Superior vena cava syndrome and pacemaker leads. Explant by mechanical dissection system of extraction and percutaneous recanalization with stents for new device implantation
ORIGINAL RESEARCH ARTICLE
Superior vena cava syndrome and pacemaker leads. Explant by mechanical dissection system of extraction and percutaneous recanalization with stents for new device implantation
Article Summary
- DOI: 10.24969/hvt.2023.372
- CARDIOVASCULAR DISEASES
- Published: 27/01/2023
- Received: 28/12/2022
- Revised: 15/01/2023
- Accepted: 16/01/2023
- Views: 6996
- Downloads: 4448
- Keywords: transvenous lead extraction, balloon angioplasty, cardiac implantable electronic devices, superior vena cava stent. superior vena cava syndrome, Mustard procedure
Address for Correspondence: Elkin Gonzalez Villegas, Department Cardiac Surgery University Hospital La Paz, Paseo de la Castellana 261,28046, Madrid, Spain Email: elgovi17@hotmail.com
ORCID: 0000-0002-4128-4631
Elkin Gonzalez Villegas1, Joan Novo Torres2, Enrique Jose Balbacid Domingo3, Maria Dolores Ponce Dorrego 2, Jose Ignacio Juarez del Rio1, Ulises Ramirez Valdiris1, Jose Carlos Romero Carmona1, Isabel Franco Fernandez1, Rafael Peinado4
1Cardiac Surgery Department, University Hospital La Paz, Madrid, Spain
2Interventional Radiology Department Hospital La Paz, Madrid, Spain
3Pediatric Hemodynamic Department Hospital La Paz, Madrid, Spain
4Electrophysiology Department University Hospital La Paz, Madrid, Spain
Abstract
Objective: Superior venous system stenosis (superior vena cava (SVC) - right subclavian vein - innominate vein - left subclavian vein) is a clinical situation that frequently appears in patients with long-term implanted cardiac stimulation devices, due to venous system thrombosis and in those with congenital heart disease who need corrective surgery, due to chronic complications inherent to surgical techniques. In clinical practice, venous system stenosis may manifest as a SVC syndrome. In many cases, we are not able to correct stenosis or obstructions, since it is impossible to cross them.
In this article, we describe the surgical technique that we have implemented in our hospital to solve this challenge, especially in those patients with pacing/defibrillation devices who present with this pathology.
Our objective was to perform an extraction of the pacemaker and defibrillation electrodes, to allow the passage of a support wire to achieve the implantation of the endovascular stent(s) to correct the SVC syndrome.
Methods: We present a retrospective series of six consecutive patients with SVC syndrome studied in a single center from 2012 to 2021.Three of them presented with thrombosis related to pacing or defibrillation electrodes and the other three presented with complications derived from Mustard or Senning techniques in patients with pacemakers and D-transposition of the great arteries.
Results: In all cases, a complete re-vascularization of the SVC system was achieved using a stent, and new leads could have been implanted through it. Combined treatment of lead extraction and endovascular stent implantation corrected the syndrome in all cases.
Conclusions: Angioplasty and stenting of the central venous system is a standardized technique with validated results, in acute, for the recanalization of chronic occlusions secondary to transvenous devices.
Key words: transvenous lead extraction, balloon angioplasty, cardiac implantable electronic devices, superior vena cava stent. superior vena cava syndrome, Mustard procedure
Introduction
During last decades, implantation of cardiostimulation/defibrillation devices has been gradually increasing as well as indications and surgical techniques complexity.
Consequently, complications rate has also increased, and new clinical situations have been appearing during the last years. Veins obstruction due to thrombosis or surgical duct stenosis after atrial repair techniques such as Mustard or Senning, in patients with D-transposition of the great arteries (D-TGA), is an example of one of these new complications that we are observing in daily clinical practice (1–3).
The incidence of partial venous obstruction in patients with pacing and defibrillation devices is usually between 31% and 64%, although most patients remain asymptomatic because of collateral circulation developing. Superior vena cava syndrome (SVCS) is only observed in 0.6%-3.5% of the patient population (2, 4, 5). The most frequent symptomatology is dyspnea, accompanied by dizziness and vision disorders. Clinical examination is characterized by edema in the upper trunk (face, neck and upper limbs) and jugular engorgement (4, 5).
The SVCS treatment includes corticosteroids and anticoagulation, although angioplasty and surgery with endovascular stents is the preferred therapeutic option (1, 6, 7). It is important to remark that venous accesses must be permeable in the cases when endovascular stents are needed.
In this article, we describe the surgical technique that we have implemented in our hospital to solve this challenge, especially in those patients with pacing/defibrillation devices who present with this pathology.
Our main objective was to remove the electrostimulation system, leave a guidewire in the venous system, which would allow the stent to be implanted, which, in turn, would allow the venous system to be re-vascularized and the new pacing system to be implanted through the venous system once it was permeable.
Methods
Study design and population
This is a retrospective, single-center study, of a population of patients consecutively included, from January 2012 to December 2021. Data were collected from the prospective database (BSICCS) of our Service. The study was approved by the hospital's Clinical Research Ethics committee.
Inclusion criteria: We included all patients referred to our department with implanted pacing/defibrillation devices with SVC syndrome and severe vein obstruction that did not allow the passage of a guidewire for the re-vascularization of the venous system with an endovascular stent.
Description of the procedure and material used
Patients were admitted 48 hours before the procedure, and before the intervention, blood count, biochemistry, coagulation study and transesophageal echocardiogram were performed. Patients with valve prostheses underwent antibiotic prophylaxis according to the American Heart Association (AHA) endocarditis guidelines (8, 9). Anticoagulation was discontinued 48 hours before procedures performed. A chest X-ray was also performed in postero-anterior and lateral projections, as well as computed axial tomography (CT), angiography and vascular Doppler ultrasonography.
All procedures were performed in the operating room, under general anesthesia and transesophageal echocardiography as a control method. Two peripheral routes (right and left arm), arterial monitoring and jugular venous access were cannulated. A temporary pacemaker was implanted by femoral approach, even if the patient was not pacemaker dependent (see details of procedure in each patient below).
All the procedures were performed after obtaining informed consent of patients and study complies with Helsinki declaration on human studies (2013).
Definitions
Superior vena cava syndrome: SVCS is a mechanical obstruction of the superior vena cava (SVC) by venous thrombi or extrinsic compression by intrathoracic tumors in most cases. The main associated symptoms are: dyspnea, Stokes' neck, distension of the neck veins and venous network in the thorax (5, 10).
Lead extraction and stent implantation - surgical technique
Lead extraction was performed in all cases using Evolution® mechanical dissection tools (Cook Medical, USA), which, with its external sheath, allowed us to implant the stent and subsequently implant the new stimulation device.
The procedures were performed in hybrid operating rooms, under general anesthesia, together with the interventional radiology and pediatric hemodynamic services. Wallstent-type coaxial prostheses (Boston Scientific, USA), with an adequate size for each case or balloon expandable stents (CP stent®, Numed USA) "of greater radial strength" were used in the patients with congenital heart disease where stenosis predominated. Finally, the device (pacemaker/ implantable cardioverter-defibrillator, ICD) is implanted coaxially, according to the usual technique, through the stent lumen.
In all patients, the normal stylet was used to verify the permeability of the leads and subsequently the locking stylet (Liberator, Cook Medical) was introduced.
In case of complete obstruction of the lumen, it was of great help that through the electrode itself a wire was left, and once extracted, enabled to advance the sheath and place the stent. Subsequently, the device (pacemaker or ICD) was re-implanted, according to the usual technique, coaxially through the stent lumen. The patency of the vein, closure of the defect and normal function of the cardiostimulation device were verified by means of phlebography.
Statistical analysis
The descriptive statistics is used. Data are presented as number and mean (SD).
Results
A series of 6 pacemaker or ICD patients with SVCS are presented (Table 1). Patient´s ages were between 18 and 76 years old (mean 47.1 and standard deviation of 22.78). They all underwent an extraction of the electrostimulation leads and implantation of endovascular stent. Three of them suffered a stenosis after the repair of atrial switch in D-TGA (patients 1, 3 and 4). The other three patients suffered SVC thrombosis. (Patients 2, 5 and 6). All had symptoms consistent with SVCS, dyspnea, facial edema and venous neck distention.
The complementary examinations that allowed the diagnosis were CT, angiography and vascular Doppler ultrasonography, which allowed us to determine the degree of obstruction, the place and the flow (Fig. 1, 2).
Electrostimulation electrodes were responsible for chronic central venous thrombosis in the three patients previously referred. The first presented with thrombosis 48 months after implantation and the second after 204 months (Fig. 3). The third patient had undergone a left subclavian lead extraction procedure, with a 252-month-old lead and 312-month ventricular lead (mean 282 months). The extraction of the leads was carried out with a mechanical dissection tool. After 1 month, the patient presented with symptoms compatible with SVCS despite anticoagulation and venous balloon dilation (Fig. 4), and required the implantation of a stent in the SVC to recanalize the venous system.
The common feature of the cases was the need for a hybrid procedure in a single time to remove the old electrodes, recanalize the SVC and subsequently carry out a new implant of electrodes. The main difficulties of the procedure were the endovascular stent implantation, as well as the stimulation leads extraction. The severe obstruction did not allow the passage of a wire.
In the case of the 18-year-old patient (Patient 1) Table 1, the 96-month-old atrial lead was removed by laser technique at age 14, due to obstruction of the SVC canal. The post-extraction venography initially showed permeabilization of the canal, so stent implantation was not necessary (Fig. 5). Four years later the patient returned, showing obstruction of the canal (despite the anticoagulation), so a stent was implanted.
In the other 2 congenital patients, 24 and 47 years old (patients 3 and 4) (Table 1), the obstruction was due to dehiscence of the atrioventricular patch which generated a stenosis of the superior cava canal.
Procedural outcomes
In patients with D-TGA (patients 1, 3 and 4), a re-permeabilization of the surgical defects was achieved and new cardiostimulation device was implanted. They are currently asymptomatic.
In patients with SVC syndrome, patient 5 presented in-stent restenosis after 60 days. The patient required explantation of the defibrillator lead and implantation of a subcutaneous ICD. He is now under anticoagulation therapy.
In our series, one death was recorded after 30 days from the procedure in a pluripathological patient, with heart and kidney failure, and liver stasis. (Patient 2). Patient number 6 is asymptomatic. Table 1.
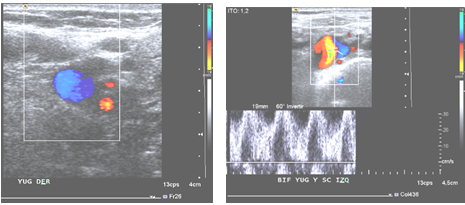
Figure 1. Doppler ultrasound view of right jugulo-subclavian junction, where permeability of the axis is appreciated in its visualizable portion, with spontaneous phasic flow related to respiratory maneuvers
|
Table 1. Patients, procedure characteristics and outcomes |
|||||||
|
Patient number |
Age and gender |
Electrode location |
Chronicity of leads (months) |
Indication of intervention |
Surgical technique |
Outcome |
|
|
Patient 1 |
18 years. Female |
RA |
144 (Laser technique) 48 (mechanical dissection) |
D-TGA. AV canal obstruction |
Laser technique for extraction. Re-stenosis 4 years later. Lead removal with dissection tool. VCS canal stent implant |
Asymptomatic 2 years |
|
|
Patient 2 |
76 years. Female |
RA |
252 |
SVC system thrombosis |
Covered stent implant in SVC |
Mortality 30 days in hospital |
|
|
RV |
204 |
||||||
|
Patient 3 |
24 years. Male |
RV |
288 |
D-TGA Dehiscence of the atrial patch and stenosis of the upper cava canal. |
Covered stent implant in SVC canal |
Asymptomatic 7 years |
|
|
Patient 4 |
47 years. Male |
RA |
6 |
D-TGA. Dehiscence of the upper cava canal |
Covered stent implant in SVC canal |
Asymptomatic 4 years |
|
|
RV |
6 |
||||||
|
Patient 5 |
66 years. Male |
RV |
54 |
SVC system thrombosis |
Covered stent implant in SVC |
In-stent restenosis 60 days |
|
|
Patient 6 |
52 years. Female |
RA |
3 (first implant 252) |
SVC system thrombosis |
Covered stent implant in SVC |
Asymptomatic 11 years |
|
|
RV |
3 (first implant 312) |
||||||
|
AV –antrioventricular, RA - right atrium, RV - right ventricle, SVC - superior vena cava, TGA –transposition of large arteries |
|||||||
Discussion
SVCS secondary to pacemaker-induced thrombosis is uncommon. In some studies (3, 11) only one case was reported in 3100 implants during 10 years of follow-up in their institution and other three cases referred to other centers. Another study cites an incidence of four in 1000 cases (12). Multiple leads presence and previous infection appear to be the most important etiological factors.
Some patients with transposition of the great arteries corrected by atrial switch techniques have pacemakers because of sinus dysfunction or AV block. Superior vena cava syndrome due to obstruction is not uncommon in these cases, but the presence of leads in the SVC canal is an added risk factor. Considering that this pathology requires a derivation of the venous blood (cava`s to the subpulmonary left ventricle), through tunnels with patches, these can become stenosed and/or present dehiscence, which can be corrected with the implantation of a stent, which solves the defect or enlarges the canal (1).
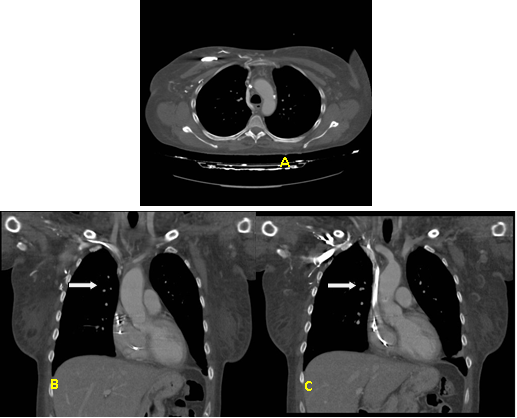
Figure 2. Thoracic computed axial tomography with intravenous contrast. A: Axial cut. B and C: Coronal reformatting MPR. Occluded left brachiocephalic venous trunk (white arrow) and decrease in caliber of the superior vena cava with repletion defects compatible with partial thrombus in its proximal third.
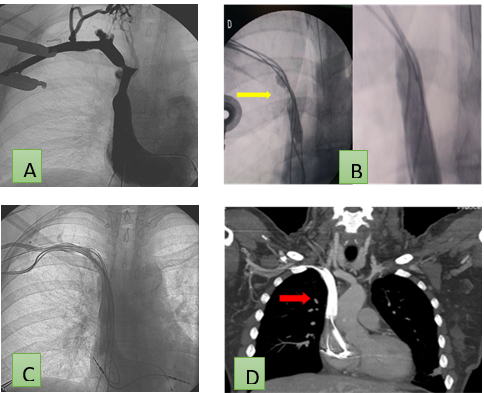
Figure 3. Phlebography of the central venous system. Significant focal stenosis in the right unnamed trunk (A). Balloon angioplasty (yellow arrow) (B) and stent implantation (C). Computed axial tomography with MPR reconstruction in coronal plane where permeable stent is observed with MP wires through it (arrow) (D)
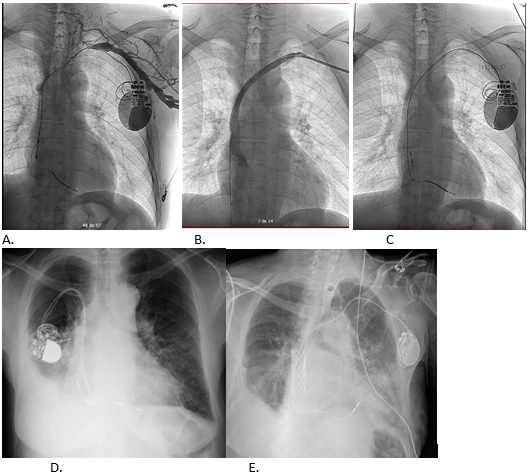
Figure 4. Phlebography of the central venous system. Long segmental occlusion in the left unnamed trunk (A). Recanalization and stent implantation (B). Re-implantation of pacemaker (C). Pacemaker inserted via right subclavian (D) and left subclavian with stent in vena cava superior (E)
Conventional angioplasty has been first choice technique in SVC syndrome cases; however, in cases of recurrence the placement of a stent in the affected venous system is an alternative, which reverses the symptomatology and allows the intra-stent implantation of stimulation leads. Traditionally, the implantation of stent in VCS has been reserved for the treatment of tumoral VCS syndrome (4, 13).
In our cases, the percutaneous extraction of the leads helped us, when leaving a wire (Fig. 6) that crosses the problem area to the ventricle and thus facilitating the implantation of the stent. There are documented cases where the caged stent implant of the electrode can cause dysfunction of the electrode (1, 15). In this type of patients, the tendency from this experience in our center is towards the systematic implantation of stents in SVC even with minimal degrees of stenosis if an intravenous stimulation system is expected to be necessary. The increase in the life expectancy of patients suffering from congenital heart disease will increase in all those susceptible to presenting this clinical situation.
Post-explant venography and transesophageal ultrasound are important tools to determine the presence of fibrin filaments and remains of intravascular adhesions generated by the extraction maneuvers, because considering the amount of them in the venous system, it is a prognostic factor for the development of venous thrombosis (12,14,16,17) (Fig. 7).
Premature anticoagulation should be considered in those patients with high risk of post-explant thrombosis (18); such as young patients under 30 years of age with long-lasting leads, multiple leads carriers or patients with partial venous system thrombosis, corroborated with venography before new implant.
In our center, we also developed an action algorithm, which guides us in cases of superior vena cava syndrome and the removal of pacing/defibrillation leads (Table 2).
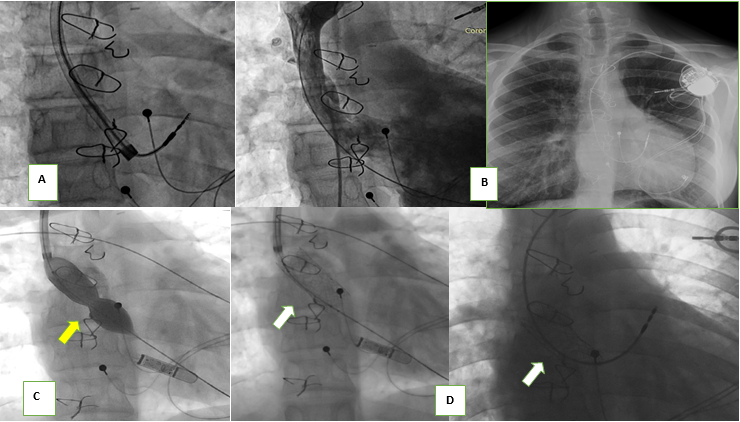
Figure 5. Atrial electrode explantation with laser system (A) in a patient with D-TGA and VCS-left atrium shunt (Mustard) who had associated stenosis/thrombosis. After the extraction of the electrode, VCS permeability (B) is observed instead and a new intravenous electrode is implanted (C) but 4 years later restenosis of the VCS canal is demonstrated, thus requiring stent implantation (D).
DTGA – D- transposition of great vessels, VCS – vena cava superior
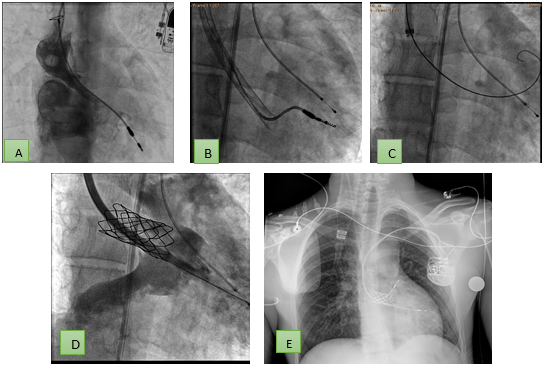
Figure 6. Stenosis associated with VCS canal dehiscence in a patient with D-TGA that requires removal of the ventricular electrode (A) and upgrade is performed after stent placement (C and D) to a dual chamber system (E). Note that the wire allowing stent angioplasty is advanced through the extraction system itself facilitating the procedure. DTGA – D- transposition of great vessels, VCS – vena cava superior
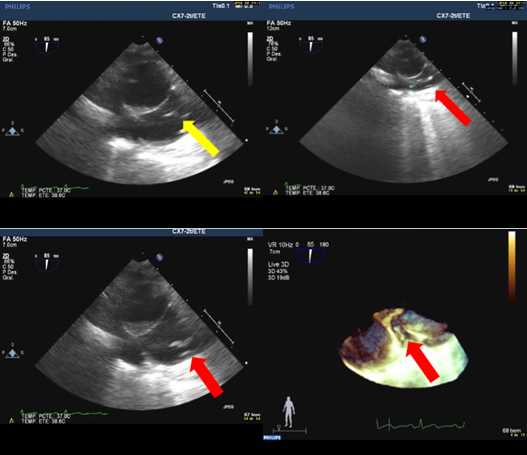
Figure 7. Transesophageal ultrasound of a 53-year-old patient who has an ICD lead extraction, 12 years of chronicity. Fibrin paths can be seen at the superior vena cava and at the mouth of the cava in the right atrium, as a product of the dissection of the lead. ICD – implantable cardioverter-defibrillator
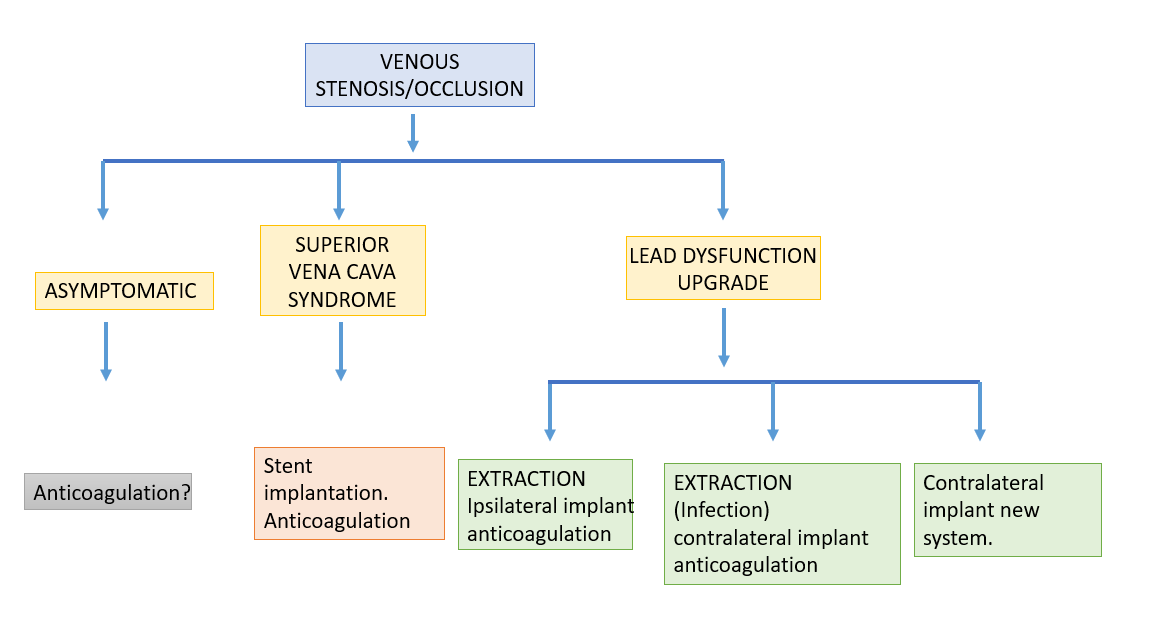
Figure 8. Algorithm of pacing/defibrillation leads removal in cases of superior vena cava syndrome
Study limitations
The main limitation of our study is the retrospective design, although the data were collected from a prospective database and the inclusion of patients was consecutive. The patients with D-TGA (patients 1, 3 and 4) were referred to our center from other hospitals. Follow-up was not carried out in our center, although, we have corroborated any information by phone with the patients` reference center.
Conclusion
Venous thrombosis and stenosis secondary to transvenous pacemakers/ICDs is relatively common, especially when previous surgical modifications were performed. Clinical manifestations are unusual and superior vena cava syndrome is rare. Although electrodes are still functional, explant them as a way to create a new venous access in order to implant an endovenous stent, appears as a valid surgical technique.
Angioplasty and stenting of the central venous system is a standardized technique with validated results, in acute, for the recanalization of chronic occlusions secondary to transvenous devices.
Ethics: Informed consent was obtained from all patients before procedure. The study protocol was approved by hospital's Clinical Research Ethics committee.
Peer-review: External and internal
Conflicts of interest: None to declare
Authorship: E.G.V., J.N.T., E.J.B.D., M.D.P.D., J.I.J..del R., U.R.V., J.C.R.C., I.F.F., R.P.P. equally contributed to the study and manuscript preparation.
Acknowledgement and funding: None to declare. This research received no specific grant for funding agency in the public, commercial or not-for-profit sectors.
References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

