead dislodgement; 1 high pacing threshold
Deshmukh (18)
2004
54
70±8
CMP LVEF 23±11%, persistent AF, QRS <120ms
De novo HBP
Observational
42
39/54 (72)
(12 pts - RV apical lead)
NR
None had QRS widening
LVEF improved from 23 ± 11% to 33 ± 15%
dP/dt, NYHA, exercise time, oxygen uptake all improved
Barba-Pichardo (19)
2012
16
67.56 ± 5.81
CHF population CRT indication (failed LV lead implantation)
CRT Alternative
Prospective cohort
31.33+21
9/16 (56.3)
3.09±0.44V at implant;
3.7±0.54V at follow-up
Intrinsic 166±9
HBP 97±9
P = 0.01
HBP corrected conduction disturbance in 81%. Improved NYHA, LV dimension, LVEF
Lustgarten (1)
2015
29
NR
CRT candidate QRS >130ms
97% had LBBB
CRT alternative
RCT
Single blinded HBP vs BiVP
12
28/29 (96.6)
At implant:
HBP <1.5V
RVP <1V
LVP <1.5V
At FU:
HBP <2.5V, RVP <1V, LVP <2V
Intrinsic 169±16
NS HBP
160±25
Selective HBP
131±35
Improved NYHA class, LVEF with both BiVP and HBP
No significant difference between BiVP and HBP
Ajijola (20)
2017
21
62±
18
All patients with CRT indication (BBB, HF)
CRT alternative
Observational
12
12/16 (75)
1.9±1.2 at 0.6±0.2ms
Intrinsic 180±23
HBP 129±13
p<0.0001
Improved NYHA class and LVEF from 27±10% to 41±13% (p<0.001)
Decreased LV dimension; No lead dislodgment
Shan (21)
2017
18
70.6± 12.9
PICM
LVEF<50% requiring CRT upgrade
(5/16 were CRT non-responders)
CRT alternative
Observational
36.2
16/18 (88.9)
At implant: PICM group
0.8±0.4V
BiVP non-responder
group 1.1±0.6V
At FU: PICM group 1.2±0.8V
BiVP non-responder group 1.7±0.8V
QRS baseline 156.9± 21.7ms to 107.1± 16.5 ms;
P <0.01)
HBP associated with decreased LVEDD, improved LVEF, improved MR, decreased BNP, improved NYHA class
Table 2. Continued from page 55
Study
Year
Pts
n= 651
Age, yrs
Patient
selection
Study design
FU, mo
Success
Rate,
n (%)
Pacing
threshold
QRS duration, ms
Long-term outcome
Sharma (28)
2018
106
71±12
All patients with CRT indication (Failed CRT or new implant)
CRT alternative
Multicenter cohort
14
95/106 (90)
1.4±0.9 at 1ms (HBP)
2±1.2 at 1ms (narrowing of BBB)
Intrinsic 157±33
HBP 117±18
Improved NYHA class and LVEF in both groups.7 Lead related complications
Sharma (22)
2018
39
72+/10
Impaired LVEF, RBBB, QRS>=120ms, NYHA II-IV
CRT alternative
Retrospective observational multicenter cohort
15±23
37/39 (95)
At implant
HBP
1.1±0.6V at 1ms
At FU
1.3±0.9V at 1ms
Intrinsic
158±24
HBP
127±17
Improved NYHA class, LVEF, narrowing of RBBB in 78%
Huang (23)
2019
74
69.6±9
CHF and LBBB
CRT alternative
Single center cohort
37.1
72/74 (97.3)
At implant:
LBBB correction threshold: 2.13±1.19 V at 0.5 ms
At FU:
2.29±0.92 V at 0.5 ms p>0.05
Intrinsic 170.9±18ms vs 113.8±24ms after HBP (p<0.001)
Improved NYHA, decreased LVESV, and improved LVEF.
Upadhyay (24)
(His-Sync)
2019
41
64±13
CHF, wide QRS, CRT candidate
CRT alternative
RCT
Single-blinded
His-CRT vs BiVP-CRT
6.2
40/40
His-CRT: 1.7 V
BiVP-CRT 0.9 V
p=0.046;
Threshold stable at 12-months follow-up
Intrinsic 172±16
His-CRT 144±30
p=0.002
but no QRS shortening in BiVP-CRT
Improved LVEF with both His-CRT and BiVP-CRT. His-CRT was not superior to BiVP-CRT with regard to LVEF improvement or rate of echo response. No lead dislodgement. No difference in CV hospitalization or death
Vijayaraman (25)
2019
85
72.4±13.2
AVB and chronic RVP and/or PICM in need for CRT
CRT alternative
Multi-center cohort
25±24
79/85 (93)
At implant:
1.47±0.9V at 1ms
At FU: 1.9±1.3V at 1ms
Intrinsic
123±31
RVP 177±17
HBP 115±20
p<0.001
Improved LVEF
Table 2. Continued from page 55
Study
Year
Pts
n= 651
Age, yrs
Patient
selection
Study design
FU, mo
Success
Rate,
n (%)
Pacing
threshold
QRS duration, ms
Long-term outcome
Su (26)
2020
94
70.1±
10.5
AF with CHF and narrow QRS requiring AVN ablation
CRT alternative
Observational cohort
36
89/94 (94.7)
At implant
1±0.7V at 0.5ms
Stable threshold during FU
NR
Improved LVEF
Heart failure
hospitalization or all-cause mortality occurred in 21 (25.9%)
Singh (27)
2020
7
59
CRT eligible candidates LBBB mediate CMP
CRT alternative
Multicenter observational cohort
14.5
7/7 (100)
At implant
1.99V at 1ms
At follow-up
2V at 1ms
Intrinsic
152
HBP
115
LVEF improvement from 25% to 50% p=0.0001; LVESD & LVEDD decreased, improved NYHA class
AF - atrial fibrillation. AVB - atrioventricular block, AVN - atrioventricular node, BBB - bundle branch block, BiVP - biventricular pacing, CMP - cardiomyopathy, CRT - cardiac resynchronization therapy, FU – follow-up, HBP - His bundle pacing, IVCD - interventricular conduction delay, LBBB - left bundle branch block, LVEDD - left ventricular end-diastolic diameter, LVEF - left ventricular ejection fraction, LVESD - left ventricular end-systolic diameter, LV P- left ventricular pacing, mo-months, NYHA - New York Heart Association, NS - non-selective, NR - not reported, PICM - pacing induced cardiomyopathy, pts – patients, RVP - right ventricular pacing, yrs- years
Electrical resynchronization with QRS narrowing was achieved in 72% of patients at implant.
Quality of life, NYHA class, 6MWT and LVEF were significantly improved in both pacing modes compared with baseline. The other single-blinded RCT, the His-Sync study, was conducted by Upadhyay et al (29). They studied 41 CRT eligible candidates. Patients were randomized to His-CRT and biventricular pacing CRT (BiV-CRT). Cross over occurred in 48% of His-CRT arm and 26% in the BiV-CRT arm. At median follow-up of 6.2 months, His-CRT resulted in significant QRS narrowing (172±16 ms to 144±30 ms, p=0.002), while BiV-CRT did not. Both His-CRT and BiV-CRT resulted in similar improvement in LVEF (median +9.1% (5-14.4%) vs +5.2% (1.5-11.3%), p=0.33). The His-CRT group had higher pacing threshold than BiV-CRT group (median 1.7V versus 0.9V, p=0.046). Overall, cardiac event rates were low (6 cardiovascular hospitalizations and 2 deaths). There was no lead dislodgement reported. The study was underpowered to detect clinical outcome difference. Other studies (19-22, 27) recruited CRT eligible patients with heterogeneous backgrounds including heart failure with BBB, PICM and AF post AVN ablation, with narrow or wide QRS complexes. The longest follow-up periods were up to approximately 3 years (19, 21, 23).
Most studies have demonstrated that HBP results in electrical resynchronization (QRS narrowing or normalization of BBB), improvement of NYHA class, LVEF, and left ventricular reverse remodelling in CRT eligible candidates. However, these studies had small sample-sizes and were underpowered to detect statistically significant difference in clinical outcome in terms of mortality and heart failure hospitalization.
HBP as a stand-alone therapy in non-heart failure patients with pacing indications
To study the effect of HBP as a stand-alone therapy in non-HF patients with pacing indications, we reviewed 11 observational cohort studies (Table 3) (30-40) (with sample size >100 patients) involving 3195 patients (conducted over the past 10 years) and 3 RCTs (Table 4)(41-43) involving 122 patients.
Observational cohorts on HBP (Table 3)
The largest multicenter observational cohort was conducted by Zanon et al. (36). Eight hundred and forty-four patients (AVB in 41.2%, sinus node dysfunction in 17.4%, AF with bradycardia in 39.7% and CRT in 1.7%) received HBP. The mean HBP pacing threshold was 1.6V at implant and 2V at follow-up. In the first 368 patients, HBP was achieved using deflectable curve delivery sheaths. In the subsequent 476 patients, HBP was achieved using fixed-curve delivery system (p<0.001). The fixed-curve delivery system was associated with lower pacing threshold (1.7±1.1 V vs 2.4±1.0 V, p<0.001) and lower complication rate (4.2% vs 11.9%, p<0.001). The paced QRS was 123±29 ms vs 112±28 ms at baseline. The 64 (7.6%) patients had interruption of HBP pacing at 3 years follow-up due to elevated capture thresholds, sensing issues, infection, lead dislodgement, lead fracture and upgrade to biventricular devices.
Keene et al. (35) conducted a multicenter observational cohort study involving 529 patients with persistent or intermittent high grade AVB. His bundle pacing was successful in 87% of patients. Pacing threshold was 1.4±0.9V at 0.8±0.3ms at implant and 1.3±1.2V at 0.9±0.2ms at follow-up. His bundle pacing preserved electrical synchrony (Intrinsic QRS 116 ± 31 ms vs HBP paced QRS 115 ± 24 ms (p=0.5)). Lead re-intervention or deactivation rate was 7.5% at 7.2±10 months follow-up (mostly related to lead dislodgement or rise in capture threshold). Five patients died within the follow-up period (3 died of progressive heart failure, 2 died of unknown cause). Zanon et al. (33) conducted a prospective cohort of 307 patients with pacemaker indications. Selective HBP and para-Hisian HBP were performed in 87 and 220 patients respectively. The capture thresholds for selective HBP and para-Hisian HBP were 2.5±2.3V and 1.3±1.1V at implant, and 3.2±2.9V and 1.6±1.5V at 24 months (at 0.5ms) respectively. His bundle pacing resulted in narrow paced QRS (108±21ms vs intrinsic QRS 104±31ms). Lead complication rate was 3.9% at 20±10 months follow-up. Beer et al. (30) performed HBP in 294 patients with pacing indications. Pacing threshold was 1.6±1V at implant. Six percent of patients required lead revision at follow-up. His bundle capture threshold remained stable in 85% of patients. Vijayaraman et al. (31) studied 100 patients with advanced AVB and preserved LVEF. His bundle pacing normalized His-Purkinje conduction in 76% of patients with infra-nodal block. However, these 5 large HBP observational cohorts did not include control groups to assess the comparative benefit of HBP over conventional RVP, in terms of HF hospitalization, LVEF improvement, left ventricular reverse remodelling and mortality.
Observational HBP studies reporting clinical outcome, in non-heart failure patients with pacing indications
Abdelrahman et al. (34) performed HBP in 332 consecutive pacemaker recipients. Pacemakers were indicated for sinus node dysfunction and AVB in 35% and 65% of patients respectively. The implant success rate was 92%. The clinical outcome was compared with 443 RVP patients.
Table 3. Studies of HBP as a stand-alone therapy in non-heart failure patients with pacing indications
Study
Year
Pts
n = 3195
Age,
yrs
Patient selection
Study Design
FU, mo
Success Rate, n(%)
Acute / Chronic
threshold
QRS duration
Long-term outcome
Zanon (33)
2011
307
72±12
Pacemaker indications
Prospective cohort
20±10
HBP
87/307 (28)
NS HBP
220/307 (72%)
At implant
Selective HBP
2.5±2.3 V
NS HBP
1.3±1.1 V
At FU:
Selective HBP
3.2±2.9V
NS HBP
1.6±1.5V at 0.5ms
Intrinsic
104±31
HBP 108±21
Lead related complications
12/307 (3.9%)
Vijayaraman (31)
2015
100
75+/12
46% AVN block; 54% infranodal block, or AVN ablation; narrow and wide QRS
Single center cohort
19±12
84/100 (84)
At implant
HBP 1.3±0.9V
At FU
1.7±1V
at 0.5ms
Intrinsic 122±27
HBP 124± 22
His Purkinje conduction normalized in 76% patients with infranodal block
Pastore (32)
2016
148
74+8.5
Complete / advanced AVB
Retrospective observational
58.5±26.5
148/148 (100)
NR
NR
HBP associated with lower risk of AF vs RVP
HR = 0.28
p=0.0001
Abdelrahman (34)
2018
332
76±11
All pts requiring pacemaker implant
Multicenter prospective cohort
Non-randomized
HBP 332 vs RVP 433
24
304/332 (92)
At implant:
HBP 1.3±0.85
RVP 0.59±0.42V
At FU:
HBP
1.56±0.95V
RVP 0.76±0.29V
Intrinsic
105-110
HBP 128±27
RVP 166±22
HBP reduced CHF hospitalization (primarily in pts with VP >20%), a trend towards reduced mortality
Bhatt (40)
2018
101
76 ± 9.8
All pts with pacing indication
Single center observational
24
76/101 (75)
At implant:
1.2 ±0.8 V at 1.0 ms
At FU:
1.8 ± 1.5V at 0.6 ± 0.2ms
Intrinsic (with BBB)
156 +/- 48ms;
HBP 83+/-2ms
Narrowing of QRS in pts with BBB. Rising threshold in 30% and lead intervention in 8%
Table 3. Continued from page 58
Study
Year
Pts
n = 3195
Age,
yrs
Patient selection
Study Design
FU, mo
Success Rate, n(%)
Acute / Chronic
threshold
QRS duration
Long-term outcome
Keene (35)
2019
529
75 ± 11
Persistent or intermittent high grade AVB
Multi-center observational study
(7 centers)
7.2±10
332/410 (81)
At implant
1.4±0.9V at 0.8±0.3 ms
At FU
1.3±1.2V at 0.9±0.2ms
Intrinsic 116 ± 31 ms
HBP
115 ± 24 ms
p=0.5
HBP lead re-intervention or deactivation rate of 7.5% (lead dislodgement or rise in threshold).
Death (n=5); progressive heart failure, 2 unknowns)
Zanon (36)
2019
844
75±9
AVB 41.2%
SND 17.4%
AF with bradycardia 39.7%
Multicenter cohort
36
844/844 (100)
2.4+/1V (with deflectable curve delivery system)
1.7±1.1V with fixed curve sheath p<0.001
Intrinsic 112 ± 28
HBP
123 ± 29ms
Complication rate 8.4%
Beer (30)
2020
294
75±11
Bradycardia / pacing indication
Single center cohort
39.5±16.8
294/294 (100)
At implant
1.6±1V
At FU 1.6±0.8V
NR
Threshold increase 41% by 8 weeks, 66% by 1y; 6% require lead revision
HBP capture threshold stable in 85% patients
Dawson (37)
2020
140
76
Pts with pacing indications
Multicenter cohort
0.5-2
122/140 (87)
At implant
Intrinsic 110
HBP 110
NR
Ravi (38)
2020
105
72.65±11.04
Pts with pacemaker indications:
HBP (105) vs RVP (120)
Observational cohort
23.4±10.8
105/105 (100)
NR
NR
HBP lowered risk of new-onset AF in pts with >20% pacing dependence.
HBP -lower risk of AF progression in pts with pacing burden ≥40%
Teigeler (39)
2021
295
69±15
SSS 41%
AVB 36% CRT 7%
AF 15%
Single center prospective observational cohort
23±20
274/295 (93)
At implant
1.1±0.9V at 0.8± 0.2ms
At FU 1.7±1.1V at 0.8±0.3ms
Threshold ≥2.5V in 24%; ≥1V in 28%.
Loss of HBP capture in 17%.
Total 11% lead revision, primarily for high thresholds
AF - atrial fibrillation, AVB - atrioventricular block, AVN - atrioventricular node, CRT - cardiac resynchronization therapy, FU – follow-up, HBP - His bundle pacing, HR - hazard ratio, mo –months, NS - non-selective, NR - not reported, pts – patients, RVP - right ventricular pacing, SSS - sick sinus syndrome, yrs -years
His bundle pacing was associated with a decrease in combined endpoint of death from any cause, HF hospitalizations or upgrade to BiVP compared with RVP (25% vs 32%; hazard ratio (HR) 0.71, 95% CI 0.534-0.944; p=0.02).
The primary outcome was predominantly driven by significant reduction in HF hospitalizations (12.4% vs. 17.6%; HR: 0.63; 95% CI: 0.430 to 0.931; p = 0.02). There was a trend towards reduced mortality in the HBP group (17.2% vs. 21.4%, respectively; p=0.06). Patients with >20% ventricular pacing burden benefited most from HBP. Pastore et al. (32) performed HBP in 148 patients with complete or advanced AVB. His bundle pacing was associated with lower risk of AF (16.9% vs right ventricular septal pacing - 25.7% vs right ventricular apical pacing - 28.0%, p=0.049.). Ravi et al. (38) compared the effect of HBP (n=105) with RVP (N=120) on the risk of new onset AF and AF progression. In patients with no history of AF, HBP was associated with lower risk of new onset AF (adjusted HR 0.53; 95% CI 0.28-0.99; p=0.046) compared with RVP, especially in patients with RVP burden >20%. In patients with prior history of AF, there was no difference in the risk of AF progression between the 2 groups. In patients with pacing burden >=40%, HBP showed a trend towards lower risk of AF progression versus RVP (HR 0.19; 95% CI 0.03-1.16; p=0.072).
Sharma et al. (44) studied 94 pacemaker recipients without HF, HBP significantly reduced HF hospitalization (2% vs 15% in RVP patients, p=0.02) in those requiring >40% ventricular pacing (in >60% of patients), during a mean follow-up period of 25.5±8.6 months. There was no difference in mortality between HBP and RVP patients.
Randomized control trials (RCT) on HBP in non-heart failure patients with pacing indications (Table 4)
Occhetta et al. conducted two small RCTs on HBP. In the first study (41), 16 patients with chronic AF requiring AVN ablation were implanted with a RVA pacing lead and a para-Hisian pacing lead. Patients were randomized and received crossover to two 6-month periods of para-Hisian pacing and conventional RVA pacing. Para-Hisian pacing resulted in improvement of interventricular electromechanical delay, NYHA class, QoL score, 6MWT, mitral and tricuspid regurgitation. Another RCT by Occhetta et al. (42) randomized 17 patients with chronic AF requiring AVN ablation or sinus rhythm with AVB and narrow QRS to HBP and RVA pacing. At 21 months follow-up, HBP was associated with improved NYHA, exercise tolerance, QoL, interventricular mechanical delay, mitral and tricuspid regurgitation. Left ventricular ejection fraction was preserved versus baseline. Kronborg et al. (43) randomized 38 patients with high grade AVB, narrow QRS and preserved LVEF >40% to HBP and right ventricular septal pacing. At follow-up of 24 months, there was no difference in NHYA class, 6MWT, QoL and device-related complications. The mean threshold was higher in HBP. His bundle pacing was associated with better preserved LVEF than right ventricular septal pacing (55±10% vs 50±11%, p=0.005).
Systematic review and meta-analysis of His Bundle Pacing Studies
Six meta-analysis were reviewed. Zanon et al (45) performed the first systematic review and meta-analysis of 1438 patients who received permanent HBP over a period of nearly 20 years, in 16 centers around the world. The average implant success rate was 84.8%. The LVEF of HBP patients improved from 42.8% at baseline to 49.5% at 16.9 months’ follow-up.
Table 4. Randomized controlled trials (RCT) on HBP in non-heart failure patients with pacing indications
Study
Year
Pts
n = 222
Age,
yrs
Patient selection
Study Design
FU, mo
Success Rate, n(%)
Pacing threshold
QRS duration
Long-term outcome
Occhetta (41)
2006
16
71±5
Chronic AF narrow QRS post AVN ablation
Cross over blinded RCT
12
16/16
(100)
0.92±0.7 V at 0.5 ms
Intrinsic
88.3±7
HBP
121 ± 10,
p<0.05
Improved QoL, NYHA, 6MWT, interventricular EMD
Occhetta (42)
2007
68
79±6
AVN ablation for chronic AF, narrow QRS; sinus rhythm with AVB and narrow QRS
RCT (first 17 patients)
Cross-over HBP vs RV apical pacing
21
NR
At implant
0.7±0.5V
At FU
0.9±0.7V
p = 0.08
Intrinsic 91±13.5
HBP 123±14
RVP 164.5±18,
p<0.05
Improved NYHA, exercise tolerance, QoL, MR, TR and ICVD; preserved LVEF vs baseline
Kronborg (43)
2014
38
67±10
High grade AVB, narrow QRS
LVEF >40%
Crossover double blinded RCT (His bundle vs RV septal pacing)
24
17/19 (89)
At implant
1V
At FU
1.5V
Intrinsic 93±16
HBP
111±19
RVP
153±12
No difference in NYHA class, 6MWT, QOL
HBP was associated with better preserved LVEF and mechanical synchrony vs RV septal pacing
6MWT - six-minute walk test, AF - atrial fibrillation, AVB - atrioventricular block, AVN - atrioventricular node, EMD- electromechanical delay, FU – follow-up, HBP - His bundle pacing, IVCD - interventricular conduction delay, LVEF - left ventricular ejection fraction, mo –months, MR- mitral regurgitation, NYHA - New York Heart Association, pts - patients, QoL- quality of life, RV = right ventricular, TR -tricuspid regurgitation, yrs -years
Among the 907 patients in the 17 studies, which reported safety information, implant complication rate was 4.7%.
There were 26 lead revisions due to lead dislodgement (n = 6) and elevated threshold (n=20). Early device replacement due to battery depletion was uncommon (0.66%).
Qian et al. (46) systematically reviewed 11 HBP studies including 494 patients with HF. The mean follow-up duration was 23.7 months. In CRT candidates who received HBP, the paced QRS duration decreased from 165.4 ± 8.7 ms at baseline to 116.9 ± 15.8 ms after HBP (p<0.0001). Left ventricular ejection fraction significantly improved from 36.9±3.3% at baseline to 48.1 ± 3.0% at follow-up (p <0.0001). Left ventricular end-diastolic volume decreased from 58.2±1.7 mm at baseline to 52.8 ± 1.7 mm (p<0.0001). His bundle pacing also improved LVEF in patients with AF who had received AVN ablation.
Slotwiner et al. (47) performed a systematic review on physiologic pacing versus RVP among patients with LVEF > 35%. The review included 679 patients in 8 HBP studies.
HBP was associated with higher LVEF compared with RVP (mean difference [MD] 4.33% 95% CI: 0.85-7.81%; p<0.01) at 8.36 months follow-up. However, the HBP did not demonstrate consistent benefit in QoL and 6MWT distance.
Pooled analysis of BiVP and HBP recipients showed that physiologic pacing improved left ventricular reverse remodelling as shown in Figure 2 (left ventricular end-systolic volume and left ventricular end-diastolic volume reduced by -7.09 ml, p=0.0009; I2=12.98%; and –2.77 mL; p=0.001; I2= 0% respectively) and LVEF (LVEF improved by 5.328%; 95% CI: 2.86–7.8; p<0.0001; I2=39.11%) compared with RVP at mean follow-up of 1.64 years. His bundle pacing did not demonstrate consistent benefit over RVP in terms of functional status, quality of life and survival. Patients with LVEF between 36% and 52% were more likely to derive cardiovascular benefit from physiologic pacing. Patients with chronic AF who underwent AVN ablation derived improvement of LVEF from physiologic pacing versus RVP.
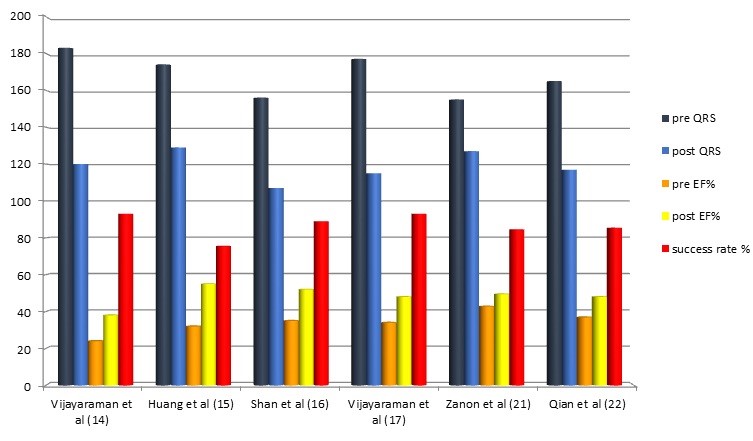
Figure 2. QRS duration, ejection fraction before and after His-bundle pacing along with success rate in reported in various studies
Sun et al. (48) performed a systematic review and meta-analysis of 13 HBP studies (comprising 2348 patients) reporting long-term clinical outcome. His bundle pacing had improved LVEF (MD, 5.65; 95% confidence interval [CI], 4.38-6.92), shorter paced QRS width (MD, - 39.29; 95% CI, - 41.90 to - 36.68), higher pacing threshold (MD, 0.8; 95% CI, 0.71-0.89) and lower rate of heart failure hospitalization (odds ratio [OR], 0.65; 95% CI, 0.44-0.96) compared with RVP. There were no statistically significant differences in left ventricular volume and all-cause mortality between the two groups.
Qi et al. (49) reviewed 13 studies (involving 503 patients) on the effect of HBP in CRT candidates. His bundle pacing resulted in narrowing of QRS duration from 165.5 ± 8.7 to 122.9 ± 12.0 ms (MD = 43.5, 95%Cl: 36.34 ~ 50.56, p < 0.001), improvement in NYHA class (MD = 1.2, 95% CI: 1.09 ~ 1.31, p < 0.001), LVEF (MD = - 12.60, 95% Cl: - 14.32 ~ - 10.87, p < 0.001), and left ventricular end-diastolic dimension (MD = 4.30, 95% Cl: 3.05 ~ 5.55, p < 0.001) at > 3 months follow-up compared with that at baseline (p<0.001). The most commonly reported complication was HBP capture threshold rise.
Fernandes et al. (50) compared the effect of HBP with BiVP and RVP in patients with normal or mildly reduced LVEF. Six studies comparing 704 BiVP patients with 614 RVP patients and four studies comparing 463 HBP patients with 568 RVP patients were included. Both HBP and BiVP increased LVEF and decreased QRS duration (MD, 5.27 [3.86-6.69], p<0.001; MD -42.2 [-51.2 to -33.3], p<0.001, respectively). In HBP or BiVP patients, mortality and HF hospitalization rate was lower compared with RVP patients (odds ratio [OR], 0.66, [0.51-0.85], p =0 .002; OR, 0.61 [0.45-0.82], p<0.001, respectively]. No significant clinical outcome difference was demonstrated between BiVP and HBP.
Guideline recommendations
Latest guideline has given HBP a class IIa recommendation in patients with reduced LV ejection fraction (LVEF) between 36% and 50% who require chronic ventricular pacing (51).
Limitations of HBP
There are certain limitations for HBP. Firstly, the implant success varies considerably in early studies, ranging from 56% to 95%. The success rate in later studies (1, 19, 20, 31, 41) improved with accumulation of operator experience. Secondly, HBP patients have higher pacing threshold compared with conventional RVP. Some patients encountered chronic threshold elevation at follow-up.
Vijayaraman et al. (52) reported that His-bundle capture threshold at 5-year follow-up was significantly higher than that in RVP patients (1.62± 1.0 V vs. 0.84± 0.4 V at 0.5 ms, p <0.05). Moreover, 5-year lead revisions rate (6.7% vs 3%) and generator replacement rate (9% vs 1%) were higher in HBP patients compared with RVP patients.
Thirdly, the concern of lead instability/ dislodgement often requires an additional backup pacing lead in some patients. The early lead revision rate was higher in HBP patients (4.2% versus 0.5% in RVP) (34). In a latest study of 295 HBP patients, Teigeler et al. (39) has shown that loss of HBP capture and lead revision occurred in 17% and 11% of patients respectively at long term follow-up (~23 months). Finally, the progression of infra-Hisian His / Purkinje system conduction disease distal to the sight of HBP might result in unpredictable ventricular non-capture at follow-up. The advent of left bundle branch pacing might potentially alleviate some of the above limitations of HBP.
Ongoing HBP studies
The His-bundle pacing vs RVA pacing in patients with reduced ejection fraction (HIS-PrEF) study (ClinicalTrials.gov Identifier: NCT04529577) is a double-blinded, RCT with crossover design. It aims to compare the effect of HBP with RVA pacing in patients with slightly or moderately reduced ejection fraction and AVB with pacing indication. The primary outcome is LVEF at 6 months.
The His bundle pacing in bradycardia and HF study (ClinicalTrials.gov Identifier: NCT03008291) by the Mayo Clinic group is a prospective cohort study. It aims to study the effect of HBP on normalization of atrioventricular conduction in heart failure, CRT candidates with conduction disease. The mapping and pacing of the His bundle for HF patients with LBBB (MAP HIS HF) study (ClinicalTrials.gov Identifier: NCT03803995) is a prospective, single-arm, non-randomized study to assess the locations of HBP that results in correction of electrical dyssynchrony using electroanatomical mapping system.
What our paper adds?
Our paper has reviewed the latest and most large-scale observational studies of HBP, RCTs and meta-analysis on HBP over the 2 decades. It has systematically summarized the major HBP trials, study sample sizes, study design, patient characteristics, follow-up duration, pacing thresholds, QRS duration at implant and follow-up, long term clinical outcome and complication rates. It has also summarized conclusion from major meta-analysis regarding the benefit and limitations of HBP.
Knowledge gap and future research directions – what is still unknown
Most of the published HBP studies are observational cohort studies and small-scale randomized trials. The patient populations in these studies were heterogeneous. The long-term beneficial effect of HBP over RVP or CRT, in terms of heart failure hospitalization and mortality remains to be demonstrated by future large-scale randomized control trials. The long-term durability and stability of HBP leads and the effect of high pacing threshold on current drain and device longevity requires long-term follow-up evaluation.
Conclusion
His bundle pacing restores physiological electrical conduction and mechanical synchrony in patients with pacemaker indications and/or heart failure with CRT indications.
In pacing-dependent patients without heart failure, HBP improves electrical synchrony and reduces the risk of pacing induced left ventricular dysfunction. In heart failure patients with CRT indications, HBP as an adjunct or alternative therapy improves left ventricular systolic function, reduces left ventricular remodelling, heart failure hospitalization and mortality.
It is a viable alternative for CRT eligible patients who have failed left ventricular lead implantation or who are CRT non-responders. The current evidence and clinical guideline support the use of HBP in patients with impaired LVEF (between 36-50%) and high pacing dependence. Future randomized studies are warranted to assess the long-term clinical benefit and safety of HBP in pacing dependent and heart failure population.
Peer-review: Internal and external
Conflict of interest: None to declare
Authorship: J.K.C., S.M., N.K. are equally contributed to study and preparation of article
Acknowledgement and funding: None to declare
References
1. Lustgarten DL, Crespo EM, Arkhipova-Jenkins I, Lobel R, Winget J, Koehler J, et al. His-bundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: A crossover design comparison. Heart rhythm 2015; 12: 1548-57. doi: 10.1016/j.hrthm.2015.03.048. PubMed PMID: 25828601. https://doi.org/10.1016/j.hrthm.2015.03.048 PMid:25828601 2. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003; 107: 2932-7. doi: 10.1161/01.cir.0000072769.17295.b1. PubMed PMID: 12782566. https://doi.org/10.1161/01.CIR.0000072769.17295.B1 PMid:12782566 3. Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. Jama 2002; 288: 3115-23. doi: 10.1001/jama.288.24.3115. PubMed PMID: 12495391. https://doi.org/10.1001/jama.288.24.3115 PMid:12495391 4. Sharma AD, Rizo-Patron C, Hallstrom AP, O'Neill GP, Rothbart S, Martins JB, et al. Percent right ventricular pacing predicts outcomes in the DAVID trial. Heart rhythm 2005; 2: 830-4. doi: 10.1016/j.hrthm.2005.05.015. PubMed PMID: 16051118. https://doi.org/10.1016/j.hrthm.2005.05.015 PMid:16051118 5. Khurshid S, Epstein AE, Verdino RJ, Lin D, Goldberg LR, Marchlinski FE, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart rhythm 2014; 11: 1619-25. doi: 10.1016/j.hrthm.2014.05.040. PubMed PMID: 24893122. https://doi.org/10.1016/j.hrthm.2014.05.040 PMid:24893122 6. Kiehl EL, Makki T, Kumar R, Gumber D, Kwon DH, Rickard JW, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart rhythm 2016; 13: 2272-8. doi: 10.1016/j.hrthm.2016.09.027. PubMed PMID: 27855853. https://doi.org/10.1016/j.hrthm.2016.09.027 PMid:27855853 7. Auricchio A, Prinzen FW. Non-responders to cardiac resynchronization therapy: the magnitude of the problem and the issues. Circ J 2011; 75: 521-7. doi: 10.1253/circj.cj-10-1268. PubMed PMID: 21325727. https://doi.org/10.1253/circj.CJ-10-1268 PMid:21325727 8. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. New Engl J Med 2009; 361: 1329-38. doi: 10.1056/NEJMoa0906431. PubMed PMID: 19723701. https://doi.org/10.1056/NEJMoa0906431 PMid:19723701 9. Curtis AB, Worley SJ, Chung ES, Li P, Christman SA, St John Sutton M. Improvement in clinical outcomes with biventricular versus right ventricular pacing: the BLOCK HF Study. J Am Coll Cardiol 2016; 67: 2148-57. doi: 10.1016/j.jacc.2016.02.051. PubMed PMID: 27151347. https://doi.org/10.1016/j.jacc.2016.02.051 PMid:27151347 10. Funck RC, Mueller HH, Lunati M, Piorkowski C, De Roy L, Paul V, et al. Characteristics of a large sample of candidates for permanent ventricular pacing included in the Biventricular Pacing for Atrio-ventricular Block to Prevent Cardiac Desynchronization Study (BioPace). Europace 2014; 16: 354-62. doi: 10.1093/europace/eut343. PubMed PMID: 24200715. https://doi.org/10.1093/europace/eut343 PMid:24200715 11. Deshmukh P, Casavant DA, Romanyshyn M, Anderson K. Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation 2000; 101: 869-77. doi: 10.1161/01.cir.101.8.869. PubMed PMID: 10694526. https://doi.org/10.1161/01.CIR.101.8.869 PMid:10694526 12. Sohaib SMA, Wright I, Lim E, Moore P, Lim PB, Koawing M, et al. atrioventricular optimized direct his bundle pacing improves acute hemodynamic function in patients with heart failure and pr interval prolongation without left bundle branch block. JACC Clin Electrophysiol 2015; 1: 582-91. doi: 10.1016/j.jacep.2015.08.008. PubMed PMID: 29759412. https://doi.org/10.1016/j.jacep.2015.08.008 PMid:29759412 13. Padeletti L, Pieragnoli P, Ricciardi G, Innocenti L, Checchi L, Padeletti M, et al. Simultaneous His bundle and left ventricular pacing for optimal cardiac resynchronization therapy delivery: acute hemodynamic assessment by pressure-volume loops. Circulation Arrh Electrophysiol 2016; 9. doi: 10.1161/CIRCEP.115.003793. PubMed PMID: 27162032. https://doi.org/10.1161/CIRCEP.115.003793 PMid:27162032 14. Lustgarten DL, Calame S, Crespo EM, Calame J, Lobel R, Spector PS. Electrical resynchronization induced by direct His-bundle pacing. Heart Rhythm 2010; 7: 15-21. doi: 10.1016/j.hrthm.2009.09.066. PubMed PMID: 19914142. https://doi.org/10.1016/j.hrthm.2009.09.066 PMid:19914142 15. Boczar K, Sławuta A, Ząbek A, Dębski M, Vijayaraman P, Gajek J, et al. Cardiac resynchronization therapy with His bundle pacing. Pacing Clin Electrophysiol 2019; 42: 374-80. doi: 10.1111/pace.13611. https://doi.org/10.1111/pace.13611 PMid:30659629 16. Vijayaraman P, Herweg B, Ellenbogen KA, Gajek J. His-optimized cardiac resynchronization therapy to maximize electrical resynchronization: a feasibility study. Circulation Arrh Electrophysiol 2019; 12: e006934. doi: 10.1161/CIRCEP.118.006934. PubMed PMID: 30681348. https://doi.org/10.1161/CIRCEP.118.006934 PMCid:PMC6420120 17. Deshmukh A, Sattur S, Bechtol T, Heckman LIB, Prinzen FW, Deshmukh P. Sequential His bundle and left ventricular pacing for cardiac resynchronization. J Cardiovasc Electrophysiol 2020; 31: 2448-54. doi: 10.1111/jce.14674. https://doi.org/10.1111/jce.14674 PMid:32666630 18. Deshmukh PM, Romanyshyn M. Direct His-bundle pacing: present and future. Pacing Clin Electrophysiol 2004; 27: 862-70. doi: 10.1111/j.1540-8159.2004.00548.x. PubMed PMID: 15189517. https://doi.org/10.1111/j.1540-8159.2004.00548.x PMid:15189517 19. Barba-Pichardo R, Manovel Sánchez A, Fernández-Gómez JM, Mori-a-Vázquez P, Venegas-Gamero J, Herrera-Carranza M. Ventricular resynchronization therapy by direct His-bundle pacing using an internal cardioverter defibrillator. Europace 2012; 15: 83-8. doi: 10.1093/europace/eus228. https://doi.org/10.1093/europace/eus228 PMid:22933662 20. Ajijola OA, Upadhyay GA, Macias C, Shivkumar K, Tung R. Permanent His-bundle pacing for cardiac resynchronization therapy: Initial feasibility study in lieu of left ventricular lead. Heart Rhythm 2017; 14: 1353-61. doi: 10.1016/j.hrthm.2017.04.003. PubMed PMID: 28400315. https://doi.org/10.1016/j.hrthm.2017.04.003 PMid:28400315 21. Shan P, Su L, Zhou X, Wu S, Xu L, Xiao F, et al. Beneficial effects of upgrading to His bundle pacing in chronically paced patients with left ventricular ejection fraction <50. Heart Rhythm 2018; 15: 405-12. doi: 10.1016/j.hrthm.2017.10.031. PubMed PMID: 29081396. https://doi.org/10.1016/j.hrthm.2017.10.031 PMid:29081396 22. Sharma PS, Naperkowski A, Bauch TD, Chan JYS, Arnold AD, Whinnett ZI, et al. Permanent His bundle pacing for cardiac resynchronization therapy in patients with heart failure and right bundle branch block. Circulation Arrh Electrophysiol 2018; 11: e006613. doi:10.1161/CIRCEP.118.006613. https://doi.org/10.1161/CIRCEP.118.006613 PMid:30354292 23. Huang W, Su L, Wu S, Xu L, Xiao F, Zhou X, et al. Long-term outcomes of His bundle pacing in patients with heart failure with left bundle branch block. Heart 2019; 105: 137-43. doi: 10.1136/heartjnl-2018-313415. PubMed PMID: 30093543. https://doi.org/10.1136/heartjnl-2018-313415 PMid:30093543 24. Upadhyay GA, Vijayaraman P, Nayak HM, Verma N, Dandamudi G, Sharma PS, et al. His corrective pacing or biventricular pacing for cardiac resynchronization in heart failure. J Am Coll Cardiol 2019; 74: 157-9. doi: 10.1016/j.jacc.2019.04.026. PubMed PMID: 31078637. https://doi.org/10.1016/j.jacc.2019.04.026 PMid:31078637 25. Vijayaraman P, Herweg B, Dandamudi G, Mittal S, Bhatt AG, Marcantoni L, et al. Outcomes of His-bundle pacing upgrade after long-term right ventricular pacing and/or pacing-induced cardiomyopathy: Insights into disease progression. Heart Rhythm 2019; 16: 1554-61. doi: 10.1016/j.hrthm.2019.03.026. PubMed PMID: 30930330. https://doi.org/10.1016/j.hrthm.2019.03.026 PMid:30930330 26. Su L, Cai M, Wu S, Wang S, Xu T, Vijayaraman P, et al. Long-term performance and risk factors analysis after permanent His-bundle pacing and atrioventricular node ablation in patients with atrial fibrillation and heart failure. Europace 2020; 22: ii19-ii26. doi: 10.1093/europace/euaa306. PubMed PMID: 33370800. https://doi.org/10.1093/europace/euaa306 PMid:33370800 27. Singh R, Devabhaktuni S, Ezzeddine F, Simon J, Khaira K, Dandamudi G. His-bundle pacing: A novel treatment for left bundle branch block-mediated cardiomyopathy. J Cardiovasc Electrophysiol 2020; 31: 2730-6. doi: 10.1111/jce.14692. https://doi.org/10.1111/jce.14692 PMid:32713017 28. Sharma PS, Dandamudi G, Herweg B, Wilson D, Singh R, Naperkowski A, et al. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: A multicenter experience. Heart Rhythm 2018 https://doi.org/10.1016/j.hrthm.2017.10.014 PMid:29031929 15: 413-20. Epub 2017/10/17. doi: 10.1016/j.hrthm.2017.10.014. PubMed PMID: 29031929. https://doi.org/10.1016/j.hrthm.2017.10.014 PMid:29031929 29. Upadhyay GA, Vijayaraman P, Nayak HM, Verma N, Dandamudi G, Sharma PS, et al. On-treatment comparison between corrective His bundle pacing and biventricular pacing for cardiac resynchronization: A secondary analysis of the His-SYNC Pilot Trial. Heart Rhythm 2019; 16: 1797-807. doi: 10.1016/j.hrthm.2019.05.009. PubMed PMID: 31096064. https://doi.org/10.1016/j.hrthm.2019.05.009 PMid:31096064 30. Beer D, Subzposh FA, Colburn S, Naperkowski A, Vijayaraman P. His bundle pacing capture threshold stability during long-term follow-up and correlation with lead slack. Europace 2020; doi: 10.1093/europace/euaa350. PubMed PMID: 33236070. https://doi.org/10.1093/europace/euaa350 PMid:33236070 31. Vijayaraman P, Naperkowski A, Ellenbogen KA, Dandamudi G. Electrophysiologic insights into site of atrioventricular block: lessons from permanent His bundle pacing. JACC Clin Electrophysiol 2015; 1: 571-81. doi: 10.1016/j.jacep.2015.09.012. https://doi.org/10.1016/j.jacep.2015.09.012 PMid:29759411 32. Pastore G, Zanon F, Baracca E, Aggio S, Corbucci G, Boaretto G, et al. The risk of atrial fibrillation during right ventricular pacing. Europace 2016; 18: 353-8. doi: 10.1093/europace/euv268. PubMed PMID: 26443444. https://doi.org/10.1093/europace/euv268 PMid:26443444 33. Zanon F, Svetlich C, Occhetta E, Catanzariti D, Cantù F, Padeletti L, et al. Safety and performance of a system specifically designed for selective site pacing. Pacing Clin Electrophysiol 2011; 34: 339-47. doi: 10.1111/j.1540-8159.2010.02951.x. PubMed PMID: 21070258. https://doi.org/10.1111/j.1540-8159.2010.02951.x PMid:21070258 34. Abdelrahman M, Subzposh FA, Beer D, Durr B, Naperkowski A, Sun H, et al. Clinical outcomes of his bundle pacing compared to right ventricular pacing. J Am Coll Cardiol 2018; 71: 2319-30. doi: 10.1016/j.jacc.2018.02.048. https://doi.org/10.1016/j.jacc.2018.02.048 PMid:29535066 35. Keene D, Arnold AD, Jastrzębski M. His bundle pacing, learning curve, procedure characteristics, safety, and feasibility: Insights from a large international observational study. J Cardiovasc Electrophysiol 2019; 30: 1984-93. doi: 10.1111/jce.14064. PubMed PMID: 31310403. https://doi.org/10.1111/jce.14064 PMid:31310403 PMCid:PMC7038224 36. Zanon F, Abdelrahman M, Marcantoni L, Naperkowski A, Subzposh FA, Pastore G, et al. Long term performance and safety of His bundle pacing: A multicenter experience. J Cardiovasc Electrophysiol 2019; 30: 1594-601. doi: 10.1111/jce.14063. https://doi.org/10.1111/jce.14063 PMid:31310410 37. Dawson LP, Cadden J, Pol D, Wynn G, Grigg L, Kalman J, et al. Learning curve and initial experience with implementation of a his-bundle pacing program in an Australian setting. Heart Lung Circ 2020; 29: 1493-501. doi: 10.1016/j.hlc.2020.01.003. PubMed PMID: 32089490. https://doi.org/10.1016/j.hlc.2020.01.003 PMid:32089490 38. Ravi V, Beer D, Pietrasik GM, Hanifin JL, Ooms S, Ayub MT, et al. Development of new onset or progressive atrial fibrillation in patients with permanent His bundle pacing versus right ventricular pacing: results from the RUSH HBP registry. J Am Heart Assoc 2020; 9: e018478. doi:10.1161/JAHA.120.018478. https://doi.org/10.1161/JAHA.120.018478 PMid:33174509 PMCid:PMC7763709 39. Teigeler T, Kolominsky J, Vo C, Shepard RK, Kalahasty G, Kron J, et al. Intermediate-term performance and safety of His-bundle pacing leads: A single-center experience. Heart Rhythm 2021; doi: 10.1016/j.hrthm.2020.12.031. PubMed PMID: 33418127. https://doi.org/10.1016/j.hrthm.2020.12.031 PMid:33418127 40. Bhatt AG, Musat DL, Milstein N, Pimienta J, Flynn L, Sichrovsky T, et al. The efficacy of His bundle pacing: lessons learned from implementation for the first time at an experienced electrophysiology center. JACC Clin Electrophysiol 2018; 4: 1397-406. doi: 10.1016/j.jacep.2018.07.013. https://doi.org/10.1016/j.jacep.2018.07.013 PMid:30466843 41. Occhetta E, Bortnik M, Magnani A, Francalacci G, Piccinino C, Plebani L, et al. Prevention of ventricular desynchronization by permanent para-Hisian pacing after atrioventricular node ablation in chronic atrial fibrillation: a crossover, blinded, randomized study versus apical right ventricular pacing. J Am Coll Cardiol 2006; 47: 1938-45. doi: 10.1016/j.jacc.2006.01.056. PubMed PMID: 16697308. https://doi.org/10.1016/j.jacc.2006.01.056 PMid:16697308 42. Occhetta E, Bortnik M, Marino P. Permanent parahisian pacing. Indian Pacing Electrophysiol J 2007; 7: 110-25. PubMed PMID: 17538702; PubMed Central PMCID: PMCPmc1877829. 43. Kronborg MB, Mortensen PT, Poulsen SH, Gerdes JC, Jensen HK, Nielsen JC. His or para-His pacing preserves left ventricular function in atrioventricular block: a double-blind, randomized, crossover study. Europace 2014; 16: 1189-96. doi: 10.1093/europace/euu011. PubMed PMID: 24509688. https://doi.org/10.1093/europace/euu011 PMid:24509688 44. Sharma PS, Dandamudi G, Naperkowski A, Oren JW, Storm RH, Ellenbogen KA, et al. Permanent His-bundle pacing is feasible, safe, and superior to right ventricular pacing in routine clinical practice. Heart Rhythm 2015; 12: 305-12. doi: 10.1016/j.hrthm.2014.10.021. PubMed PMID: 25446158. https://doi.org/10.1016/j.hrthm.2014.10.021 PMid:25446158 45. Zanon F, Ellenbogen KA, Dandamudi G, Sharma PS, Huang W, Lustgarten DL, et al. Permanent His-bundle pacing: a systematic literature review and meta-analysis. Europace 2018; 20: 1819-26. doi: 10.1093/europace/euy058. PubMed PMID: 29701822. https://doi.org/10.1093/europace/euy058 PMid:29701822 46. Qian Z, Zou F, Wang Y, Qiu Y, Chen X, Jiang H, et al. Permanent His bundle pacing in heart failure patients: A systematic review and meta-analysis. Pacing Clin Electrophysiol 2019; 42: 139-45. doi: 10.1111/pace.13565. PubMed PMID: 30515877. https://doi.org/10.1111/pace.13565 PMid:30515877 47. Slotwiner DJ, Raitt MH, Del-Carpio Munoz F, Mulpuru SK, Nasser N, Peterson PN. Impact of physiologic pacing versus right ventricular pacing among patients with left ventricular ejection fraction greater than 35%: a systematic review for the 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019; 74: 988-1008. doi: 10.1016/j.jacc.2018.10.045. https://doi.org/10.1016/j.jacc.2018.10.045 PMid:30412708 48. Sun JY, Sha YQ, Sun QY, Qiu Y, Shao B, Ni YH, et al. The long-term therapeutic effects of His-Purkinje system pacing on bradycardia and cardiac conduction dysfunction compared with right ventricular pacing: A systematic review and meta-analysis. J Cardiovasc Electrophysiol 2020; 31: 1202-10. doi: 10.1111/jce.14445. PubMed PMID: 32162743. https://doi.org/10.1111/jce.14445 PMid:32162743 49. Qi J, Jia X, Wang Z. His bundle pacing for cardiac resynchronization therapy: a systematic literature review and meta-analysis. J Interv Card Electrophysiol 2020; 59: 463-70. doi: 10.1007/s10840-020-00827-6. PubMed PMID: 32748157. https://doi.org/10.1007/s10840-020-00827-6 PMid:32748157 50. Fernandes GC, Knijnik L, Lopez J, Rivera M, Fernandes A, Lambrakos LK, et al. Network meta-analysis of His bundle, biventricular, or right ventricular pacing as a primary strategy for advanced atrioventricular conduction disease with normal or mildly reduced ejection fraction. J Cardiovasc Electrophysiol 2020; 31: 1482-92. doi: 10.1111/jce.14490. PubMed PMID: 32275339. https://doi.org/10.1111/jce.14490 PMid:32275339 51. Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. Circulation 2019; 140: e333-e81. doi: 10.1161/CIR.0000000000000627. PubMed PMID: 30586771. https://doi.org/10.1161/CIR.0000000000000627 52. Vijayaraman P, Dandamudi G, Zanon F, Sharma PS, Tung R, Huang W, et al. Permanent His bundle pacing: Recommendations from a Multicenter His Bundle Pacing Collaborative Working Group for standardization of definitions, implant measurements, and follow-up. Heart Rhythm 2018; 15: 460-8. doi: 10.1016/j.hrthm.2017.10.039. PubMed PMID: 29107697. https://doi.org/10.1016/j.hrthm.2017.10.039 PMid:29107697

Lake Higgins in Northern Michigan, USA. National Geographic rated it as the sixth most beautiful lake in the world.
It is a glacier formed lake, and is spring fed, and is incredibly clear with an underwater visibility of 42 feet. It has an average depth of 35 feet and is 135 feet deep at most. It freezes sufficiently in winter that one can walk and snowmobile and ice fish on it. Dan Hermes, Michigan, USA.
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.Archive of Issues
AUTHOR'S CORNER
 Authors having problems with submissions please notify editor: editor@hvt-journal.com
Authors having problems with submissions please notify editor: editor@hvt-journal.com
