Subclavian coronary steal syndrome in a post coronary artery bypass grafting patient: A case report
CASE REPORT
Subclavian coronary steal syndrome in a post coronary artery bypass grafting patient: A case report
Article Summary
- DOI: 10.24969/hvt.2023.385
- CARDIOVASCULAR DISEASES
- Published: 02/05/2023
- Received: 12/03/2023
- Revised: 25/04/2023
- Accepted: 26/04/2023
- Views: 6486
- Downloads: 4081
- Keywords: coronary steal, post coronary artery bypass grafting, refractory angina, subclavian steal
Address for Correspondence*: Riyaz Charaniya, Department of Cardiology, U. N. Mehta Institute of Cardiology and Research Centre, Civil Hospital Campus, Asarwa, Ahmedabad-380016, Gujarat, India Email: riyaz.doc@gmail.com
Mobile: +919910198836, Fax: +9107922682092
Rujuta Parikh, Jayal Shah, Abhishek Shah, Riyaz Charaniya*
Department of cardiology, U. N. Mehta Institute of Cardiology and Research Centre (UNMICRC), Civil Hospital Campus, Asarwa, Ahmedabad-380016, Gujarat, India
Abstract:
Objective: “Subclavian coronary steal” refers to diversion of blood flow from coronary bed to subclavian artery. In patients receiving internal mammary grafts during coronary artery bypass grafting surgery (CABG), aorta and its proximal branches become part of coronary circulation. Atherosclerotic occlusion of subclavian artery can manifest as subclavian coronary steal syndrome and patients may present with angina, myocardial infarction and even sudden cardiac death. The objective of our case report is to identify and present important non-conventional causes of angina for better management of patients.
Case presentation: A 50-year-old, hypertensive, male patient with a prior history of having undergone CABG, presented with CCS class III angina of one-month duration. He was found to have a completely occluded left subclavian artery from origin with retrograde flow of blood in left internal mammary artery from coronary to subclavian artery. Percutaneous transluminal angioplasty with stenting to subclavian artery was performed. Antegrade flow was established and coronary steal through left internal mammarian artery graft was thus abolished with subsequent resolution of symptoms.
Conclusion: We have presented a case of refractory angina in post coronary artery bypass grafting patient who was eventually treated with percutaneous stent implantation to treat the subclavian stenosis; and had complete resolution of symptoms post intervention.
Take home message: Patients undergoing CABG should be screened for a possible asymptomatic subclavian stenosis that may become symptomatic after CABG. Also, subclavian coronary steal syndrome should be suspected in patients presenting with refractory angina post CABG.
Key words: coronary steal, post coronary artery bypass grafting, refractory angina, subclavian steal
Introduction
Coronary steal refers to the phenomenon of diversion of blood flow away from coronary vasculature. In a patient with a prior history of coronary artery bypass surgery (CABG) with internal mammary graft, subclavian coronary steal occurs due to development of severe stenosis in subclavian artery proximal to the graft. Subclavian stenosis may develop as a result of atherosclerosis, Takayasu arteritis, radiation, fibromuscular dysplasia or extrinsic compression. The proximal subclavian artery is three times more prone to atherosclerosis due to shear stress occurring as a result of acute angulation of subclavian artery from aorta (1). Subsequent reduction in blood flow to upper limb can cause the retrograde blood flow in internal mammary graft from coronary to subclavian artery, thus causing a steal phenomenon (Fig. 1).
Herein, we present a case of such coronary steal presenting to us with refractory angina and subsequent resolution by percutaneous stenting of subclavian artery.
Case report
A 50-year-old, hypertensive, male patient with a prior history of non-ST-elevation myocardial infarction (NSTEMI), having undergone coronary artery bypass grafting (CABG) six months previously, presented with angina of one-month duration, in functional class III. Despite being on maximal antianginal therapy in the form of beta blockers, nitrates, nicorandil, trimetazidine and ranolazine, he had persistent chest pain on minimal exertion with radiation to left arm. During CABG he had received left internal mammary artery graft to left anterior descending artery (LAD) and saphenous vein grafts to posterior descending artery (PDA) and obtuse marginal branch (OM).
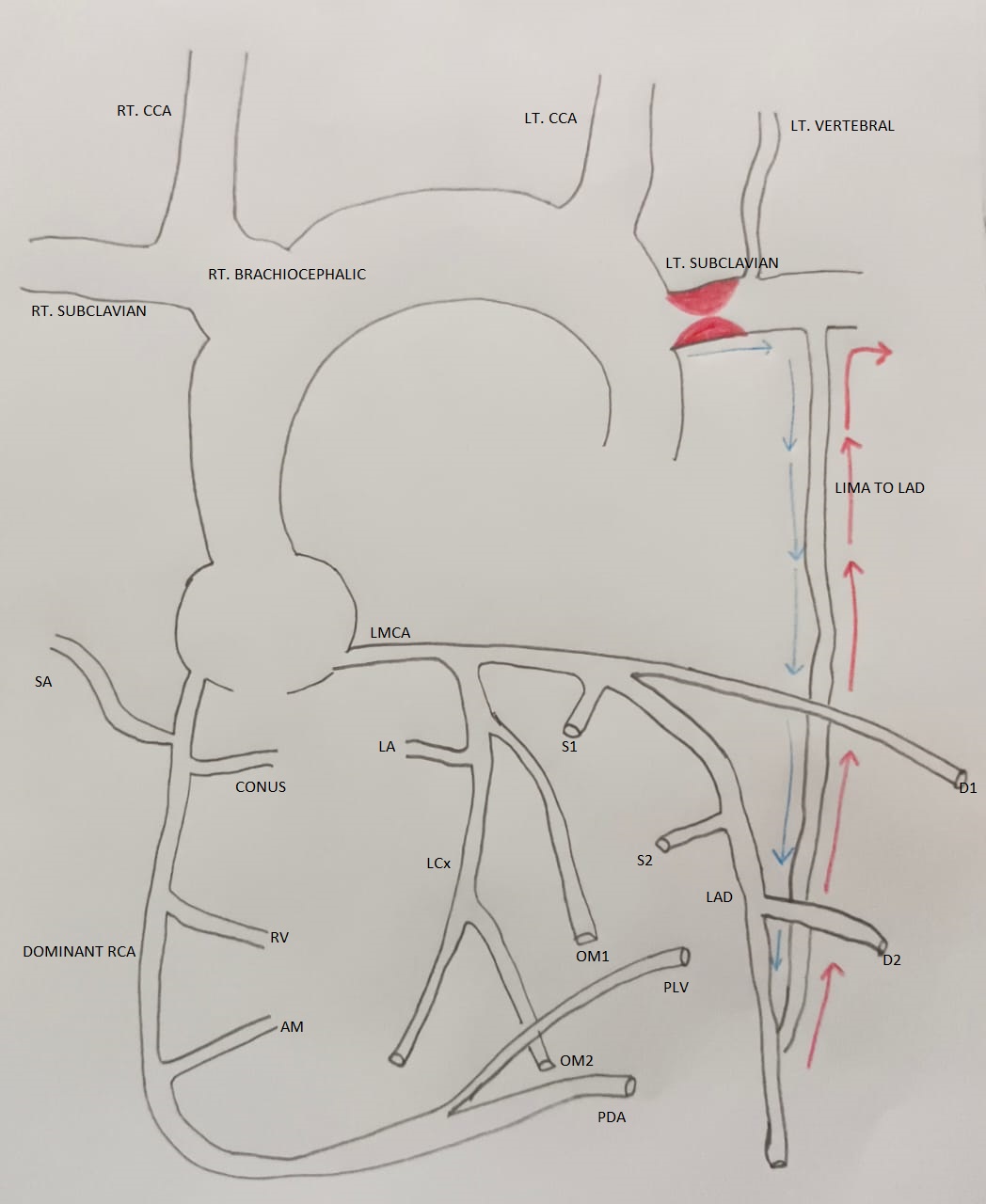
Figure 1. Pathophysiology of subclavian coronary steal syndrome
AM - acute marginal, artery, CABG – coronary artery bypass surgery, CCA - common carotid artery, D – diagonal branch, LA - left atrial branch, LAD - left anterior descending artery, LCx - left circumflex artery, LIMA – left internal mammary artery, LMCA - left main coronary artery, LT - left, OM- obtuse marginal branch, PDA - posterior descending artery, PLV - posterior left ventricular artery, RCA - right coronary artery, RT - right, RV - right ventricle, OM S – septal branch, SA - SA node
*Blue arrow indicates expected flow through LIMA post CABG, red arrow indicates reversal of blood from coronary to subclavian due to stenosis proximal to LIMA
On physical examination, left radial pulse was absent and right radial pulse had normal volume with equal pulses in both lower limbs. There was no supraclavicular bruit. His electrocardiogram (ECG) showed non-specific ST segment changes (Fig. 2) and echocardiogram showed normal left ventricle size with ejection fraction of 55%. Troponin I was <1.9 ng/mL (negative).
In view of persistent angina, coronary angiogram was done, which showed native triple vessel disease with patent left internal mammary artery (LIMA) to LAD graft with retrograde flow in LIMA from LAD to subclavian artery with completely occluded left subclavian artery from origin (Fig. 3).
Patient was taken for percutaneous transluminal angioplasty (PTA) to subclavian artery. Right femoral artery was accessed and Judkins right catheter was used to cross the lesion with 0.035" Terumo wire. Pre-dilation was achieved with 5x20mm balloon (Fig. 4) and 7x23mm stent (Fig. 5) was deployed in the subclavian artery. Antegrade flow was established and coronary steal through LIMA graft was thus abolished (Fig. 6).
Post procedure, patient had an uneventful recovery with complete resolution of symptoms.
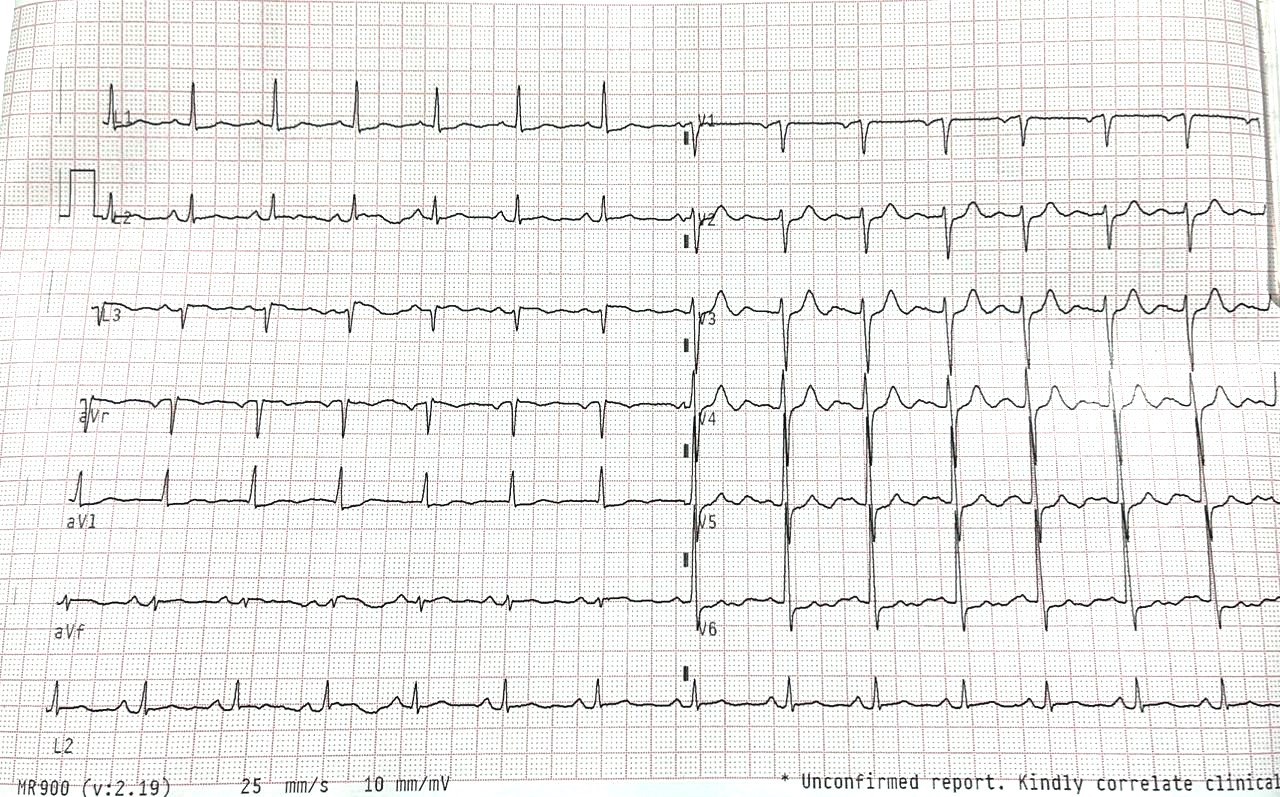
Figure 2. Electrocardiogram on presentation
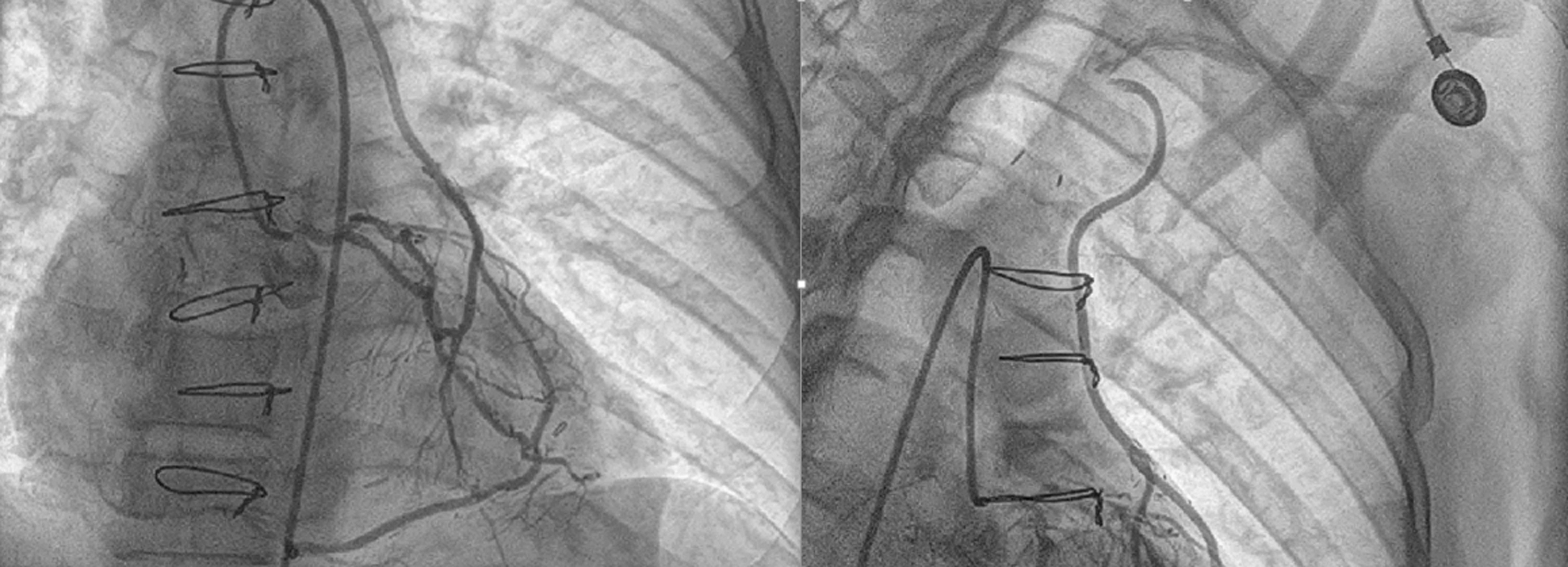
Figure 3. Retrograde filling of LIMA from LAD to left subclavian artery
LAD – left anterior descending artery, LIMA – left internal mammary artery
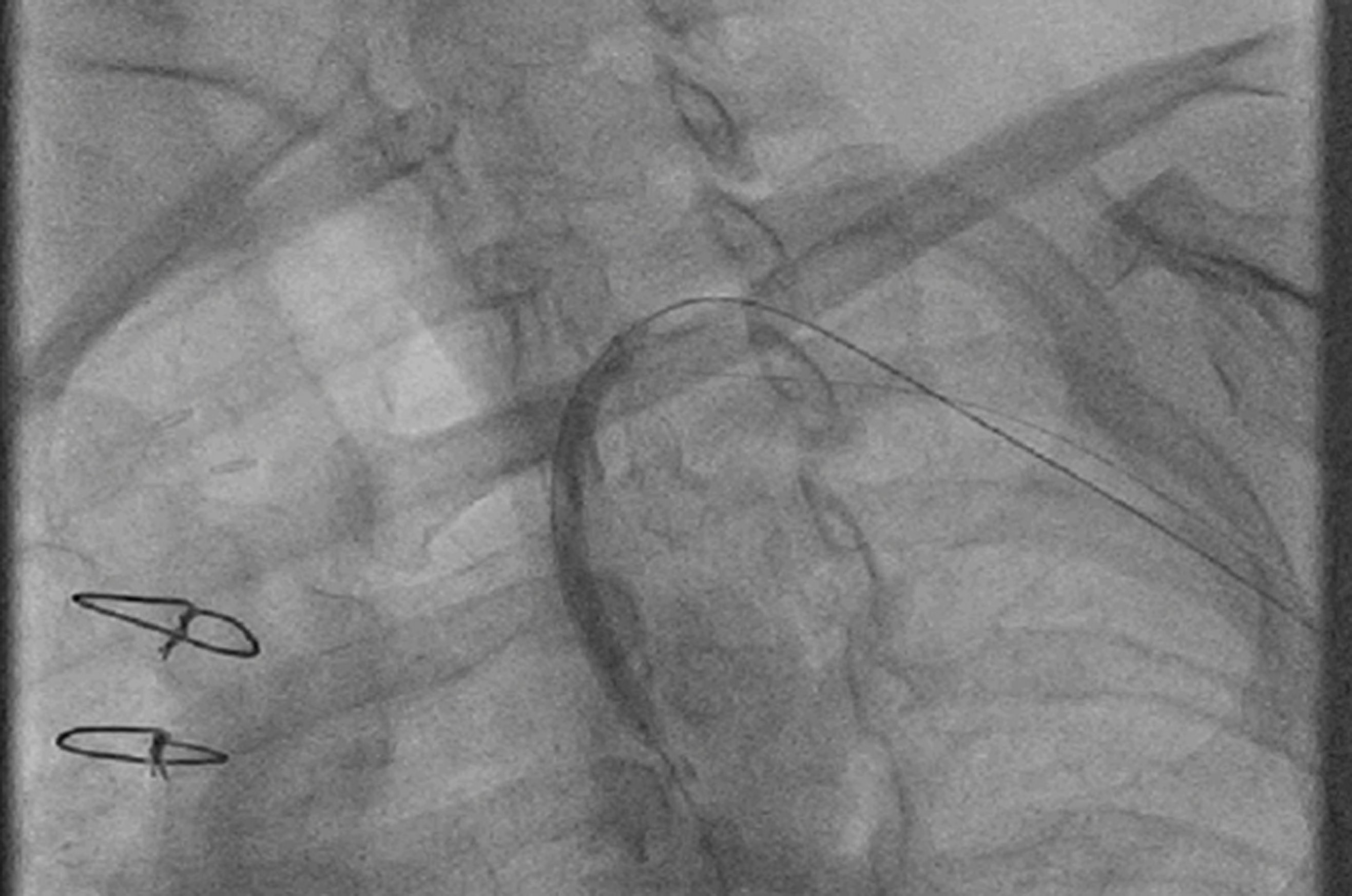
Figure 4. Pre-dilation after crossing of wire
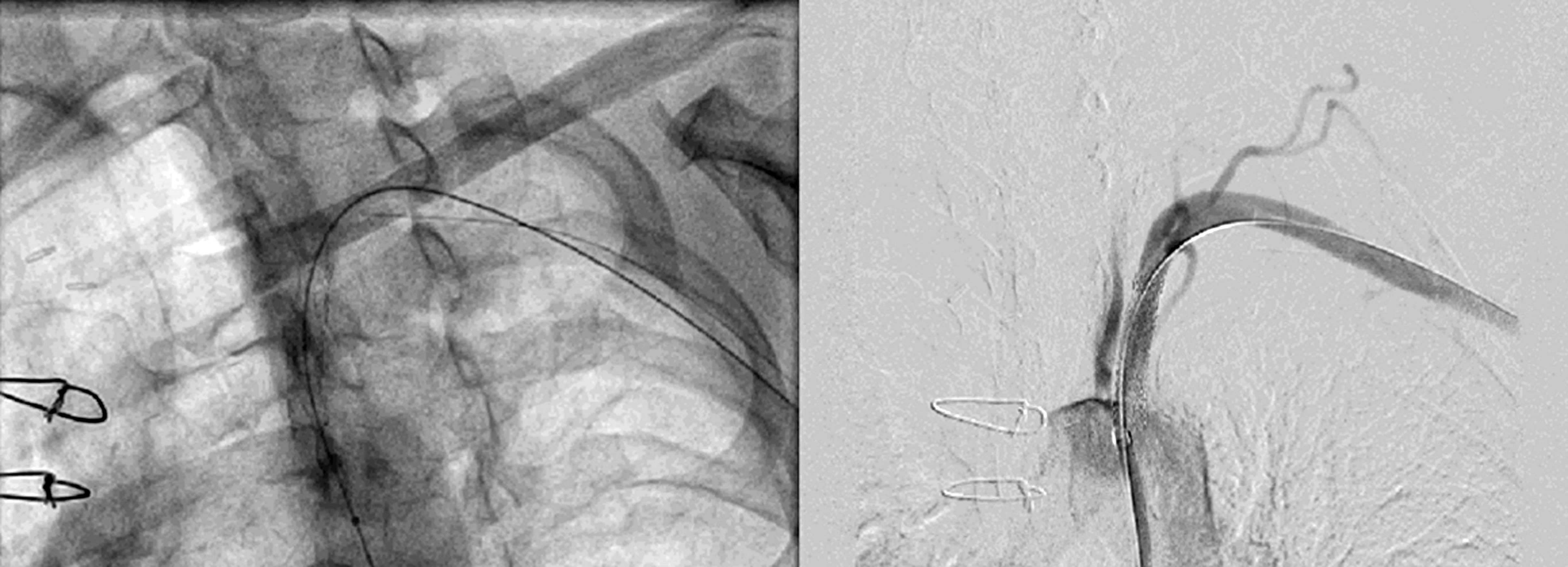
Figure 5. Post stent placement antegrade flow was established in left subclavian artery
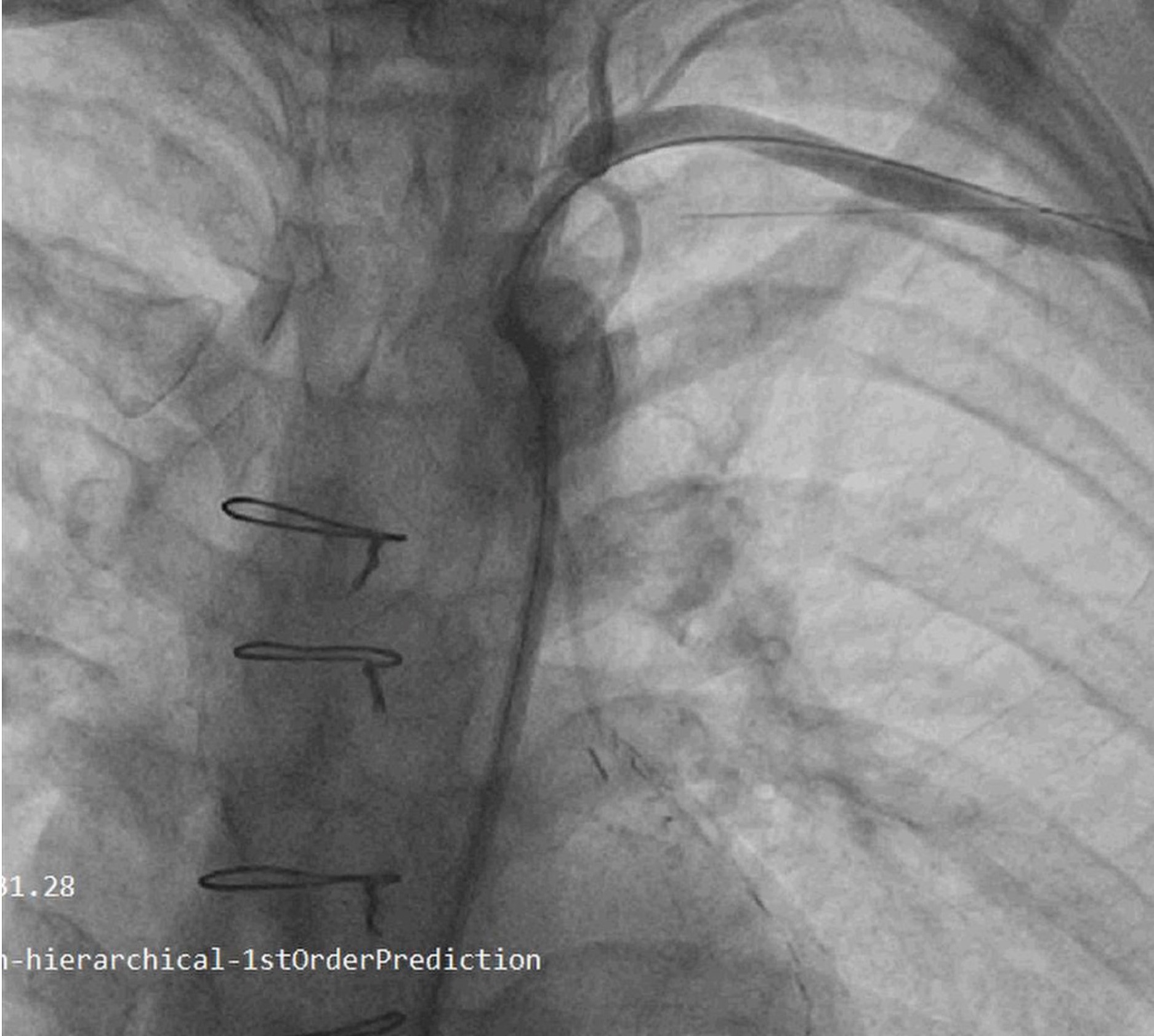
Figure 6. Subclavian angiogram showing antegrade flow through LIMA to LAD
LAD – left anterior descending artery, LIMA – left internal mammary artery
Discussion
The prevalence of flow limiting stenosis in subclavian artery has been estimated to be 1.46% in patients planned for CABG (2). Subsequent development of coronary steal syndrome post CABG is a rare complication occurring in 0.44% of patients (3). Previously similarly reported cases are shown in Table 1.
Our case report suggests the importance of screening for subclavian stenosis in patients undergoing CABG and also development of new atherosclerotic subclavian lesion may be suspected in patients presenting with angina post CABG.
|
Table 1. Previously reported similar cases |
||||||||
|
No. |
Author and year |
Patient details |
Pre CABG Subclavian assessment |
CABG details |
Time interval and symptoms post CABG |
Diagnostic modality for SCS |
Treatment |
Follow-up |
|
1 |
Monteagudo-Vela et al4, 2023 |
62 year, male, prediabetes, smoker, STEMI, LVEF 30% |
Clinical examination and bilateral equal pulse |
LIMA to LAD, SVG to RI and PDA |
48 hours, Cardiogenic shock |
CAG |
Coiling and microvascular plugging of LIMA with PTCA with sirolimus DES from LMCA to LAD |
6 months follow up, NYHA I |
|
2 |
Steinberger et al 5, 2022 |
73 yo, female, ischemic cardiomyopathy, LVEF 35%, AICD (primary prevention) |
Not known |
LIMA to LAD, SVG to OM |
Angina, sustained VT, ineffective AICD |
CAG |
PTCA to LAD with DES |
Discharged |
|
3 |
Gill et al,6 2022 |
69 yo, male |
Not known |
LIMA to LAD |
Angina and left arm claudication |
CTA |
PTA to LSA with atherectomy and filter wires |
Discharged |
|
4 |
Waduud et al7, 2018 |
68 years, male, hypertensive, dyslipidemic, smoker, unstable angina |
Clinical examination |
LIMA to Diagonal, SVG to LAD, OM and RCA and PTCA to SVG to OM |
16 years, anterior wall STEMI |
CAG |
Primary PTCA to SCG to LAD and PTA to subclavian artery |
Discharged |
|
5 |
Vasigh et al8, 2020 |
64 years, female, diabetic, smoker, dyslipidemic, with h/o PAOD and HFrEF with h/o coronary subclavian steal |
Clinical examination |
SVG to RCA, D1, LIMA-to-LAD T-graft (radial artery) to LCx followed by PTCA to SVG twice and PTA to LSA |
15 years, Inducible ischemia on stress test with technetium 99-m sestamibi |
CAG |
PTA to LSA, ISR |
Discharged |
|
6 |
Walensi et al9, 2021 |
71 years old, female, h/o AF, hypertension, diabetes, hypercholesterolemia, obesity, PAOD, PAH, chronic kidney disease, hepatitis B and ICA stenosis with thrombendarterectomy |
Not known |
LIMA to Ramus circumflexus (RCX), RIMA to LAD |
11 years, NSTEMI, syncope, chest pain after left arm straining |
Duplex ultrasound, CTA and CAG |
left-sided carotid-subclavian bypass |
12 months, NYHA I |
|
Table 1 - continued |
||||||||
|
No. |
Author and year |
Patient details |
Pre CABG Subclavian assessment |
CABG details |
Time interval and symptoms post CABG |
Diagnostic modality for SCS |
Treatment |
Follow Up |
|
7 |
Baghaffar et al10 |
71 year, male, hypertensive, diabetic |
Not known |
LIMA to LAD, SVG to OM |
2 years, NSTEMI with heart failure |
CAG |
left carotid to subclavian artery bypass |
Discharge |
|
70 yo, female, hypertensive, h/o, dyslipidemia, diabetic |
Not known |
LIMA to LAD, SVG to diagonal, OM, and PDA |
10 years, NSTEMI with heart failure |
CAG |
left carotid to subclavian artery bypass |
Discharge |
||
|
AF – atrial fibrillation, CAG – coronary angiography, CABG – coronary artery bypass surgery, CTA – computed tomography angiography, D – diagonal branch, DES – drug-eluting stent, h/o – history of, HFrEF – hear failure with reduced ejection fraction, ICA – internal carotid artery, LAD - left anterior descending artery, LCx - left circumflex artery, LIMA – left internal mammary artery, LMCA - left main coronary artery, LVEF – left ventricular ejection fraction, NSTEMI – non-ST-elevation myocardial infarction, OM- obtuse marginal branch, PAOD – peripheral arterial obstructive disease, PAH – pulmonary arterial hypertension, PDA - posterior descending artery, PTA – percutaneous transluminal angioplasty, PTCA – percutaneous coronary transluminal angioplasty, RCA - right coronary artery, RI – ramus interventricularis, RIMA- right internal mammary artery, SCS – subclavian coronary steal syndrome, STEMI – ST-elevation myocardial infarction, SVG – saphenous vein graft, yo – years old |
||||||||
Routinely, history and physical examination findings such as history of upper limb claudication, cerebrovascular ischemia, supraclavicular bruit and difference in both upper limb blood pressure >15 mmHg may be useful (11). Duplex imaging can provide reliable information on internal mammary artery diameter, peak systolic blood flow and mean blood flow and has been found to correlate with intraoperative measurements (3). Other methods like computed tomography coronary angiography and magnetic resonance angiography may be used for diagnosis. Definitive diagnosis of subclavian stenosis is made by peripheral angiogram.
Treatment options of coronary subclavian steal syndrome include endovascular or surgical intervention. Percutaneous transluminal angioplasty has a technical success rate of 98% in critical stenosis and 90% in cases of total occlusion. A patency rate of 89% is observed at 3 years follow up (12). Complications like access site hematoma, distal embolization, in-situ thrombosis, dissection and stroke has been reported in 0.9 to 1.4% of patients (12). Restenosis rate was reported to be 12% at 5-year follow up (13).
In patients with lesion length more than 5 cm, severe calcification, and lesion near to ostium of vertebral artery or in patients with concomitant brachiocephalic artery disease, surgical intervention in the form of carotid-subclavian, carotid-axillary or aorto-axillary bypass may be required. Carotid-subclavian bypass has a 10-year patency rate of 92% (14).
Conclusion
Subclavian steal occurring from vertebral circulation has been well documented. However, subclavian steal occurring from coronary circulation is not that common an entity. Coronary subclavian steal should be suspected in refractory angina occurring in post CABG patient. Asymptomatic subclavian stenosis should be ruled out in all patients prior to undergoing CABG.
Take home message: Patients undergoing CABG should be screened for a possible asymptomatic subclavian stenosis that may become symptomatic after CABG. Also, subclavian coronary steal syndrome should be suspected in patients presenting with refractory angina post CABG.
Ethics: Written informed consent was obtained from patient before all procedures.
Peer-review: Internal and external
Conflict of interest: None to declare
Authorship: R.P., J.S., A.S, and R.C. equally contributed to the management of patient and preparation of manuscript.
Acknowledgement and funding: This work was supported by U. N. Mehta Institute of Cardiology and Research Centre itself and received no specific grant from any funding agency, commercial or not for profit sectors.
References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

