Effect of percutaneous balloon mitral valvuloplasty on left ventricular function in rheumatic mitral stenosis
ORIGINAL RESEARCH ARTICLE
Effect of percutaneous balloon mitral valvuloplasty on left ventricular function in rheumatic mitral stenosis
Article Summary
- DOI: 10.24969/hvt.2022.353
- Cardiac Surgery
- Published: 26/11/2022
- Received: 15/10/2022
- Revised: 15/11/2022
- Accepted: 19/11/2022
- Views: 5934
- Downloads: 4717
-
Citations
- Keywords: mitral stenosis, percutaneous balloon mitral valvuloplasty, echocardiography, left ventricular function, tissue Doppler, mitral annular systolic velocity, myocardial performance index
PDF PRINT VERSION CommentsAddress for CorrespondenceAddress for Correspondence: Rujuta Parikh, Department of Cardiology, UNMICRC, Civil Hospital Campus,
Asarwa, Ahmedabad-380016, Gujarat, India Email: rujutaparitkh1992@gmail.com
Mobile: +91-9427475822, Fax:+91- 079-22682092
Gaurav Singh1a, Jayesh Prajapati1a, Rujuta Parikh1a, Kamal Sharma1a, Iva Patel1b, Ashish Mishra1a, Lalan Singh2, Utsav Patel1a, Jaykumar Vadodariya1a
1a Department of Cardiology and 1bDepartment of Research , U. N. Mehta Institute of Cardiology and Research Centre (UNMICRC), Civil Hospital Campus, Asarwa, Ahmedabad-380016, Gujarat, India
2Department of Cardiology, Shyam Shah Medical College, Rewa-486001, India
Abstract:
Objective: Patients with rheumatic mitral stenosis, despite having normal left ventricular ejection fraction (LV EF), have ventricular dysfunction in the form of impaired longitudinal excursion. Tissue Doppler velocity is a useful indicator for assessment of long-axis ventricular shortening and lengthening.
The aim of our study was to evaluate the effect of percutaneous balloon mitral valvuloplasty (PBMV) on LV function in rheumatic MS and to study echocardiographic parameters with M-Mode and Tissue Doppler Imaging pre PBMV, post PBMV and on follow-up to determine predictors of LV function.
Methods: We analysed 52 patients with severe mitral stenosis with normal LV EF, who underwent PBMV at our institute. Baseline parameters of LV function were compared with immediate post PBMV and at three months follow up.
Results: The mean age of the patients was 33.73 (10.87) years with female preponderance. The mean mitral valve area before PBMV was 0.92 (0.13) cm2 which increased to 1.65 (0.21) cm2 after PBMV and at 3 month it was 1.61 (0.23) cm2 (p<0.001). LVEF before PBMV by modified Simpson’s method was 55.45 (8.44)% and after PBMV, it was 55.58 (3.46)% and at 3 month it was 56.62 (2.46)% (p>0.05). Mitral valve E’ was 8.71 (1.54) cm/s which increased to 10.13 (1.68) cm/s post PBMV and at 3 month it was 10.83 (1.34) cm/s (p<0.001).. Mitral annular systolic velocity (MASV), before PBMV was 7.90 (0.96) cm/s which increased to 9.31 (1.68) cm/s after PBMV and at 3 month it was 10.13 (0.96) cm/s (p<0.001). Myocardial performance index (MPI) before PBMV was 0.54 (0.48) which decreased post PBMV to 0.47 (0.06) and at 3 month it was 0.38 (0.04) (p=0.01). Pre PBMV MPI value <0.48 predicted improvement in LV function (sensitivity: 81%, specificity: 58.1%).
Conclusion: Thus, PBMV leads to improvement in LV function in patients with severe MS with normal LV EF.
Introduction
Up to 21% of patients with mitral stenosis (MS) have impaired left ventricular (LV) function (1). Different mechanisms of LV dysfunction have been elucidated. Decreased compliance due to recurrent myocardial inflammation as in recurrent carditis, is one such mechanisms (2). Also, rheumatic affection of subvalvular apparatus can lead to tethering of postero-basal LV and wall motion abnormalities (3). Abnormal interventricular interdependence due to paradoxical movement of septum and abnormal right ventricular (RV) function due to pulmonary hypertension (PH) has also been shown to cause LV dysfunction (4). Atrial fibrillation has also been shown to cause impairment of LV function. Comorbidities such as systemic hypertension and coronary artery disease (CAD) may also lead to LV dysfunction.
Even with normal ejection fraction (EF) (indicating preserved global left ventricular function), subclinical LV dysfunction has been reported in multiple studies (5, 6). Strain rate imaging was shown to be lower in patients with MS with normal LVEF, as compared to a healthy cohort, thus demonstrating subclinical LV dysfunction (5).
Another study showed improvement in strain rate post percutaneous balloon mitral valvuloplasty (PBMV) and used it for quantitative evaluation of PBMV (6). Impairment in long-axis function as measured by tissue Doppler echocardiography was first evaluated by Ozdemir et al . in patients with moderate to severe MS with normal LVEF (7). Furthermore, Sengupta et al. showed improvement in peak annular velocity of systolic excursion (S’) and peak annular velocity in early diastole (E’) in patients with severe MS undergoing PBMV (8).
Altered LV long-axis movement has been shown to be a sensitive indicator of early myocardial dysfunction. Pulsed-wave Doppler tissue velocities have been proven to be a good tool for assessment of long-axis ventricular shortening and lengthening (9,10).
The aim of our study was to evaluate the effect of PBMV on LV function in rheumatic MS and to study echocardiographic parameters with M-Mode and Tissue Doppler Imaging pre PBMV, post PBMV and on follow-up to determine predictors of LV dysfunction.
Methods
Study design and population
This was a prospective observational study conducted U.N. Mehta Institute, Ahmedabad, Gujarat, India, which is a tertiary care centre. Patients with severe MS with normal LVEF undergoing PBMV from August 2016 to March 2019 were screened. Patients with concomitant moderate to severe mitral regurgitation, concomitant involvement of other valves, coronary artery disease as evidenced by electrocardiogram (ECG), 2D echocardiogram or coronary angiogram (CAG), hypertension and diabetes mellitus were excluded. Of a total of 72 patients were screened, 5 had hypertension, 3 had diabetes mellitus, and 12 patients were excluded due to significant other valve involvement.
A total of 52 patients with severe mitral stenosis of rheumatic affection were enrolled.
Data were collected after obtaining written, informed consent from the patients and their one close relative; and after approval from institutional Ethics committee.
All patients underwent clinical and laboratory examinations and following data were collected: demographic parameters as age, sex, INR level, and presence of atrial fibrillation and duration of disease.
Echocardiography
Echocardiography was done by Vivid GE machine, using probe frequency range of 2–4 MHz by a single investigator. For patients in atrial fibrillation, a mean of measurements in five consecutive cardiac cycles was taken.
Baseline echocardiographic parameters like mitral valve area (MVA), mitral valve (MV) gradient, PA pressure, tricuspid regurgitation and RV function were assessed (Fig. 1). Ejection fraction was assessed with modified Simpson’s method (Fig. 2).
Mitral annular plane systolic excursion (MAPSE) was measured by M-mode images obtained at the LV septal and lateral annulus in 4-chamber view, and an average MAPSE value was calculated (Fig. 3).
For peak annular velocities in systole and diastole, apical 4-chamber view and apical 2-chamber views were used. Tissue Doppler cursor was placed at the septal and lateral sides in 4-chamber view and on anterior and inferior sides of the mitral annulus in 2-chamber views. Peak velocities during systole (S’) and early diastole (E') were measured and average value from all four sites were obtained (Fig. 4).
Myocardial performance index (MPI) was calculated by conventional method by dividing the sum of isovolumic contraction time (IVCT) and isovolumic relaxation time (IVRT) by ejection time (ET). Systolic dysfunction shortens the ejection time (ET) and diastolic dysfunction prolongs the IVRT (Fig. 5).
Echocardiogram was done just before and within 24 hours after PBMV. Follow-up echocardiogram was done at 3 months and 1 year.
Percutaneous balloon mitral valvuloplasty
According to European Society of Cardiology (ESC), PBMV is a Class I indication in all symptomatic patients with MVA less than 1.5 cm2 without any unfavourable characteristics or with high surgical risk. Unfavourable characteristics for PBMV include old age, history of prior intervention, New York Heart Association (NYHA) class IV symptoms, permanent AF, severe pulmonary hypertension, Wilkins’ score >8, calcification of mitral valve as assessed by fluoroscopy, and severe tricuspid regurgitation. In asymptomatic patients, it may be considered in patients with high risk of thromboembolism or have a greater risk of hemodynamic decompensation (11).
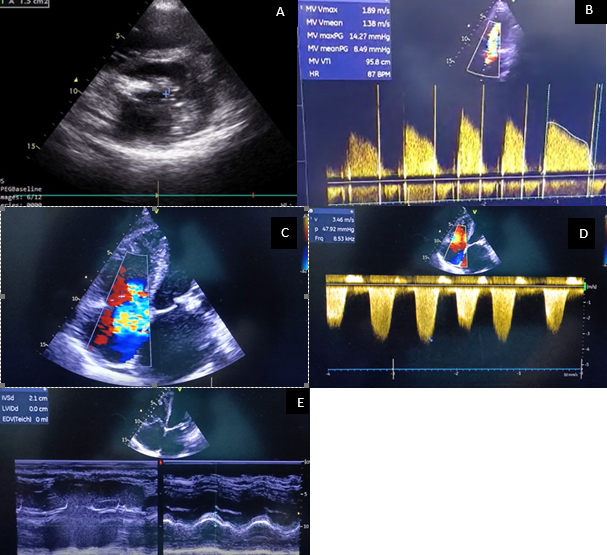
Figure 1. Baseline echocardiographic parameters. Panel A: Mitral valve area by planimetry. Panel B: Mitral valve gradient as assessed by mitral inflow velocity. Panel C: Tricuspid regurgitation. Panel D: Assessment of right ventricular systolic function. Panel E: Assessment of right ventricular function by tricuspid annular plane systolic excursion (TAPSE).
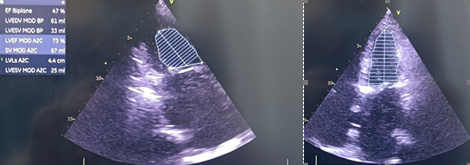
Figure 2. Assessment of left ventricular ejection fraction by Simpsons method in apical 2- chamber and 4- chamber views
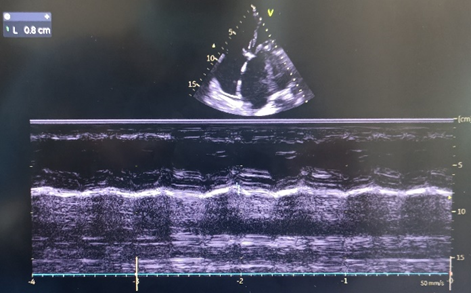
Figure 3. Assessment of mitral annular plane systolic excursion by M-Mode
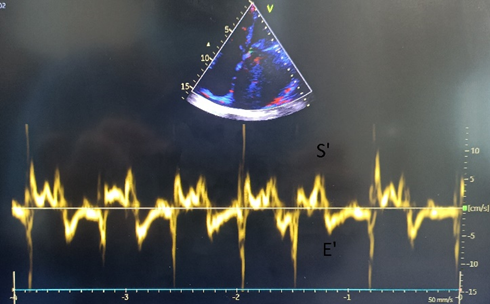
Figure 4. Assessment of peak annular velocities in systole and diastole in apical 4-chamber view by tissue Doppler velocity
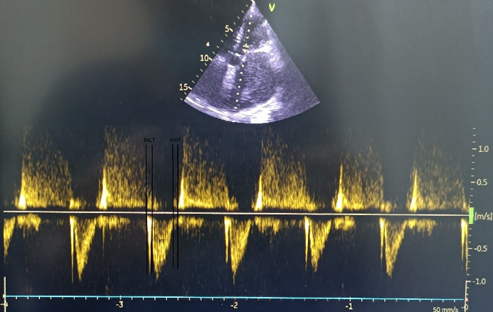
Figure 5. Assessment of myocardial performance index by pulse wave Doppler
An experienced team performed PBMV with double lumen Accura balloon while monitoring conventional hemodynamic parameters. Balloon size was chosen according to Hung’s formula.
PBMV was regarded as successful if the mitral valve area post procedure increased to >1.5 cm2; or the gain in MVA was >50% of baseline with not more than grade II mitral regurgitation (12).
Statistical analysis
All statistical studies were carried out using Statistical Package for Social Sciences (SPSS vs.22.0). Quantitative variables were expressed as the mean (standard deviation) and qualitative variables were expressed as percentage (%). A comparison of parametric values between two groups was performed using the independent sample t test. Categorical variables were compared using the Chi-square test and were presented as frequencies and percentage. Comparison of repeated measures was performed using Friedman test. Logistic regression was used to predict the different risk factors for presence of left ventricular dysfunction determined by longitudinal tissue Doppler velocity (E’ < 8 cm/s) (13). The predictive diagnostic value of pre BMV MPI for LV dysfunction was calculated using the receiver operating characteristic (ROC) curve. A nominal significance was taken as a two tailed p value<0.05.
Results
Baseline characteristics:
The baseline characteristics of study population is shown in Table 1.
In our study, mean mitral valve area, measured by planimetry, before PBMV was 0.92 (0.13) cm2 which increased to 1.65 (0.21) cm2 post PBMV; at 3 months follow up, it was 1.61 (0.23) cm2; at 1 year follow up, it was 1.60 (0.19) cm2 (p=0.01). The mean mitral valve gradient before PBMV was 16.23 (5.97) mm Hg, which reduced to 6.27(2.45) mm Hg after PBMV; at 3 months follow up, was 6.47 (2.23) mm Hg and at 1 year follow up, it was 6.89 (2.66) mm Hg (p<0.001). Mitral valve E’ was 8.71 (1.54) cm/s which increased to 10.13 (1.68) cm/s post PBMV and at 3 month it was 10.83 (1.34) cm/s (p<0.001).. Mitral annular systolic velocity (MASV), before PBMV was 7.90 (0.96) cm/s which increased to 9.31 (1.68) cm/s after PBMV and at 3 month it was 10.13 (0.96) cm/s (p<0.001). Myocardial performance index (MPI) before PBMV was 0.54 (0.48) which decreased post PBMV to 0.47 (0.06) and at 3 month it was 0.38 (0.04) (p=0.01). The parameters of LV function of the study population are described in Table 2.
There was no significant difference in the baseline LV parameters of patients in atrial fibrillation and those in sinus rhythm as shown in Table 3.
Table 1: Baseline characteristics of study population
Variables
n=52
Age, years*
33.73 (10.87)
Male, n(%)
14 (26.92)
Female, n(%)
38 (73.08)
Atrial fibrillation on oral anticoagulation, n(%)
24 (46.1)
PT INR in therapeutic range, n(%)
11 (45.83)
RHD Prophylaxis, n(%)
41 (78.85)
Duration of illness, years*
6.16 (3.95)
*Mean (SD)
INR – international normalized ratio, PT – prothrombin time, RHD – rheumatic heart disease
Table 2. Parameters of LV function of the study population before and after PBMV
Variables
Baseline
Post BMV
3 months follow up
1 year follow up
p*
MV area by planimetry, cm2
0.92 (0.13)
1.65 (0.21)
1.61 (0.23)
1.60(0.22)
0.01
MV gradient, mmHg
16.23 (5.97)
6.27 (2.45)
6.47 (2.23)
6.53 (2.65)
<0.001
LV ejection fraction, %
55.45 (8.44)
55.58 (3.46)
56.62 (2.46)
56.23 (2.11)
0.46
MAPSE, mm
10.50 (1.94)
11.00 (1.74)
11.04 (1.48)
11.01 (1.21)
0.21
MASV (S’), cm/s
7.90 (0.96)
9.31 (1.68)
10.13 (0.96)
10.22 (0.92)
<0.001
MPI
0.54 (0.07
0.47 (0.06
0.38 (0.04)
0.31 (0.06)
0.01
E’, cm/s
8.71 (1.54)
10.13 (1.68)
10.83 (1.34)
10.91 (1.22)
<0.001
TAPSE, mm
19.67 (2.22)
19.48 (2.69)
19.37 (1.96)
19.32 (1.63)
0.79
*p – Friedman test
E’ – mitral annular velocity during early diastole, LV – left ventricle, MAPSE- mitral annular plane systolic excursion, MASV – mitral annular systolic velocity, MPI – myocardial performance index, MV – mitral valve, PBMV – percutaneous balloon mitral valvuloplasty, TAPSE- tricuspid annular plane systolic excursion
Predictors of improvement in LV function
LV dysfunction as determined by baseline longitudinal tissue Doppler velocity (E’ < 8 cm/s) (13) was found in 31 (59.6%) patients. The baseline characteristics of patients with LV dysfunction and normal LV function are summarized in following Table 4. Cardiovascular risk factors and echo parameters such as MV gradient, pre PBMV LVEF, Pre PBMV MAPSE, and MASV did not differ between both groups. However, MPI (p=0.03) was significantly higher and E’ markedly lower in patients with LV dysfunction as compared with patients without LV dysfunction.
Immediately post PBMV, LV function improved in 24 (77.4%) of patients with LV dysfunction and the remaining 7 patients had improved function at 3 months follow up.
Regression analysis for predictor variables of LV dysfunction is shown in Table 5. As can be seen from Table 5 among echocardiographic variables MPI was found to be a predictor of LV dysfunction after PBMV – the probability of LV dysfunction was 1.83 times higher for patients with abnormal MPI (95%CI – 0.53-6.33, p<0.05).
ROC analysis showed pre PBMV MPI value < 0.48 to be predictive of improvement in LV function with a sensitivity of 81% and specificity of 58.1% (AUC=0.687 95% CI 0.54-0.81 with p=0.02) (Fig. 6).
Table 3. Comparison of echocardiographic and ECG parameters between patients with sinus rhythm and patients with atrial fibrillation
Variables
Sinus rhythm (n=41)
Atrial fibrillation (n=11)
p
Echocardiographic parameters
MV area by planimetry, cm2
0.91 (0.11)
0.92 (0.14)
0.78
MV gradient. mmHg
16.43 ( 6.34)
15.98 (4.87)
0.65
LA size. mm
44.5 (3.2)
51.1 (2.87)
0.061
PAP, mmHg
48 (12)
52 (10)
0.24
MAPSE, mm
10.56 (2.00)
10.27 (1.85)
0.669
MASV. cm/s
8 (0.99)
7.55 (0.82)
0.169
LVEF. %
55.88 (4.64)
57.45 (3.45)
0.300
MPI
0.29 (0.46)
0.18 (0.46)
0.471
E’, cm/s
8.80 (1.60)
8.36 (1.29)
0.404
TAPSE, mm
19.77 (2.56 )
19.23 (2.87)
0.525
Baseline ECG parameters
Heart Rate, beats/min
84
81
0.98
QRS Axis
106.4
110.2
0.62
E’ – mitral annular velocity during early diastole, ECG – electrocardiogram, LA- left atrium, LVEF– left ventricular ejection fraction, MAPSE- mitral annular plane systolic excursion, MASV – mitral annular systolic velocity, MPI – myocardial performance index, MV – mitral valve, PAP – pulmonary arterial pressure, TAPSE- tricuspid annular plane systolic excursion
Table 4. Baseline characteristics of patients with LV dysfunction and normal LV function
Variables
Normal LV function (n=21)
LV dysfunction (n=31)
p
Age, years
33.36(12.14)
34.17(9.43)
0.7919
Male, n(%)
07 (33.3
07 (22.5)
0.5465
Female, n(%)
14 (66.6)
24 (77.4)
Diabetes, n(%)
06 (21.43)
04 (16.67)
0.9351
Hypertension, n(%)
07 (25)
03 (12.5)
0.4311
MV gradient, mmHg
16.32 (6.09)
16.13 (5.95)
0.907
Pre PBMV LVEF, %
55.82 (5.01)
56.67 (3.70)
0.489
Pre PBMV MAPSE, mm
10.79 (1.20)
10.17 (2.56)
0.259
MASV, cm/s
8.07 (0.86)
7.70 (1.06)
0.169
E’, cm/s
9.50 (1.71)
7.79 (0.41)
0.025
MPI
0.21 (0.30)
0.33 (0.48)
0.034
E’ – mitral annular velocity during early diastole, LV – left ventricular, LVEF– left ventricular ejection fraction, MAPSE- mitral annular plane systolic excursion, MASV – mitral annular systolic velocity, MPI – myocardial performance index, MV – mitral valve, PBMV – percutaneous balloon mitral valvuloplasty
Table 5. Predictors of LV dysfunction - logistic regression analysis
Variables
Odds
95% CI
p
MAPSE
0.836
0.61-1.15
0.273
MASV
0.651
0.35-1.20
0.169
MPI
1.833
0.53-6.33
0.05
E’
0.205
0.007-0.53
0.001
CI – confidence interval, E’ – mitral annular velocity during early diastole, LV – left ventricular, MAPSE- mitral annular plane systolic excursion, MASV – mitral annular systolic velocity, MPI – myocardial performance index
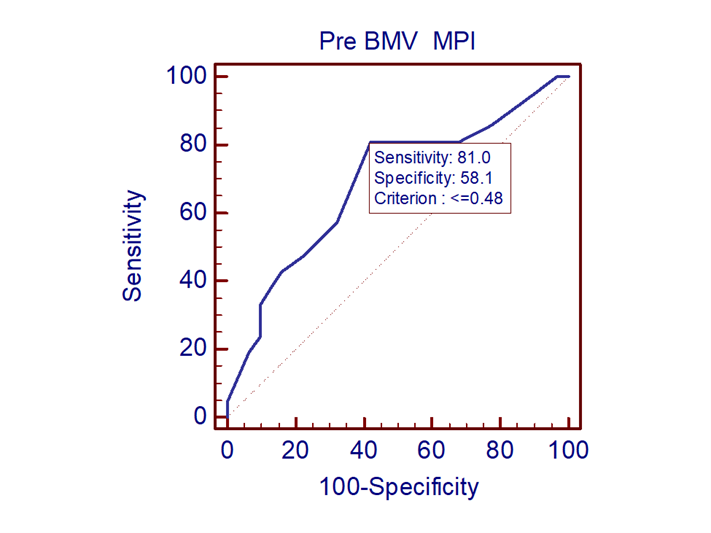
Figure 6. ROC analysis of myocardial performance index for prediction of left ventricular dysfunction
(AUC - 0.687, 95% CI 0.54-0.81, p=0.02)
AUC- area under the ROC curve, CI – confidence interval
Discussion
Our study demonstrated that among various echocardiographic indices MPI was a predictor of LV dysfunction defined by low mitral annular velocity during diastole after PBMV. Pre PBMV MPI value < 0.48 predicted improvement of LV function after procedure with good sensitivity.
The evaluation of effect of valve dysfunction on isovolumic contraction time, isovolumic relaxation time and ejection time has not been well studied before. Analysis of MPI, thus helps in understanding the pathophysiological changes in LV due to valve dysfunction. Since it takes into account both systolic and diastolic function of LV, it is a better predictor of global LV function.
Diagnostic accuracy of imaging of LV dysfunction in rheumatic mitral stenosis:
2D Echocardiography has been the gold standard for evaluation of valvular heart disease. When transthoracic echocardiogram (TTE) images are inadequate, transesophageal echocardiography (TEE) is useful for assessing global as well as regional systolic and diastolic LV function, valve area and morphology (14). 3D echocardiography and 3D strain parameters provide valuable assessment of LV myocardial dynamics (15). Furthermore, unlike 2D echocardiogram and M-mode, which makes incorrect geometric assumptions about LV, 3D echocardiography allows us to see the LV as it is. It has better reproducibility and good correlation with LV mass, volume and LVEF (16). With respect to LV function, mitral valve area and aortic valve area, cardiac magnetic resonance imaging has also been found to be accurate with a coefficient of correlation being 0.82, 0.98 and 0.92 respectively. However, due to limitations of technology and lengthy imaging time, the role of cardiac magnetic resonance imaging is, at best, only complimentary to 2D Echocardiography (17).
LV dysfunction in rheumatic mitral stenosis
Multiple hemodynamic and myocardial factors have been implicated in the causation of LV dysfunction in patients with mitral stenosis. Table 6 shows the various studies implicating pathophysiology of LV dysfunction in patients with mitral stenosis.
In our study, we have used tissue Doppler imaging to quantify MAPSE, peak systolic velocity (S’), peak early diastolic velocity (E’) and MPI to identify impairment of longitudinal LV function and subclinical LV dysfunction.
Similar to our study, Ozer et al. (5) demonstrated in their study, that the displacement of mitral annulus towards the apex, denoting the longitudinal motion of LV is impaired, as measured by tissue Doppler velocity, despite normal global systolic functions.
This was attributed to the longitudinal orientation of myofibrils in the endocardium and the chief affection of rheumatic activity at the endocardium. Guven et al. (23) also showed impaired LV longitudinal motion in patients with moderate to severe MS; by using tissue Doppler velocity, and attributed it to discordant longitudinal and circumferential fibres of LV. Hady et al. (20) demonstrated LV dysfunction to be present in rheumatic MS, irrespective of the patient being in sinus rhythm or atrial fibrillation.
Table 6. Studies on LV dysfunction in rheumatic mitral stenosis
No.
Study
Pathophysiology of LV dysfunction
Assessment of LV function
Conclusion
1
Gash et al, 1983 (18)
increased afterload without adequate Frank-Starling compensation
Invasive haemodynamic
Patients with MS have reduced ejection performance and preload as compared to healthy controls
2
Mohan et al, 1989 (2)
Depressed inotropic state of LV in presence of increased afterload
M-mode, Doppler and colour flow mapping
Patients with isolated rheumatic MS have decreased myocardial contractility as compared to healthy controls.
3
Gaasch et al, 1991 (19)
Tethering effects of mitral apparatus, RV pressure overload, Paradoxical septal motion, LV diastolic suction due to elastic recoil
Left heart catheterization
LV dysfunction is independent of rheumatic myocardial factor.
4
Ozdemir et al, 2002 (7)
chronic decrease in
preload, increase of afterload, reduced left-ventricular thickness, altered LV suction, altered interventricular interdependence
Doppler tissue imaging
Patients with MS have decreased longitudinal function when compared to healthy controls
5
Ozer et al, 2004 (9)
Insufficient pre-load, afterload mismatch, RV pressure overload, myocardial factors
M-Mode and tissue Doppler velocity
Patients with MS have reduced septal and lateral annular velocity as compared to healthy controls.
6
Klein et al, 2006 (3)
Impaired diastolic filling and myocardial contractility, rigidity and fixation of the
postero-basal myocardium due to scarring or inflammation, pressure overload effects from the right ventricle
Tissue Doppler imaging and strain rate imaging
All invasive treatment including surgical replacement and PBMV (except chordal excision) helps in improving LV function.
7
Buyukkaya et al, 2008 (10)
Tensed mitral valve apparatus and altered interventricular interdependence
Tissue Doppler
In rheumatic mitral stenosis, especially with atrial fibrillation, significant impairment of left ventricular long-axis function is noted with normal global systolic function.
8
Simsek et al, 2010 (5)
Ultrastructural pathological changes in LV muscle cells
Tissue Doppler imaging and strain rate echocardiography
Subclinical LV dysfunction exists in patients with MS as compared to healthy control
9
Hady et al, 2011 (20)
Tension created by a thickened and fibrosed mitral valve apparatus, passive elastic changes of LV due to the chronic decrease in LV filling and to myocardial fibrosis
Tissue Doppler and strain imaging
Rheumatic MS patients have both systolic and diastolic dysfunction. Diastolic function is more affected in patients with sinus rhythm and systolic function is more affected in patients with AF.
10
Bilen et al, 2011 (21)
Reduced in LV filling, chronic myocardial inflammation and
scarring of mitral subvalvular apparatus, diastolic dysfunction, increased afterload, abnormal septal interaction, pulmonary hypertension and loss of myofibrils
Peak longitudinal strain and strain rate
Independent of hemodynamic severity patients with MS have subclinical LV dysfunction
11
Mukherjee et al, 2018 (22)
Atrial fibrillation, chronic decrease in preload, myocardial fibrosis, change in interaction between LV and RV
Tissue Doppler, Doppler Strain, 2D strain
2D strain and strain rate gives a quantitative assessment of global and regional LV function
LV-left ventricle, MS- mitral stenosis, PBMV – percutaneous balloon mitral valvuloplasty, RV – right ventricle
Table 7. Studies comparing LV function pre and post PBMV
No.
Study
LV function parameter
Pre PBMV
Post PBMV
3 -month
Follow-up
p
Conclusion
1
Sengupta et al, 2004
(8)
Peak annular velocity of systolic excursion in ejection
6.15 (1.06)
6.77
(1.21)
NA
NA
A significant improvement was seen in the mitral peak annular velocities
of systolic excursion in ejection and of early diastole in both lateral and medial walls. Serial evaluation of mitral annular velocities correlates with immediate improvement in left ventricular function after PBMV.
Peak annular velocity in early diastole
6.74 (1.56)
8.98
(2.45)
NA
NA
Peak annular velocity in late diastole
6.56 (3.18)
6.07 (2.24)
NA
NA
2
Sowdagar et al, 2017 (24)
Peak myocardial velocity during systole
5.8 (0.7)
9.9
(1.6)
NA
<0.001
Mobilization of the mitral apparatus post PBMV can lead to reversal of the myocardial stiffness which explains the immediate improvement in mitral annular velocities seen
Peak myocardial velocity during early diastole
6.4
(0.6)
13.1
(2.1)
NA
<0.001
MPI
0.68
(0.1)
0.39
(0.03)
NA
<0.001
3
Rajesh et al, 2016
(25)
MPI
0.587 (0.020)
0.582 (0.028)
0.488 (0.012)
<0.001
Immediately post PBMV, improvement in LV long axis function was seen. A gradual improvement in global left ventricular function at three months follow up was noted
MASV, cm/s
7.274 (0.201)
7.951
(0.195)
8.015 (0.187)
<0.001
E’, cm/s
7.677 (0.234)
8.015 (0.226)
8.331 (0.229)
0.04
4
Our study
MPI
0.54 (0.07)
0.47 (0.06)
0.38 (0.04)
0.01
PBMV improves LV function immediately and on further improvement was noted on follow up.
MASV, cm/s
7.90 (0.96)
9.31 (1.68)
10.13 (0.96)
<0.001
E’, cm/s
8.71 (1.54)
10.13 (1.68)
10.83 (1.34)
<0.001
E’ – mitral annular velocity during early diastole, LV – left ventricle, , MASV – mitral annular systolic velocity, MPI – myocardial performance index, NA – not available, PBMV – percutaneous balloon mitral valvuloplasty
Improvement in LV function post PBMV (Table 7)
In a study by Sengupta et al. (8), peak annular velocity improved significantly, immediately post PBMV; and correlated with improvement in the mitral valve area. In another study by Rajesh et al. (25), MAPSE and LVEF did not show improvement immediately post PBMV. While, MASV improved immediately post PBMV; MPI improved only at 3 month follow up (25). Similarly, our study found that MASV, MPI and longitudinal motion improved immediately post PBMV and showed similar trend at 3-month follow up.
MAPSE is an M-mode parameter correlating with movement of mitral annulus. On the other hand, S’ and E’ correlate with mitral annular velocity. Patients with preserved LV EF are likely to have normal overall excursion of mitral annulus as measured by MAPSE. Despite no significant increase in MAPSE, PBMV increases the velocity of excursion, thus decreasing the isovolumic contraction time and increasing the ejection time. Hence, there is an increase in S’, E’ and decrease in MPI.
Commissurotomy results in mechanical relief of LV inflow obstruction and increased preload of LV that could explain the immediate improvement in LV function and continuous benefit noted on follow up. Mobilization of mitral apparatus may result in better compliance of the ventricle. The improvement in MPI suggests improvement in both systolic and diastolic functions of LV.
Predictors of improvement in LV function
Post intervention LV dysfunction is estimated to be seen in 16.8% of patients (26). Hence, prediction of improvement in LV function is important in prognostication of patients. In our study, pre BMV MPI < 0.48 correlated with better post PBMV outcomes because overall preserved LV systolic and diastolic function can be expected to have better outcomes. This is in concordance to a study by Rajesh et al., who also suggested that a PBMV done prior to a significantly worsened LV function yielded better outcomes.
Study limitations
1.The study was done in a single centre providing care to relatively racially homogenous population due to a restricted geographic area; and therefore the results might not be applicable in other settings.
2.LV strain imaging which is a good indicator of LV function was not done.
3. The sample size of the study was relatively small, further studies on larger population should be sought.
Conclusion
Patients with mitral stenosis have impaired LV function despite having normal LV EF. Longitudinal motion improves immediately post PBMV and can be assessed by MASV and MPI. An MPI < 0.48 predicts better LV function post PBMV with a sensitivity of 81% and specificity of 58.1%.
Ethics: Informed consent was obtained from patients before enrollment to the study and study protocol was approved by Ethics committee of our institution
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: G.S., J.P., R.P., K.S., I.P., A.M., L.S., U.P. and J. V. equally contributed to the study and preparation of manuscript
Acknowledgement and funding: None to declare
References
1.Shikano M, Nakatani S, Kim J, Hanatani A, Hashimura K, Yasumura Y, et al. Impaired left ventricular systolic function in mitral stenosis. J Cardiol 2003; 42: 75-9. 2.Mohan JC, Khalilullah M, Arora R. Left ventricular intrinsic contractility in pure rheumatic mitral stenosis. Am J Cardiol 1989; 64: 240-2. https://doi.org/10.1016/0002-9149(89)90469-4 PMid:2741834 3.Klein AJP, Carroll JD. Left ventricular dysfunction and mitral stenosis. Heart Fail Clin 2006; 2: 443-52. https://doi.org/10.1016/j.hfc.2006.09.006 PMid:17448431 4.Curry GC, Elliott LP, Ramsey HW. Quantitative left ventricular angiocardiographic findings in mitral stenosis. Am J Cardiol 1972; 29: 621-7. https://doi.org/10.1016/0002-9149(72)90162-2 PMid:5021491 5.Simsek Z, Karakelleoglu S, Gundogdu F, Aksakal E, Sevimli S, Arslan S, et al. Evaluation of left ventricular function with strain/strain rate imaging in patients with rheumatic mitral stenosis. Anatol J Cardiol 2010; 10: 328-33. https://doi.org/10.5152/akd.2010.091 PMid:20693128 6.Gunturk EE, Baran O, Akkaya H, Orsçeli̇K O. Evaluation of the effect of percutaneous mitral balloon valvuloplasty on left ventricular systolic functions using strain and strain rate echocardiography. Turk J Med Sci 2020; 50: 724-30. https://doi.org/10.3906/sag-1901-69 PMid:32093442 PMCid:PMC7379431 7.Ozdemir K, Altunkeser BB, Gok H, İçli A, Temizhan A. Analysis of the myocardial velocities in patients with mitral stenosis. J Am Soc Echocardiogr 2002; 15: 1472-8. https://doi.org/10.1067/mje.2002.128645 PMid:12464914 8.Sengupta PP, Mohan JC, Mehta V, Kaul UA, Trehan VK, Arora R, et al. Effects of percutaneous mitral commissurotomy on longitudinal left ventricular dynamics in mitral stenosis: Quantitative assessment by tissue velocity imaging. J Am Soc Echocardiogr 2004; 17: 824-8. https://doi.org/10.1016/j.echo.2004.04.025 PMid:15282484 9.Ozer N, Can I, Atalar E, Sade E, Aksoyek S, Ovunç K, et al. Left ventricular long-axis function is reduced in patients with rheumatic mitral stenosis. Echocardiogr 2004; 21: 107-12. https://doi.org/10.1111/j.0742-2822.2004.03064.x PMid:14961787 10.Buyukkaya S, Buyukkaya E, Arslan S, Aksakal E, Sevimli S, Gundogdu F, et al. Evaluation of left ventricular long-axis function in cases of rheumatic pure mitral stenosis with atrial fibrillation. Tex Heart Inst J 2008; 35: 22. 11.Falk V, Holm PJ, Iung B, Lancellotti P, Lansac E, Munoz DR, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38: 2739-91. https://doi.org/10.1093/eurheartj/ehx391 PMid:28886619 12.Nobuyoshi M, Arita T, Shirai S ichi, Hamasaki N, Yokoi H, Iwabuchi M, et al. Percutaneous balloon mitral valvuloplasty: A review. Circulation 2009; 119: e211-9. https://doi.org/10.1161/CIRCULATIONAHA.108.792952 13.Ho CY, Solomon SD. A Clinician's guide to tissue Doppler imaging. Circulation 2006; 113: e396-8. https://doi.org/10.1161/CIRCULATIONAHA.105.579268 PMCid:PMC1475954 14.Akiyama K, Arisawa S, Ide M, Iwaya M, Naito Y. Intraoperative cardiac assessment with transesophageal echocardiography for decision-making in cardiac anesthesia. Gen Thorac Cardiovasc Surg 2013; 61: 320-9. https://doi.org/10.1007/s11748-013-0208-6 PMid:23404310 15.Oz TK, Abdelnabi M, Fiore C, Almaghraby A, Cihan D, Babazade N, et al. Assessment of sacubitril/valsartan effects on left ventricular dynamics using 3D echocardiography and 3D strain in heart failure with reduced ejection fraction patients. Minerva Cardiol Angiol 2022; 70: 431-8. https://doi.org/10.23736/S2724-5683.21.05802-6 PMid:34713680 16.Monaghan MJ. Role of real time 3D echocardiography in evaluating the left ventricle. Heart 2006; 92: 131-6. https://doi.org/10.1136/hrt.2004.058388 PMid:16365369 PMCid:PMC1861009 17.Mutnuru PC. Cardiac MR Imaging in the Evaluation of Rheumatic Valvular Heart Diseases. J Clin Diagn Res 2016; Available from: URL: http://jcdr.net/article_fulltext.asp?issn=0973-709x&year=2016&volume=10&issue=3&page=TC06&issn=0973-709x&id=7405 18.Gash AK, Carabello BA, Cepin D, Spann JF. Left ventricular ejection performance and systolic muscle function in patients with mitral stenosis. Circulation 1983; 67: 148-54. https://doi.org/10.1161/01.CIR.67.1.148 PMid:6847794 19.Gaasch WH, Folland ED. Left ventricular function in rheumatic mitral stenosis. Eur Heart J 1991; 12(suppl B): 66-9. https://doi.org/10.1093/eurheartj/12.suppl_B.66 PMid:1936029 20.Hady BMA, Al-Shahawy ES, Fereig HM. Left ventricular function in rheumatic mitral stenosis with or without atrial fibrillation: tissue Doppler and strain imaging study. ????? 2011; 9: 17. 21.Bilen E, Kurt M, Tanboga IH, Kaya A, Isik T, Ekinci M, et al. Severity of mitral stenosis and left ventricular mechanics: a speckle tracking study. Cardiology 2011; 119: 108-15. https://doi.org/10.1159/000330404 PMid:21912124 22.Mukherjee S. Left ventricular function in patients with rheumatic mitral stenosis. J Cardiol Cardiovasc Ther 2018; 11: doi: jocct/JOCCT.MS.ID.555825.php https://doi.org/10.19080/JOCCT.2018.11.555825 23.Guven S, Sen T, Tufekcioglu O, Gucuk E, Uygur B, Kahraman E. Evaluation of left ventricular systolic function with pulsed wave tissue Doppler in rheumatic mitral stenosis. Cardiol J 2014; 21: 33-8. https://doi.org/10.5603/CJ.a2013.0058 PMid:23799550 24.Sowdagar MA, Subba Reddy YV. Immediate impact of successful percutaneous balloon mitral valvuloplasty on right and left ventricular functions: An echocardiographic study using load independent tissue velocity imaging indices. Indian Heart J 2018; 70: 672-9. https://doi.org/10.1016/j.ihj.2017.11.006 PMid:30392505 PMCid:PMC6204446 25.Rajesh GN, Sreekumar P, Haridasan V, Sajeev CG, Bastian C, Vinayakumar D, et al. Effect of balloon mitral valvuloplasty on left ventricular function in rheumatic mitral stenosis. Indian Heart J 2016; 68: 612-7. https://doi.org/10.1016/j.ihj.2015.09.030 PMid:27773398 PMCid:PMC5079113 26.Ferreira MVS, Cunha CRD, Oliviera GS, Otto MEE, Atik FA. Left ventricular remodeling shortly after open mitral valve replacement for rheumatic mitral stenosis. Braz J Cardiovasc Surg 2021; 36: 468-75 https://doi.org/10.21470/1678-9741-2020-0641 PMid:34617428 PMCid:PMC8522327 Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.Archive of Issues
AUTHOR'S CORNER
 Authors having problems with submissions please notify editor: editor@hvt-journal.com
Authors having problems with submissions please notify editor: editor@hvt-journal.com
