Anatomy of the retrograde type A aortic dissection after endovascular repair of type B aortic dissection: a case report
CASE REPORT
Anatomy of the retrograde type A aortic dissection after endovascular repair of type B aortic dissection: a case report
Article Summary
- DOI: 10.24969/hvt.2021.262
- Page(s): 135-143
- CARDIOVASCULAR DISEASES
- Published: 04/08/2021
- Received: 23/06/2021
- Revised: 01/08/2021
- Accepted: 01/08/2021
- Views: 15669
- Downloads: 6042
- Keywords: Type A aortic dissection, Type B aortic dissection, thoracic endovascular aortic repair, supracoronary aortic replacement, aortic anatom
Address for Correspondence: Uliana Pidvalna, Department of Normal Anatomy, Danylo Halytsky Lviv National Medical University, 79010, Lviv, Pekarska Str., 69, Ukraine
Email: uljaska.p@gmail.com Phone: +380963551782
1Danylo Halytsky Lviv National Medical University, Lviv, Ukraine
2Ukrainian-Polish Heart Center “Lviv”, Lviv, Ukraine
3Lviv Regional Clinical Hospital, Lviv, Ukraine
Abstract
Aortic dissection requires immediate medical attention. The optimal treatment approach of Type B aortic dissection is still a matter of debate. Possible options include open surgery, endovascular aortic repair (EVAR), and hybrid procedure. The indication for surgery is the dissection that involves the ascending aorta and the aortic arch (Type A Stanford, Types I and II DeBakey). Hybrid or endovascular procedures seem to be an attractive alternative treatment for patients with the complex aortic disease and a high risk of surgery. Endovascular treatment of acute Type B aortic dissections is designed to prevent the retrograde dissection of the ascending aorta and the aortic arch. The occurrence of retrograde Type A aortic dissection (RTAD) is rare, but the mortality rate is high.
We report a case of a 55-year-old gentleman who had undergone thoracic EVAR. Thirty months after the given procedure he presented with RTAD and underwent supracoronary aortic replacement.
The article is intended to remind the clinicians of the importance of early detection of the possible complications when performing EVAR, and the significance of a rapid response.
Key words: Type A aortic dissection, Type B aortic dissection, thoracic endovascular aortic repair, supracoronary aortic replacement, aortic anatomy
Introduction
Acute aortic dissection is the most life-threatening condition which occurs in 30% of all pathological processes affecting the thoracic aorta (1). Without undergoing surgery 33% of patients in this category die within the first 24 hours, and another half of patients pass away within 48 hours (2).
Aortic dissection requires immediate medical attention. Traditional cardiac surgery, or open-heart surgery, is now partially replaced by endovascular techniques. As of today, the possible options include open surgery, endovascular aortic repair (EVAR), and hybrid procedure (combinations of the previous two). Each technique has its indications, advantages, disadvantages, and potential complications. Therefore, the optimal approach to the aortic dissection treatment is still a matter of debate (3).
The indication for surgery is the dissection that involves the ascending aorta and the aortic arch (Type A Stanford, Types I and II DeBakey). Hybrid or endovascular procedures seem to be an attractive alternative treatment for patients with a complex aortic disease and high risk of the conventional arch or thoracoabdominal surgery. Indications for the endovascular stent-grafting on a Type B dissection are “TEAR'EM”, where T stands for TEVAR expertise, E – effusion-left pleural, A – aneurysmal dilatation, R – refractory pain, E – extension proximally, and M – malperfusion syndrome.
During thoracic endovascular aortic repair (TEVAR) the endoprosthesis (a stent-graft) has to be adequately sealed at the point of the primary tear (4). It must prevent the postimplantation aortic dissection. Unfortunately, the retrograde Type A aortic dissection (RTAD) might still occur after TEVAR. Despite the fact that it is infrequent, the mortality rate remains high (5).
Retrograde progression of the dissection to the arch and to the ascending aorta requires an urgent surgical operation. Stanford Type A, De Bakey Type I or II remain the most lethal; they are the indications for a prompt surgical intervention (6).
We present the case of a 55-year-old gentleman with the acute aortic syndrome, who was diagnosed with the retrograde Type A aortic dissection 30 months after he had undergone TEVAR.
Case report
A 55-year-old gentleman presented with the acute aortic syndrome and a history of the distal aortic arch and the descending thoracic aortic dissection. The patient had been treated at another hospital (abroad), where he underwent TEVAR. Thoracic descending stent-graft by Cook device (30х380 mm) was implanted. Endoprosthesis was placed in zone 1, extending along the aortic arch and the descending thoracic aorta to the Th9 level. During TEVAR an orifice of the left subclavian artery was closed without acute ischemia of the left upper extremity. No complications were observed in the postoperative period.
Thirty months later, the patient sought medical help suffering from acute chest pain, hypotension and numbness in the right hand. The patient urgently underwent computed tomography (CT) with contrast.
Multidetector CT study protocol
Patient was scanned with a 64-CT scanner (GE Light Speed VCT, GE Healthcare). Angiographic scan parameters included the following: number of slices per rotation – 64; individual detector slice width of 0.625 mm; and 24.21 mm spatial coverage in 1 seconds at a gantry rotation speed of 0.8 seconds. After the patient was advanced into the scanner bore, the first scanning consisted of a localizer image of the thoracic and abdominal cavity and pelvis. The second scan phase was a non-contrast scan performed with the scanning parameters including gantry rotation time 0.8 seconds, tube voltage 140 kVp, tube current 250 mA, and collimation 64 × 0.625 mm. The third scan phase was the contrast-enhanced angiogram. The patient was asked to breathe deeply and then hold his breath at end-inspiration. Ultravist 370 (Bayer Healthcare, Germany) was administered according to the following protocol: 110 cc at a rate of 5 cc/second. The imaging parameters for this scan were: rotation time 0.8 seconds, tube voltage 140 kVp, tube current 250 mA, and collimation 64 × 0.625 mm. After image acquisition, images were transferred to a GE® AW workstation for analysis.
CT angiography results
In the analysis of a series of obtained tomograms of the aorta in unenhanced and arterial phases, axial, coronal and sagittal sections, we used multiplanar and volumetric reformations.
The thoracic aortic dissection from the level of the right coronary artery was visualized with spread to the brachiocephalic artery (Fig. 1, Fig. 2), as well as thrombosis of the proximal segment of the left subclavian artery and functioning of the false lumen at the level of endoprosthesis and distally to the abdominal aortic bifurcation with the partial thrombosis of the false lumen (Fig.3).
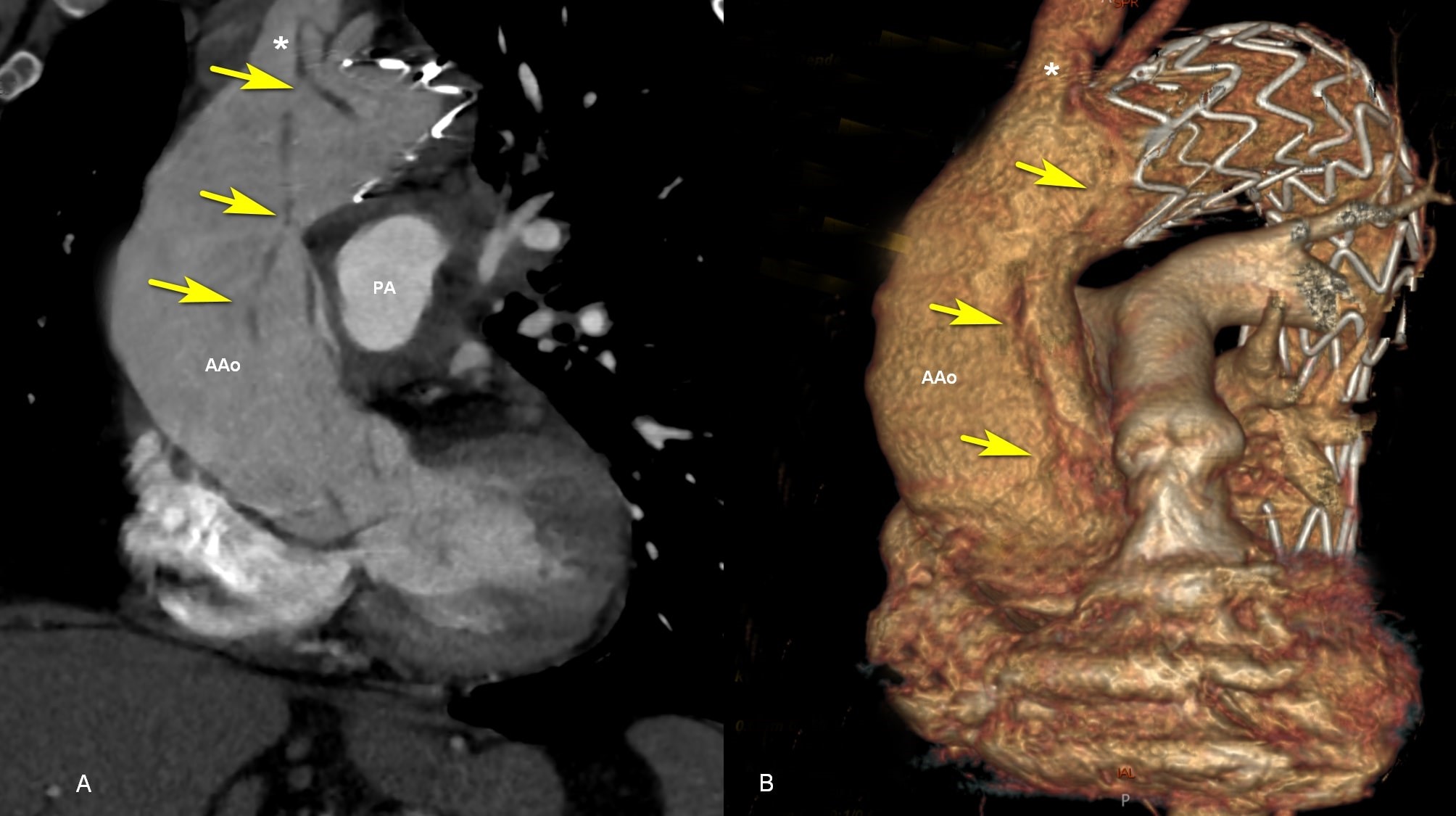
Figure 1. Contrast enhanced CT: A, An oblique coronal image demonstrates type A aortic dissection (yellow arrow) moving to the brachiocephalic artery. B, Volumetric rendering (a 3D reformatted image) shows the endoprosthesis in the arch and descending thoracic aorta, distal to brachiocephalic artery (*). AAo - ascending aorta; CT – computed toography, PA - pulmonary artery.
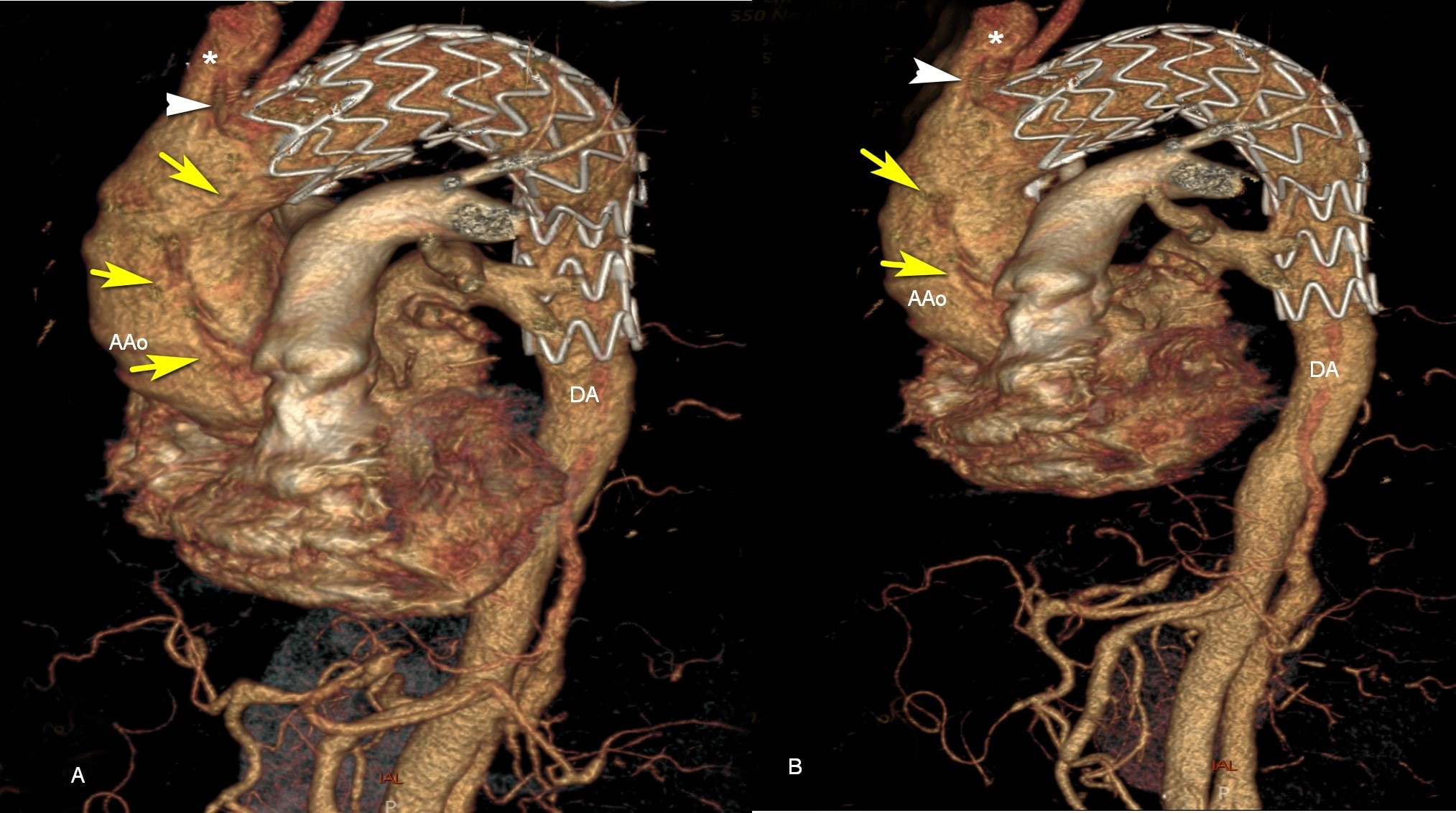
Figure 2. A, B, 3D reconstructions of contrast-enhanced CT scan. The endoprosthesis in the distal arch and descending thoracic aorta, distal to brachiocephalic artery (*). Type A aortic dissection (yellow arrow) moving to the brachiocephalic artery (white triangle). AAo - ascending aorta; DA - descending thoracic aorta
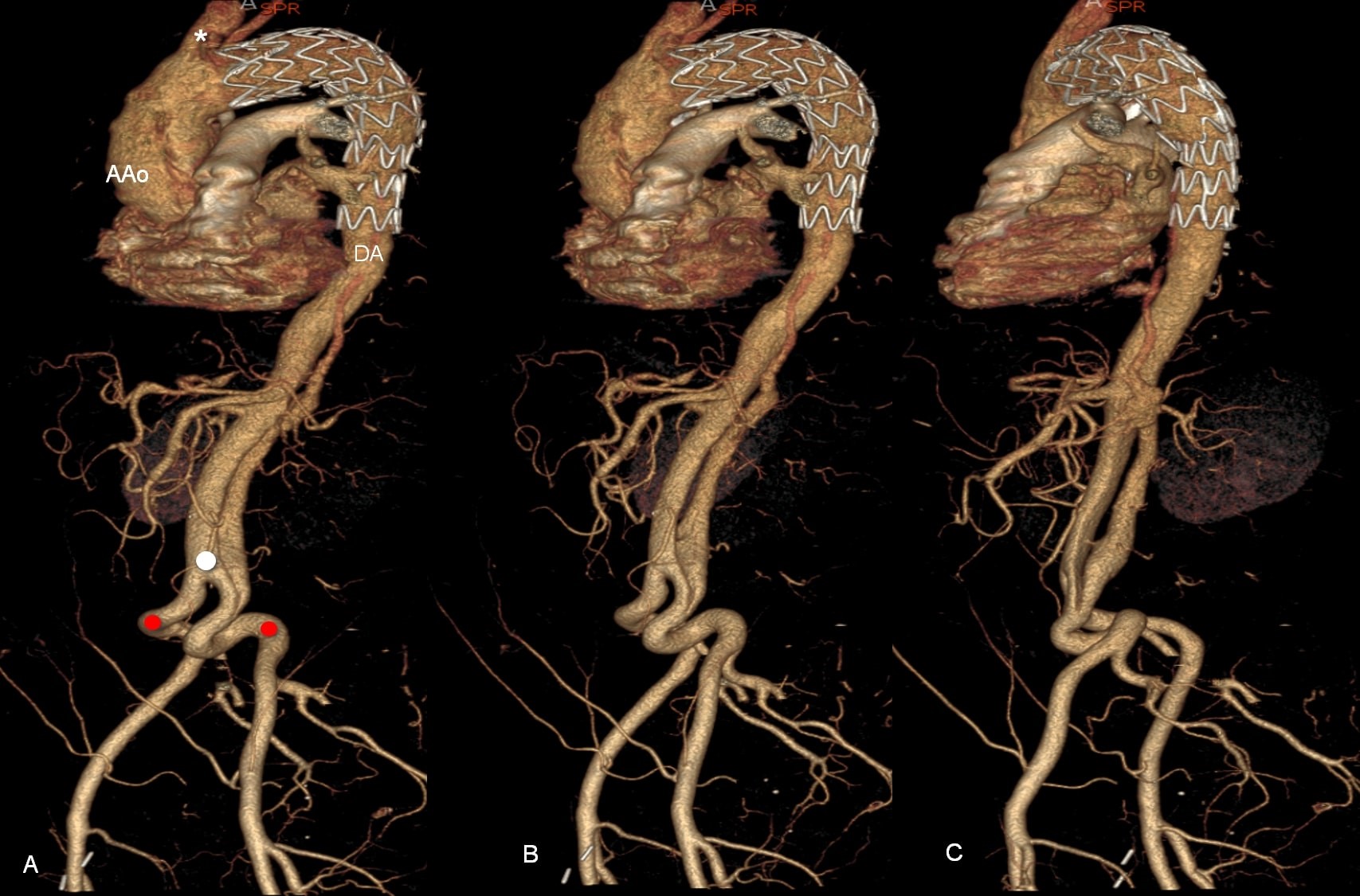
Figure 3. A, B, C, 3D reconstructions of contrast-enhanced computed tomography scan. The endoprosthesis in the distal arch and descending thoracic aorta (DA). Retrograde progression of the dissection to the arch and to the ascending aorta (AAo), dissection of the abdominal aorta. Left common iliac artery diameter with the dissection of the proximal segment. Kinking of the common iliac arteries (red circle), aortic bifurcation (white circle)
Coronary arteries were patented with good filling and appeared to be intact (Fig. 4). The aortic dissection extended to the abdominal aortic bifurcation with the transition to the proximal segment of the left common iliac artery (Fig. 5).Fluid in the pericardial cavity was present in small quantities: up to 9-10 mm with inhomogeneous density ≈ + 20- + 40 HU.
Thoracic aorta diameter: at the level of the aortic annulus ≈ 21х20 mm; at the level of the sinuses of Valsalva – 36х37х39 mm; at the supracoronary level – 51х41 mm; at the level of the sinotubular junction ≈ 57х56 mm; at level of the mid ascending aorta ≈ 53х51 mm; at level of the proximal arch (to endoprosthesis) – 46 mm; brachiocephalic artery diameter ≈ 16 mm, dissected; left common carotid artery diameter ≈ 9 mm, with the thrombosed proximal segment; left subclavian artery diameter ≈ 10 mm, free of dissection or thrombosis; distal from the left subclavian artery (at the level of endoprosthesis) – 40 mm; at the level of the mid descending aorta and the level of the pulmonary artery (at the level of endoprosthesis) – 30-37 mm, with a semilunar hypodense lesions on the left lateral contour (≈ 6-7 mm thick).
The mean aortic diameter at the level of the diaphragm – 40х30 mm, true lumen diameter – 28х20 mm, false lumen diameter ≈ 8 mm (functional) (Fig. 6).

Figure 4. Coronal oblique contrast enhanced computed tomography image shows dissection (yellow arrow) distal to the right coronary artery (*)
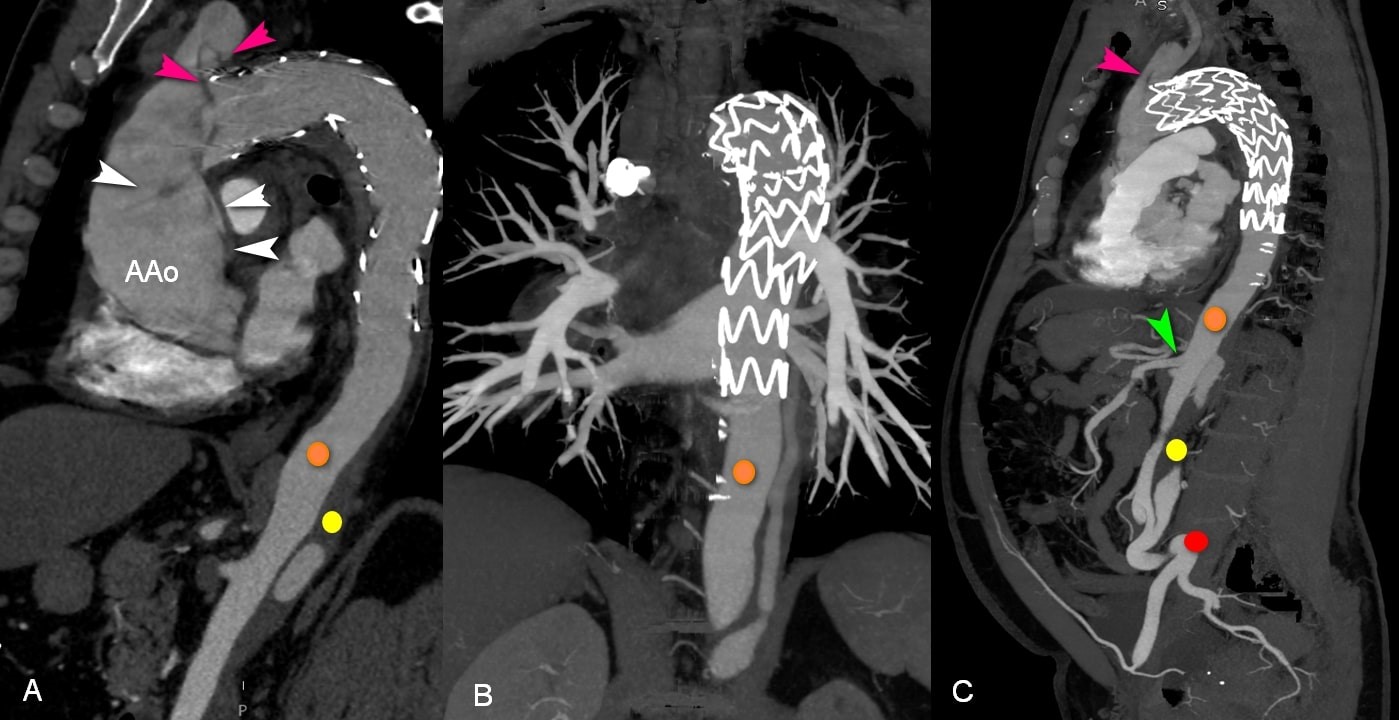
Figure 5. A, C, Sagittal and B, coronal contrast-enhanced CT scans confirming RTAD (white triangle) to the ascending aorta (AAo) and brachiocephalic artery (pink triangle). Formation of true (orange circle) and false lumen (yellow circle) in the thoracic aorta. Kinking of the common iliac arteries (red circle). Superior mesenteric artery (green triangle) arising from the true lumen. CT – computed tomography, RTAD – retrograde thoracic aorta dissection
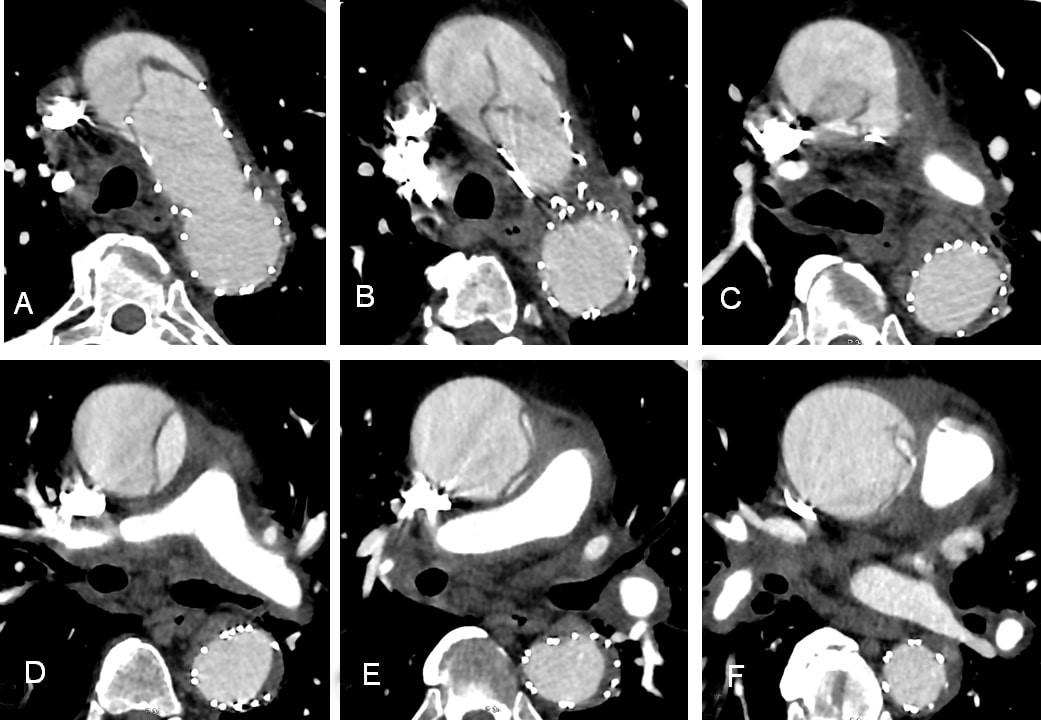
Figure 6. A-F, Contrast-enhanced CT scans after stent-graft replacement with aortic dissection. Axial cuts through ascending, arch and descending thoracic aorta. CT – computed tomography
Abdominal aorta diameter: at the level of the celiac trunk – 36х27 mm, true lumen diameter – 23х18 mm, false lumen diameter ≈ 10х18 mm (functional); at the level of the superior mesenteric artery – 41х27 mm, true lumen diameter – 23х17 mm, false lumen diameter ≈ 11х17 mm (functional); the right renal artery arising on the level of the superior mesenteric artery, supplied from the true lumen; at the level of the left renal artery origin – 34х27 mm, true lumen diameter – 18х26 mm; false lumen diameter ≈ 21х14 mm (functional); at the level of inferior mesenteric artery origin – 34х28 mm, true lumen diameter – 11х21 mm, false lumen diameter ≈ 12х14 mm (functional); abdominal aorta diameter just superior to the aortic bifurcation – 34х31 mm, true lumen diameter – 11х23 mm, false lumen diameter ≈ 21х19 mm (functional); the right common iliac artery diameter – 15 mm; left common iliac artery diameter – 16 mm (with the dissection of the proximal segment). Kinking of the common iliac arteries (Fig. 7).
The celiac trunk, the superior and inferior mesenteric arteries, the right renal artery and the right common iliac artery arisen from the true lumen. The left gastric artery, the left renal artery arisen from the false lumen.
Patient perspective
The anatomy of the aortic dissection is an indication for an urgent cardiac surgery - supracoronary aortic replacement (SCAR) using full root technique for ascending aortic dissection with cardiac tamponade. SCAR was performed through median sternotomy access. Pericardiotomy. Under high pressure lysed blood appears, blood clots were removed from the pericardial cavity. The ascending aortic dissection was visualized. Heparinization.
An end-to-side anastomosis was sewn between the left common carotid artery and the vascular graft prosthesis Vascutec 8 mm (Prolene 5-0). Cardiopulmonary bypass (CPB) was instituted by the left common carotid artery and the right atrial cannulation; cooling down the patient. After placing the aortic cross-clamp (ACC), the ascending aorta was opened. First antegrade blood cardioplegia was into coronary arteries ostia, retrograde blood cardioplegia later. The aortic valve was tricuspid without valve pathology.
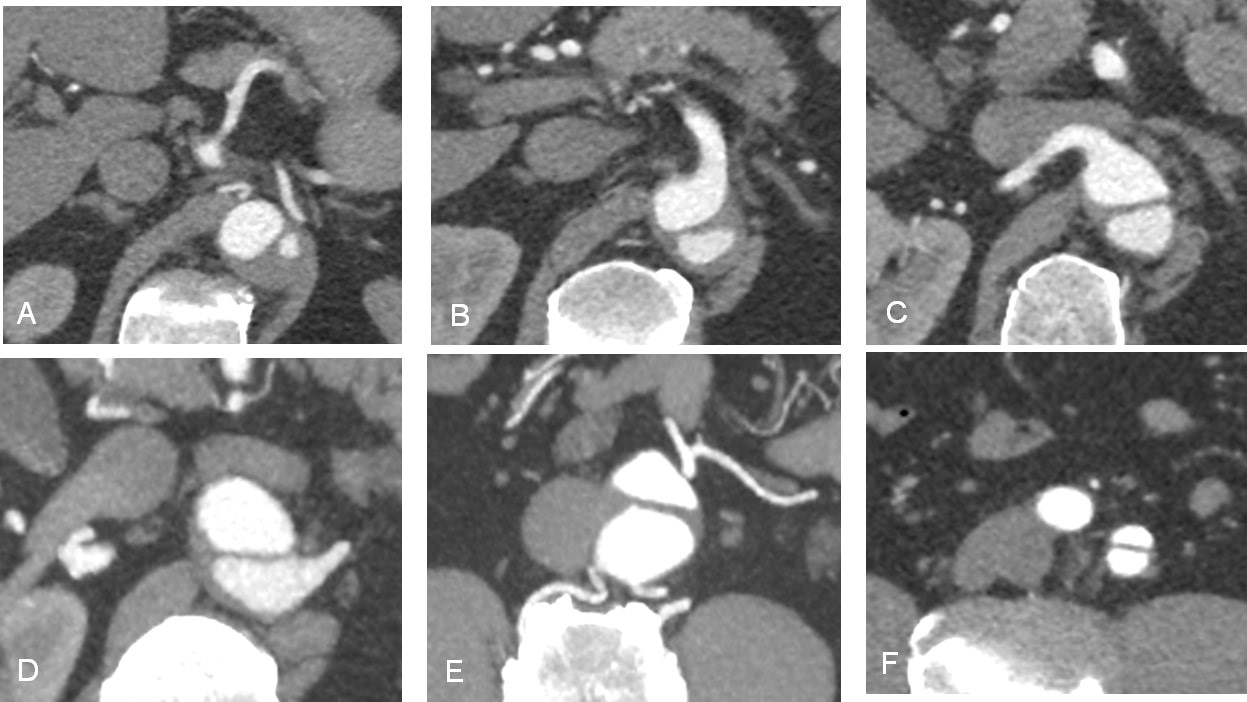
Figure 7. Contrast-enhanced CT: Continuous series of short-axis views of the abdominal aorta showing the dissection on the different levels. A, At the level of celiac trunk with partial thrombosis of the false lumen. B, At the level of the superior mesenteric artery. C, At the level of the right renal artery. D, At the level of the left renal artery. E, At the level of inferior mesenteric artery. F, At the level of the left common iliac artery, with the dissection of the proximal segment.
CT – computed tomography
The aortic valve resuspension was performed. The ascending aorta was cut 1.0 cm above the coronary ostia and the aortic valve commissures. A proximal anastomosis was placed between the ascending aorta and the vascular graft prosthesis Intergard 28 mm (Maquet, Rastatt, Germany), using the telescopic suture (Prolen 5-0). The suture line was additionally reinforced with U-shaped polypropylene sutures (Prolen 4-0) with Teflon pledgets around the perimeter. In parallel, the patient was cooled down to 18C° and the CPB changed to selective antegrade cerebral perfusion (unilateral ACP) through the left common carotid artery. The distal ascending aorta was cut just below the brachiocephalic artery. Endograft, placed 30 month ago, was visualized. It was established well, no signs of endoleak were detected, the ostium of the left subclavian artery was not visualized. Additionally, the brachiocephalic artery ostia was cannulated and switched to bilateral ACP mode. "Open" distal anastomosis was performed between the distal ascending aorta and the vascular prosthesis with a continuous double-row suture (Prolene 4-0); on the inside – additional U-shaped sutures with pledgets. Rewarming. Restoring the cardiac activity. Standard finishing procedures.
The patient successfully underwent SCAR and was discharged from the hospital without any major complications.
Discussion
There are several classifications of the aortic dissection, namely the Stanford, DeBakey, Svensson, and Penn classifications (7). The basis for the new classifications was laid by a significant difference in the effectiveness of surgical treatment of the ascending and descending aortas. In the description of this case we used Stanford (Type A, Type B) and DeBakey (Type I, Type II, Type IIIA, and IIIB) classifications.
According to Stanford classification, the aortic dissection is divided into two types: Type A – the dissection begins in the ascending aorta and ends distally anywhere in the aorta, and Type B – the dissection begins below the discharge of the left subclavian artery, it spreads and ends distally (8).
According to the classification of M. DeBakey, the following types of aortic dissection are distinguished: Type I – the primary rupture of the intima is in the ascending aorta and the dissection extends distally to the descending thoracic or abdominal aorta; Type II – the primary rupture of the intima is in the ascending aorta and the dissection ends before the discharge of the shoulder-head trunk; Type III dissection is divided into two subtypes: Type IIIA – the primary rupture of the intima is below the mouth of the left subclavian artery, and the dissection is limited to the descending thoracic aorta; Type IIIB – the primary rupture of the intima occurs below the level of discharge of the left subclavian artery, the dissection extends to the abdominal aorta (9).
Based on these classifications, a tactical division is established: Type A is an absolute surgical emergency, Type B is mainly subject to conservative therapy in the form of controlled hypotension. Exceptions to the use of surgical treatment for Type B are, in particular, cases of rupture of the descending thoracic aorta with the development of haemothorax (10).
Innovative methods in cardiology and cardiac surgery are changing the approach to the treatment of aneurysms and aortic dissections (11). While Type A dissections are still subject to open surgery only, Type B dissections are possible to be treated with the help of endovascular (stent-graft) or hybrid procedures.
The first operation of the aortic endoprosthesis (endovascular aneurysm repair, EVAR) in the world was performed by Ukrainian professor Mykola Volodos (15.05.1934 – 3.04.2016) in 1987 (12). Wider use of EVAR makes it possible to adequately place prosthesis into the segment of the aorta which was affected by the aneurysm or dissection, as well as avoid complex traumatic open operations that require thoraco-phreno-lumbotomy.
Indications for the endovascular stent-grafting on Type B dissections are “TEAR'EM”, where T stands for TEVAR expertise, E – effusion-left pleural, A – aneurysmal dilatation, R – refractory pain, E – extension proximally, and M – malperfusion syndrome. After opening, mesh endoprosthesis strengthens the aortic wall and prevents its rupture (13). Endovascular treatment of acute Type B aortic dissections is designed to prevent the retrograde dissection of the ascending aorta and the aortic arch (5). Unfortunately, postimplantation Type A aortic dissection is one of the possible complications. Although delayed rupture is uncommon, the mortality rate is high (4).
The retrograde Type A aortic dissection (RTAD) is an indication for surgery. The anatomy of the dissection is taken into account while choosing the surgical technique. If the dissection is limited to the ascending aorta or the arch without damaging the aortic valve, supracoronary aortic replacement (SCAR) with or without resuspension of the aortic valve is performed; combining the ascending aortic and hemiarch replacement. When the aortic valve is involved, Bentall procedure should be performed; David I – V operations (14), the aortic root remodelling (Yacoub Procedure, Urban operation) (15), the neocuspidation of the aortic valve (Ozaki operation) (16). It is important to ensure the anatomically correct geometry of the ascending aorta and the aortic valve.
CT angiography (CTA) allows you to choose the optimal technique to plan a surgery. It is important for the surgeons to receive information from the radiologist about the initial intimal tear, sizes of the true and false lumens, presence of thrombus in the false lumen, length of the dissection, presence of compression of the true lumen, compression of the orifice of the aortic branches, and dynamic or organic malperfusion syndrome (17). In complicated cases, for instance, after stent-graft implantation, re-entry in the thoracic and abdominal parts of the aorta, lending zones should be assessed.
In the case described, CTA showed the condition after endoprosthesis of the aortic arch and the descending thoracic aorta. Stent-graft was performed after the Stanford type B aortic dissection. CT scan was typical of the retrograde dissection of the ascending thoracic aorta to the level of the right coronary artery with the brachiocephalic artery dissection (Stanford Type A, DeBakey Type I), with the functioning of two lumens and partial thrombosis of the false lumen from the level of the endoprosthesis to the aortic bifurcation and the spread of dissection to the proximal segment of the left common iliac artery; signs of aneurysm of the ascending thoracic aorta, hydropericardium with haemorrhagic component; kinking of both common iliac arteries.
SCAR is the surgical technique chosen as the most appropriate. The supracoronary aortic prosthesis with the “full root” technique is a complete cross-section of the aortic diameter, removal of the dissected area of the ascending aorta and placement of the prosthesis of the ascending aorta above the coronary arteries (approximately 1.5-2 cm), as high as possible to the place of discharge of the brachiocephalic artery with inserting circular (360°) anastomoses, without preserving the posterior wall of the ascending aorta, using the principles of the “free tension technique” (18).
The supracoronary prosthesis of the ascending aorta is performed when there are no additional dissections of the intima in the projection of the aortic root (the sinuses of Valsalva, coronary arteries ostia), with no hemodynamically significant pathologies of the aortic valve, in patients without annulo-aortic ectasia (19) and without additional ruptures of the aortic intima in the zone of the aortic arch.
The patient initially had no indications for the hybrid treatment. Type B aortic dissection 30 months before the given event had involved the isolated implementation of the endovascular restoration of perfusion of the aortic branches by stenting. Acute onset of Type A aortic dissection caused the second stage – the classic correction of the ascending aorta and the aortic arch.
Etiology of the retrograde aortic dissection remains unknown (20). The reasons may include unfavorable anatomy, mismatch of the size of the stent-graft, technical difficulties in the implantation of endoprosthesis, etc. Imaging follow-up is important (21). Neurological deficit in the patient, uncontrolled hypertension, progressive atherosclerosis require adequate assessment of the anatomy of the stent-graft.
Conclusion
Aortic dissection is a disease with a high mortality rate; it remains one of the most difficult pathologies to treat. With the advent of TEVAR, the concept of hybrid treatment of the acute aortic dissection has emerged. The latter involves the primary endovascular restoration of perfusion of the aortic branches by fenestration or stenting, followed by the second stage of the delayed classical correction on the ascending and / or the aortic arch. Type B aortic dissection treated with TEVAR may lead to complication (RTAD); it requires an urgent open surgical approach with the use of cardiopulmonary bypass. CTA allows you to choose the optimal technique to plan both hybrid procedure and proper preoperative assessment.
The case-report provides the description of the RTAD, which required urgent SCAR after TEVAR 30 months ago. Medical staff should pay attention to the patient’s anamnesis to make a right clinical decision. Often an acute chest pain is associated with heart attack with very different diagnostic and clinical approach. It is an important fact that pathology could be verified owing to timely and modern aortic imaging diagnostic. It allows not only predicting surgical treatment, but also it is the method of detailed quality control of the completed procedure.
Ethics: Informed consent was obtained from a patient
Peer-review: Internal and external
Conflict of interest: Nothing to declare
Authorship: U.P., M.M., A.V., D.B. are equally contributed to management of case and preparation of article
Acknowledgement and funding: The study was conducted and funded within the scientific research program of the Department of Normal Anatomy at Danylo Halytsky Lviv National Medical University (Lviv, Ukraine) according to the research topic «Morphofunctional features of organs in pre- and post-natal ontogenetic periods under the influence of opioids, food additives, reconstructive surgeries and obesity»
References
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

Fort Meyers Beach, Estero Ireland, Florida, USA. Raymond Singer, Pennsylvania, PA, USA.
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

