Under pressure: The first valve-in-valve transcatheter aortic valve implantation for stenosis of a BioValsalva prosthesis using a Sapien 3 Ultra balloon expandable valve in a patient with complex congenital heart disease
CASE REPORT
Under pressure: The first valve-in-valve transcatheter aortic valve implantation for stenosis of a BioValsalva prosthesis using a Sapien 3 Ultra balloon expandable valve in a patient with complex congenital heart disease
Article Summary
- DOI: 10.24969/hvt.2022.330
- Page(s): 142-147
- Cardiac Surgery
- Published: 03/07/2022
- Received: 25/05/2022
- Revised: 02/07/2022
- Accepted: 03/07/2022
- Views: 5404
- Downloads: 4634
- Keywords: aortic stenosis, TAVI, valve-in-valve transcatheter aortic valve implantation, adult congenital heart disease
Address for Correspondence: Clare Rayner, 19 Baker Street, New Town, Tasmania, 7008, Australia. phone: +61 0400 142 791, fax:+61 036173
Email: clare.rayner@ths.tas.gov.au
Clare Rayner1, Amanda Sherwen2, Umair Hayat3, Heath Adams1
1Royal Hobart Hospital, Tasmania, Australia
2St Vincent’s Hospital, Victoria, Australia
3Royal Hobart Hospital and Launceston General Hospital, Tasmania, Australia
Abstract
Advances in the diagnosis and management of congenital heart disease (CHD) have resulted in an increased number of patients living into adulthood. Despite increased survival, these patients often require surgery at a young age and are susceptible to developing complications related to the degeneration of surgical prostheses and valves. This case describes to our knowledge, the first successful balloon expandable valve-in-valve transcatheter aortic valve implantation (ViV-TAVI) for stenosis of a BioValsalva stentless aortic graft in a patient with complex congenital heart disease and two prior sternotomies. Prior to ViV-TAVI, the patient was critically ill and unable to be weaned from intensive care unit supports due to recurrent pulmonary oedema. Our case demonstrates successful ViV-TAVI in what was considered an extreme-risk patient. This procedure enabled the patient to be rapidly weaned from respiratory supports and discharged seven days post-procedure.
Key words: aortic stenosis, TAVI, valve-in-valve transcatheter aortic valve implantation, adult congenital heart disease
Introduction
Advances in the diagnosis and management of congenital heart disease (CHD) have resulted in an increased number of patients living into adulthood (1, 2).
Transcatheter aortic valve implantation (TAVI) is now routinely used for the management in aortic stenosis. With growing experience with TAVI technology there has been an increase in the application of TAVI procedures including valve-in-valve (ViV) procedures. TAVI in patients with adult congenital heart disease (ACHD) is not well described. There has been one retrospective study looking at TAVI in patients with ACHD. In this study, 13 patients with CHD who underwent TAVI were described. Of these, five patients underwent ViV-TAVI, two of which were in patients with a homograft (3). Aside from this retrospective study, TAVI in ACHD is only described in the literature in a small number of case reports (4-7).
To our knowledge, this is the first reported case of balloon expandable ViV TAVI in this particular aortic graft.
The aim of this case report is to describe the successful use of ViV-TAVI in a patient a patient with complex congenital heart disease, and to highlight challenges in the pre-procedure planning and procedure itself, particularly in the context of a stentless graft. Specific challenges identified in this case include TAVI valve sizing; principles of coronary artery protection; and the potential complications associated with aortograms in a patient with high left ventricular end-diastolic pressure (LVEDP).
Case presentation
A 54-year-old female presented to a regional hospital with acute onset dyspnoea, she was critically unwell and admitted directly to the intensive care unit (ICU) with non-COVID19 respiratory sepsis secondary to community-acquired pneumonia, acute pulmonary oedema (APO), acute respiratory distress syndrome (ARDS) and atrial fibrillation with rapid ventricular rate.
The patient had a complex cardiac surgical history in the setting of congenital heart disease – an atrial septal defect and bicuspid aortic valve. In 1972 (age five) she had her first cardiac surgery with an atrial septal defect patch, redirection of the superior vena cava and aortic valvotomy.
In 2009 (age 42) the patient had presented with severe aortic stenosis and aortic incompetence with dilatation of the ascending aorta and arch. She underwent redo aortic valve replacement (AVR) with 25mm Biovalsalva stentless bioprosthetic valve, and replacement of the ascending aorta and transverse arch with a 26mm Dacron graft.
This procedure required reimplantation of the left main coronary artery (LMCA) and right carotid artery (RCA). Reimplantation of the RCA was done with an 8mm interposition graft. The right subclavian, right carotid and left carotid arteries were reimplanted with a trifurcation interposition Dacron graft. The patient’s other medical history comprised of hypertension, type two diabetes mellitus, asthma, depression, gastro-oesophageal reflux disease and lumbosacral spondylosis. Prior to her admission she was independent with all activities of daily living and received the disability support pension.
The patient required intubation and mechanical ventilation in ICU and transfer to a tertiary centre was arranged. Her initial transthoracic echocardiogram demonstrated normal left ventricular size and function with an ejection fraction of 55%. Her bioprosthetic AVR was well seated and she had severe aortic stenosis (AS) with a peak velocity 4.9 ms-1, mean gradient 58 mmHg, aortic valve area (AVA) 0.8 cm2 and dimensionless index (DI) 0.15 (Fig. 1). There was mild-moderate mitral regurgitation. The patient had a prolonged ICU admission of over two months requiring tracheostomy. Despite treatment of her respiratory sepsis and ARDS she was unable to be weaned from mechanical ventilation due to recurrent APO and dysrhythmia. Whilst in ICU her admission was complicated by Burkholderia species ventilator associated pneumonia, sacral pressure ulcer with subsequent pseudomonas infection, and critical illness myopathy.
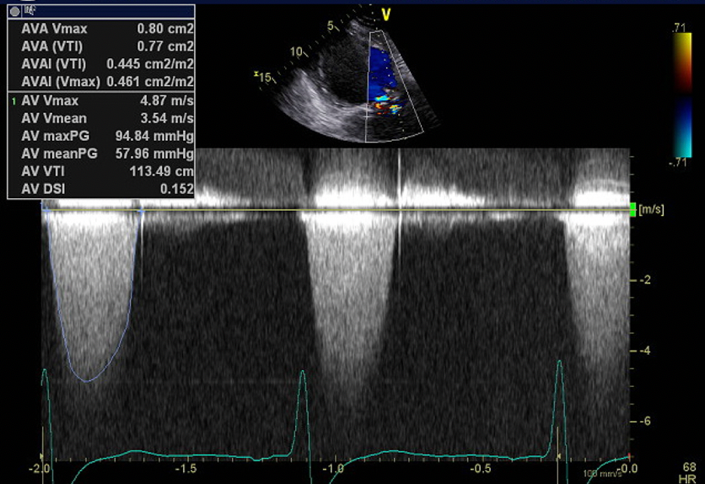
Figure 1. TTE with CW spectral Doppler profile of aortic valve demonstrating severe aortic stenosis
CW – continuous wave, TTE – transthoracic echocardiography
Further investigations performed as part of the work up for potential intervention included a computed tomography (CT) TAVI (Fig. 2-4) to assess the aortic annulus, femoral and subclavian access options. This demonstrated an internal valve diameter of 20 x 20 mm, area- 335 mm2 and perimeter - 64 mm. Annulus to LMCA implantation height was 10 mm, and annulus to RCA height of 10.5 mm. The virtual transcatheter valve to coronary ostium distance (VTC) was 4mm in on the left, and 5 mm on the right. Due to the nature of the graft, there were no true sinus landmarks, however on estimated measurements this appeared to be 26 x 27 x 26 mm, the sinotubular junction (STJ) was 28mm. Transoesophageal echocardiography (TOE) showed severe bioprosthetic AS (peak velocity 5.4 ms-1 and mean gradient 71 mmHg) with a thickened aortic root replacement. There was moderate mitral regurgitation with a short hypoplastic posterior leaflet. There was concern for vegetations present on the anterior leaflet – however, it was unclear the significance of this noting that the patient had multiple sets of negative blood cultures. Following the TOE findings, screening for culture-negative endocarditis was arranged and did not yield any results of significance. Positron emission tomography scan was performed and did not show any valvular or graft uptake, as such, it was thought that endocarditis was unlikely.
The patient was discussed the local Heart Team meeting. Initially percutaneous intervention with TAVI was contemplated, however this was not thought to be feasible following CT TAVI demonstrating borderline anatomy for left sided transfemoral access. Redo surgical AVR was then considered and discussed with a cardiothoracic surgery at a quaternity centre.
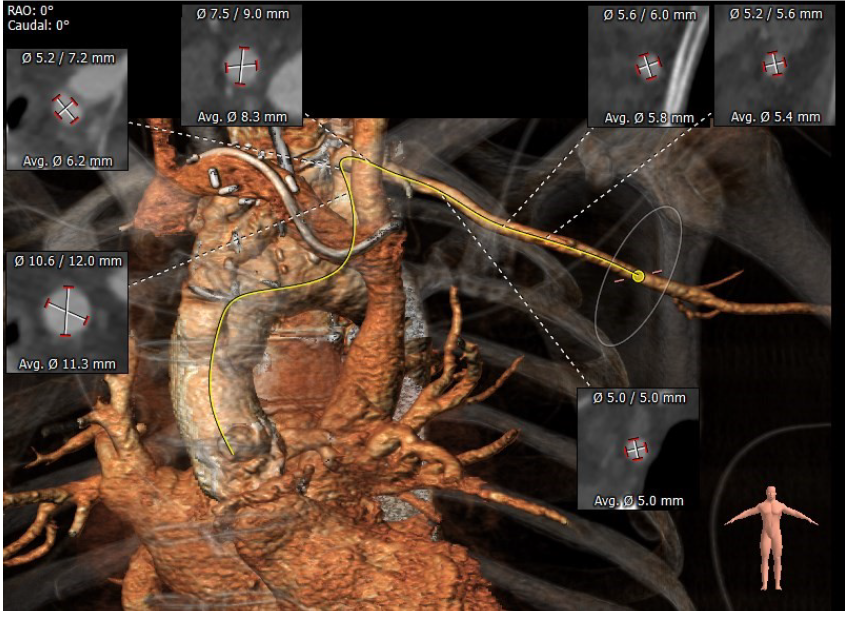
Figure 2. CT TAVI for assessment of subclavian access
CT TAVI -computer tomography transcatheter valve implantation evaluation
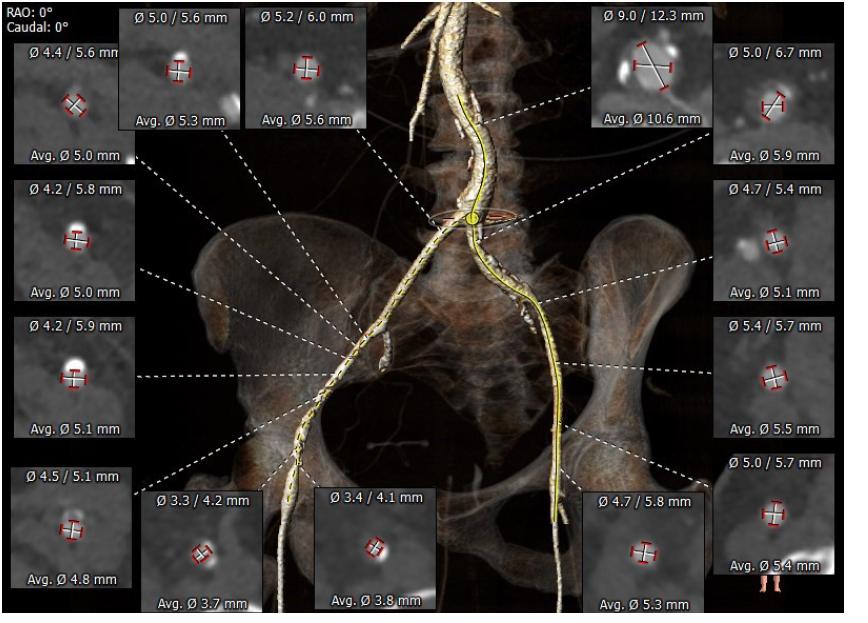
Figure 3.CT TAVI for assessment of femoral access option
CT TAVI -computer tomography transcatheter valve implantation evaluation
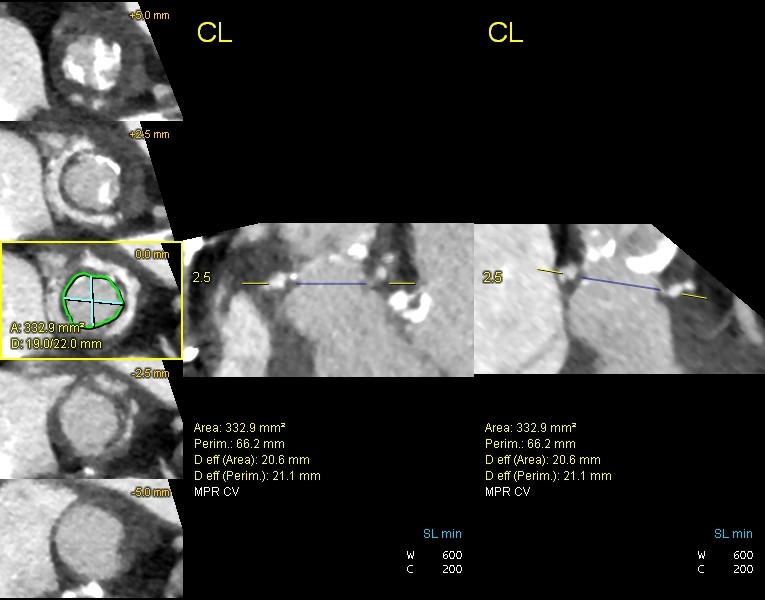
Figure 4. CT TAVI for assessment of valve annulus
CT TAVI -computer tomography transcatheter valve implantation evaluation
Her Euroscore II and STS score were calculated as 57.9% and 39.3% respectively, and she was assessed as being a prohibitive surgical risk. As such, the consensus was to proceed with high-risk ViV-TAVI. Pre-procedure, international opinions were sought regarding valve sizing and the decision made to proceed with an oversized 23 mm and 1 ml Sapien 3 Ultra balloon-expandable valve with access via the left femoral artery with concurrent coronary artery protection. Cerebral protection was considered; however, the right subclavian and brachiocephalic arteries were unsuitable.
ViV-TAVI was performed with the patient intubated by a tracheostomy. Bifemoral artery access was obtained, with tertiary access via the right ulnar artery for LMCA protection. Ulnar artery access was used due to biradial artery occlusion. Crossing the bioprosthetic valve was highly challenging and performed in systole with a 5F MPA catheter after multiple catheter and wire changes. The LVEDP was 34 mmHg prior to valve deployment, with severe aortic regurgitation (AR) induced by the prosthesis. Two aortograms were performed with subsequent pulseless electrical activity arrest, the valve was deployed with rapid pacing and cardiopulmonary resuscitation was performed for approximately 30 seconds post valve deployment and adrenaline administered after which return of spontaneous circulation was achieved.
Post-procedure the patient was transferred back to ICU where she was able to be rapidly weaned from respiratory support. Transthoracic echocardiography on day one post ViV-TAVI showed a well-seated valve with mild-moderately increased hemodynamics (mean pressure gradient 22.5 mmHg, peak velocity 3.8 cm2, AVA 0.8 cm2 and DI 0.35) (Fig. 5). There was trivial valvular AR. Following discharge to the ward, the patient was able to be down-transferred back to the original regional facility for ongoing rehabilitation on day six post ViV-TAVI. At one-month follow up, the patient had New York Heart Association class I symptoms.
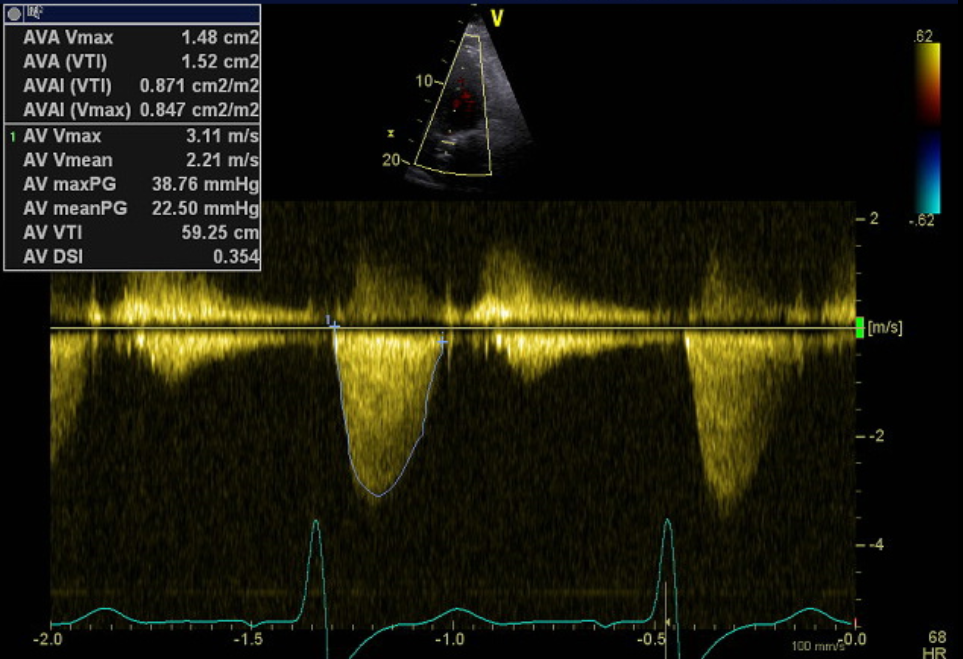
Figure 5. Post-procedure TTE with CW spectral Doppler profile of aortic valve/TAVI
CW – continuous wave, TAVI – transcatheter valve implantation, TTE – transthoracic echocardiography
Discussion
Adults with congenital heart disease who have undergone surgical repair at a young age frequently have complex cardiac anatomy. Following valvular replacement with a bioprosthetic valve, homograft or autograft – bioprosthetic valves are susceptible to structural deterioration (3). In the setting of severe and symptomatic valvular disease, patients with ACHD should be considered for re-do cardiothoracic surgery, or percutaneous intervention (8). Redo-cardiac surgery in this patient group is associated with significant morbidity and mortality (9). Despite the increased use of ViV-TAVI with growing experience in TAVI technology, the use of TAVI in patients with ACHD is not well described. Reported cases of successful TAVI cases in ACHD have highlighted the technical complexities and significant challenge associated with these procedures, which is further amplified by a high-risk patient population.
Our case posed several challenges both in the pre-procedure planning, and intra-procedure. Due to our patient having a stentless aortic graft it was particularly difficult to identify the true aortic annulus, neosinus, and obtain measurements for valve sizing on CT TAVI. Annulus measurements the patients’ CT TAVI slightly differed from the True ID of the 25mm BioValsalva graft according to the ViV TAVI App.
For the procedure, the decision was made to use an oversized 23mm balloon-expandable valve to prevent patient-prosthesis mismatch. We were not keen to use a self-expandable valve mainly for the need for multiple aortograms, and anticipated difficulty with crossing the valve with a self-expandable device and wanted to limit the need for a pre-balloon aortic valvuloplasty and avoid severe AR.
Furthermore, despite the ability to perform commissural alignment, the value of a balloon expandable device would be to preserve coronary re-access in a young patient. This was successful with post-procedure TTE demonstrating a well-seated TAVI without evidence of paravalvular AR.
ViV-TAVI is associated with an increased risk of obstruction of the coronary ostia when compared to a regular TAVI procedure (10). Recognised risk factors associated with ViV-TAVI include geometry of the aortic root (low coronary ostia height and shallow sinus of Valsalva) (3, 11). As such, coronary protection principles are an important consideration in a ViV-TAVI procedure. Our patient had an annulus to LMCA implantation height of 10 mm and a RCA height of 10.5mm. The VTC was 4 mm on the left, and 5 mm on the right. Coronary protection of the LMCA was achieved with a 3.5 non-compliant balloon into the mid-left anterior descending (LAD) coronary artery. This was done using triple vascular access from bifemoral and the right ulnar artery. The ulnar artery was used in the context of the patient having biradial artery occlusion. Our patient had Type I anatomy according to the risk of coronary obstruction classification developed by the Valve-in-Valve International Data (VIVID) registry investigators (12). Although theoretically, coronary protection was not required – identifying the true annular plane in this case was challenging due to a paucity of data, despite technically Type I anatomy.
Crossing the aortic valve in this procedure was highly challenging and resulted in severe AR induced by the balloon expandable valve within the Biovalsalva prosthesis. The quantification of procedural AR and AR index although challenging, is an important part of the TAVI procedure with moderate-severe procedural AR being associated with worse clinical outcomes and increased one-year mortality (13, 14). Aortography is a common and convenient to assess for procedural AR after TAVI, however in our case, the use of two aortograms in the context of a significantly raised LVEDP of 34 mmHg resulted in a pulseless electrical activity/ arrest. We would recommend the limited use of aortograms in a patient with elevated LVEDP and consider transoesophageal or fusion CT imaging to allow for precision VIV deployment.
Conclusion
This case describes a successful ViV-TAVI procedure in an extreme-risk patient with complex CHD. It highlights challenges that may be faced in this expanding group of patients with inoperable valvular pathology involving a bioprosthesis. Important teaching points heralded from our patient include valve-sizing for a degenerated bioprosthesis; coronary protection principles; and risks associated with the use of aortograms in a patient with severe AR and elevated LVEDP.
Ethics: Informed consent was obtained from the patient to publish this case as a case report and permission given to use relevant data/media
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: C.R. - writing – original draft, review and editing; A.Sh. writing – original draft, U.H. – supervision, H.A. - supervision, review and editing; All authors took part in care of patient and reviewed and approved final version for publication Acknowledgement and funding: None to declare
References
- 1. Baumgartner H, De Backer J, Babu-Narayan SV, Budts M, Chessa M, Diller GP, et al. 2020 ESC Guidelines for the management of adult congenital heart disease: The Task Force for the management of adult congenital heart disease of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Adult Congenital Heart Disease (ISACHD). Eur Heart J 2021; 42: 563-645.
- 2.Moons P, Bovijn L, Budts W, Belmans A, Gewillig M. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation 2010;1 22: 2264-72.
- 3.Moharem-Elgamal S, Yeong M, Veerappan S, Veerappan S, Manghat NE, Bedair R, et al. Feasibility and effectiveness of transcatheter aortic valve implantation in adults with congenital heart disease. Int J Cardiol Cong Heart Dis 2021;3:100116.
- 4.Nascimbene A, Loyalka P, Gregoric ID, Bellera R, Malahfji M, Petrovic M, et al. Transcatheter aortic valve implantation in a patient with unicuspid aortic valve. Texas Heart Inst J 2017 ;44: 127-30.
- 5.Lehner A, Herrmann FE, Mehilli J, Haas NA. Edwards Sapien 3 transcatheter aortic valve implantation for management of severe aortic regurgitation in a teenage patient with corrected atrioventricular septal defect and progressive left ventricular dysfunction. Cath Cardiovasc Interv 2019; 93: E244-E7.
- 6.Keswani A, Verma A, Dann K, et al. Transcatheter aortic valve implantation in surgically repaired double outlet right ventricle. Congenital Heart Disease 2014; 9: E153-E7.
- 7.Attia R, Visagan R, Nowell J, Chadalavada S, Thomas M, Bapat V. Transapical transcatheter aortic valve implantation in a complex aortic surgical patient: A case involving the youngest valve-in-valve implantation with a 29 mm transcather valve. Exp Clin Cardiol 2012; 17: 251.
- 8.Van Der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, Mulder BJ. The changing epidemiology of congenital heart disease. Nature Rev Cardiol 2011; 8: 50-60.
- 9.Bridgewater B, Keogh B, Kinsman R, Walton P. Sixth national adult cardiac surgical database report. Henley-on-Thames: Dendrite Clinical Systems Ltd 2008.
- 10. Nalluri N, Atti V, Munir AB, Karam B, Patel NJ, Kumar V,, et al. Valve in valve transcatheter aortic valve implantation (ViV‐TAVI) versus redo—surgical aortic valve replacement (redo‐SAVR): A systematic review and meta‐analysis. J Interv Cardiol 2018;31:661-71.
- 11.Bapat V. Technical pitfalls and tips for the valve-in-valve procedure. Ann Cardiothorac Surg 2017;6: 541.
- 12.Tang GH, Komatsu I, Tzemach L, Tzemach L, Simonato M, Wolak A, et al. Risk of coronary obstruction and the need to perform BASILICA: the VIVID classification. EuroIntervention 2020; 16: e757-e9.
- 13. Mihara H, Shibayama K, Jilaihawi H, Itabashi Y, BerdejoJ, Utsunomiya H, et al. Assessment of post-procedural aortic regurgitation after TAVR: an intraprocedural TEE study. JACC Cardiovas Imag 2015; 8: 993-1003.
- 14.Sinning J-M, Hammerstingl C, Vasa-Nicotera M, Adenauer V, Cachiguango SJL, Scheer AC, et al. Aortic regurgitation index defines severity of peri-prosthetic regurgitation and predicts outcome in patients after transcatheter aortic valve implantation. J Am Coll Cardiol 2012; 59: 1134-41.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

