Sudden cardiac arrest after late permanent pacemaker implantation in a heart transplant patient
CASE REPORT
Sudden cardiac arrest after late permanent pacemaker implantation in a heart transplant patient
Article Summary
- DOI: 10.24969/hvt.2021.259
- Page(s): 131-134
- CARDIOVASCULAR DISEASES
- Published: 05/07/2021
- Received: 23/06/2021
- Accepted: 05/07/2021
- Views: 7958
- Downloads: 5887
- Keywords: heart transplantation, permanent pacemaker, rejection
Address for Correspondence: Yavuzer Koza, Department of Cardiology, Faculty of Medicine, Ataturk University
Yakutiye, Erzurum, Turkey Phone:+904422318521 Fax:+904422361301 E-mail: yavuzerkoza@hotmail.com
Department of Cardiology, Faculty of Medicine, Ataturk University, Erzurum, Turkey
Abstract
Bradycardia during the early period following heart transplantation frequently occurs with an incidence of 14 to 44% and it is usually self-limited. The incidence of late bradycardia (from 30 days to more than 5 or 6 months after transplantation) has been reported to be 1.5%. A 33-year-old male patient with a history of orthotopic heart transplantation in 2013 presented with complaints of dizziness and near syncope. A DDDR permanent pacemaker was implanted for sinus pauses exceeding 3 seconds recorded on Holter examination. Shortly after the procedure, he developed sudden cardiovascular collapse. Cardiopulmonary resuscitation was performed and a pulse steroid treatment (2 grams of methylprednisolone) was given. After 2 days, the patient was extubated. While making preparations for re-transplantation, cardiopulmonary arrest developed again and he died. Sinus pause may be a clue for rejection and is an important finding in predicting clinical course.
Key words: heart transplantation, permanent pacemaker, rejection
Introduction
Heart transplantation remains a good option for selected patients with heart failure refractory to medical management. Bradycardia in the immediate post-transplantation period is a well-known and self-limited entity (1). However, after the first 2 weeks from the transplantation, bradyarrhythmia episodes should be promptly evaluated for causes such as sinus node dysfunction, ischemia, rejection, allograft vasculopathy, or drug effects (2).
Here, we report a case of late sinus arrest following heart transplantation that underwent a permanent pacemaker implantation and developed cardiovascular collapse shortly after the procedure.
Case report
A 33-year-old male patient who underwent a DDDR permanent pacemaker implantation in 2009 due to complete heart block and an orthotopic heart transplantation using the bicaval anastomosis technique due to dilated cardiomyopathy in 2013 was admitted with complaints of dizziness and near syncope. The patient underwent cardiac biopsy for same complaints after two years from transplantation and no evidence of rejection was detected. His medications included mycophenolate mofetil, cyclosporine and ursodeoxycholic acid. Serum concentrations of anti-rejection drugs were optimal. Thyroid function tests and serum potassium level were within normal limits. The admission electrocardiogram (ECG) revealed a sinus rhythm with complete right bundle branch block and left anterior hemiblock (Fig. 1A). A transthoracic echocardiography revealed a left ventricular ejection fraction (LVEF) of 58% and there was no diastolic dysfunction. A Holter examination revealed sinus pauses exceeding 3 seconds when he was awake (Fig. 1B) with junctional rhythm episodes and therefore, a DDDR permanent pacemaker was implanted to the patient (Fig. 2A). Post implantation pacemaker checking revealed predominantly atrial and ventricular pacing. Shortly after the procedure, the patient developed sudden cardiopulmonary arrest. An ECG showed a junctional rhythm when pacemaker was turned off (Fig. 2B). There were no any tachyarrhythmias or other ECG changes (e.g., ST elevation/depression, QRS widening ).
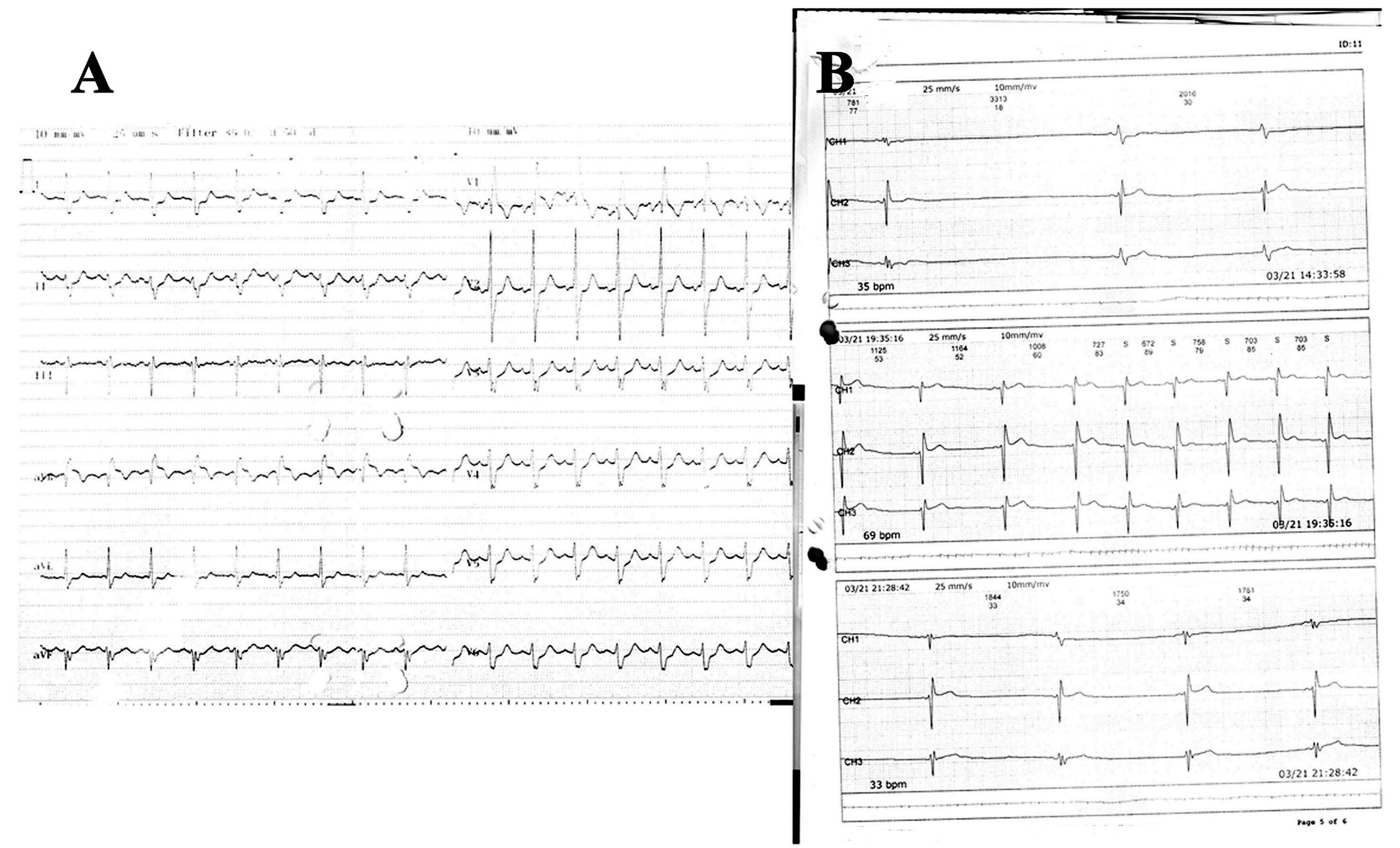
Figure 1. A) The admission electrocardiogram B) A Holter recording showing the sinus pause and junctional rhythm tracings.
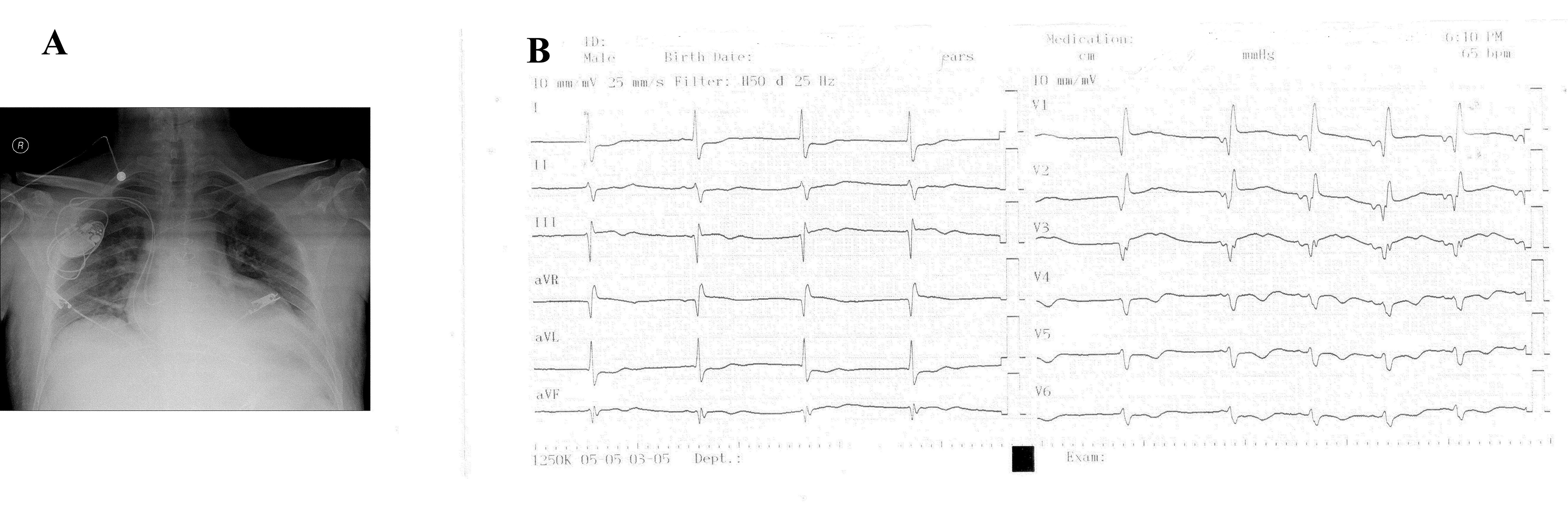
Figure 2. A) Chest radiography after pacemaker implantation B) The electrocardiogram showing a junctional rhythm when pacemaker was turned off.
Echocardiographic examination did not show any pericardial effusion and no pneumothorax was seen on chest radiography (Fig. 2A). Cardiopulmonary resuscitation was performed for 15 minutes and the echocardiogram revealed a LVEF of 30% with severe global hypokinesia of the left ventricle. Considering the fact that the pacemaker leads could aggravate an existing rejection, a pulse steroid treatment (2 grams of methylprednisolone) was given. The patient was followed by intubation for 2 days and then extubated. An optimal medical therapy for heart failure was also started. While making preparations for retransplantation, cardiopulmonary arrest developed again. Despite maximal medical and mechanical support, the patient passed away.
Discussion
This is the first case report claiming that pacemaker placement can aggravate the existing rejection following heart transplantation. Bradyarrhythmias in the immediate post-transplantation period following heart transplantation occur in up to half of patients, with incidence varying widely in different case series (2,3 ). Late bradycardia has been defined from 30 days to more than 5 or 6 months after transplantation and occurs in approximately 1.5% of patients (4). Because the increased use of bicaval anastomoses rather than atrio-atrial anastomoses is associated with a lower incidence of sinus node dysfunction, the requirement for cardiac pacing in the adult population has decreased significantly (5).
A previous study reported that six of 18 heart transplant patients underwent pacemaker implantation (three with sinus node dysfunction and three with AV block) and only two of them became pacemaker-dependent during follow-up. According to this study, the mechanism for late onset bradyarrhythmias is obscure, but 30 % of cases occurred in patients with allograft vasculopathy, while acute rejection was rarely seen (5%) (6).
In the present case, routine endomyocardial biopsy at initial presentation and postmortem examination including the conducting system could not be performed. On the other hand, rejection may occur in a patchy regional fashion. Since our patient had a history of heart block prior to transplantation, a systemic disease, involving the conduction system could be the reason for bradycardia but all investigations of such a disease were unremarkable. Unfortunately, there was no any information about the donor heart.
Knight et al. (7) suggested a management strategy for post-transplant patients with unexplained syncope for rejection, including steroid boluses and plasmapheresis, followed by photopheresis. Those authors also proposed that these patients should be treated likewise to that for hemodynamically significant rejection even if not indicated by standard right ventricular biopsy.
Denervation of the transplanted heart and potential trauma and ischemia of the sinoatrial node during the operation are the probable causes of the bradyarrhythmias in the first 6 months after transplantation. Indeed, functionally significant cardiac reinnervation of the donor sinus node does not occur in the majority of patients within the first 5 years after transplantation (6, 7).
Allograft vasculopathy also known as transplant coronary artery disease is another possible cause of bradyarrhythmia, which is the leading cause of death after the first post-transplant year and is present in up to 50% 5 years after transplantation (8). Late pacemaker requirement after heart transplantation may also predict the presence of transplant coronary artery disease. Because conventional coronary angiography shows only luminal changes and the arterial wall, it may be difficult to detect allograft vasculopathy, which is often diffuse and concentric by nature (9). As coronary allograft vasculopathy is resistant to traditional treatment modalities for coronary artery disease, we did not performed coronary angiography.
Although current guidelines recommend permanent pacing for heart transplant patients with persistent, inappropriate or symptomatic bradycardia, the optimal time for pacemaker implantation and prophylactic pacemaker implantation for bradycardia during the rejection period is not well-defined (10). In patients with late onset symptomatic bradycardia, rejection and transplant vasculopathy should be excluded. Previous studies reported that prophylactic permanent pacemaker implantation may prevent bradycardic sudden death and improve survival in heart transplant patients with coronary disease (3-6), however, as in the present case pacemaker implantation can aggravate existing rejection as an inflammatory response to a component of the leads (11). In allograft vasculopathy or rejection, retransplantation is the only definitive treatment.
Conclusion
Although a potential association of bradycardia with increased likelihood of rejection or graft vasculopathy is controversial, rejection involving the conducting system is a probable cause for bradycardia, which may be missed by endomyocardial biopsy.
Although rejection is generally more frequent and severe early after transplantation, it must be considered as a possible cause of bradyarrhythmias at any time after transplantation. Before implanting a permanent pacemaker these patients should be aggressively treated for rejection even though rejection could not be showed histopathologically.
Ethics: Informed consent was obtained from patient before all procedures.
Peer-review: Internal and external
Conflict of interest: None to declare
Authorship: Y.K., O.B., S.S.A., F.O., H.T. equally contributed to study and preparation of article
Acknowledgement and funding: None to declare
References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

Golden Bridge across Horn Bay and next is the Russky Bridge to Russky Ireland, Vladivostok, Russia, Pacific Ocean 2021. Alexander Lyakhov, Vladivostok, Russia.
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

