ESC guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery: a practical guide for clinicians
EDITORIALS
ESC guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery: a practical guide for clinicians
Article Summary
- DOI: 10.24969/hvt.2023.393
- CARDIOVASCULAR DISEASES
- Published: 31/05/2023
- Received: 28/05/2023
- Accepted: 29/05/2023
- Views: 15696
- Downloads: 4540
- Keywords: editorial
Address for Correspondence: Giuseppe Iuliano, Cardio-Thoracic-Vascular Department, University Hospital “San Giovanni di Dio e Ruggi d’Aragona”, Heart Tower – Room 807. Largo Città d’Ippocrate, 84131 Salerno, Italy.
Phone: +39 3465335232 E-mail: gi.iuliano93@gmail.com
Giuseppe Iuliano, Rodolfo Citro
Cardio-Thoracic-Vascular Department, University Hospital “San Giovanni di Dio e Ruggi d’Aragona”, Largo Città d’Ippocrate, 84131 Salerno, Italy
In 2022 European Society of Cardiology (ESC) released the new guidelines on cardiovascular (CV) assessment and management of patients undergoing non-cardiac surgery, which are substantially a deeply revised version of 2014 guidelines (1, 2). The increasing aging of the world's population and the rising burden of CV disease lead clinicians to evaluate on daily basis patients at high risk of developing CV complications in the peri-operative setting. Such complication may particularly occur in patients with significant coronary artery disease (CAD), myocardial dysfunction, valvular heart disease (VHD) and arrhythmias. In addition, the type and duration of surgery are critical in stratifying each patient peri-operative risk, as any surgery carries a specific hemodynamic stress and hemorrhagic risk. When concerning peri-operative risk of myocardial ischemia, three leading mechanisms have been pointed out: oxygen supply-demand mismatch due to peri-operative fluctuating coronary perfusion on top of non-critical coronary artery disease (type II myocardial infarction); stress induced atherosclerotic plaque erosion/rupture and thrombosis due to surgical-related prothrombotic and inflammatory state (type I myocardial infarction); stent thrombosis in patient with recent history of percutaneous coronary intervention (PCI) due to anti-platelet therapy interruption for preventing surgical-associated bleeding. These guidelines are aimed to help clinicians in standardizing an evidence-based approach to perioperative CV management, to reduce CV morbidity and mortality. A stepwise assessment of patient`s clinical risk factors and test-results, combined with a careful evaluation of the specific surgery-related risk, leads to a tailored risk stratification for each patient, thus allowing to the best preventive/therapeutic strategy before, during and after non-cardiac surgery (NCS).
Clinical risk evaluation
The first step involves the assessment of surgery-related risk and patient-related risk. All surgical operations are
classified into 3 classes of risk: low, intermediate and high, according to 30-day risk of CV death, myocardial infarction (MI) and stroke (<1% for low, 1-5% for intermediate, >5% for high risk). Another crucial issue to be considered is the timing of surgery. If the surgery is deemed emergent or urgent, cardiac testing is not feasible but close follow-up after the intervention is advisable.
In case of time-dependent surgery, the decision of performing individualized cardiac testing is taken by a multidisciplinary team on a case-by-case basis. Type and modalities of cardiac testing before elective surgery is closely related to patient characteristics, symptoms and risk profile. In patients aged 45-65 years without history of CV disease, without CV risk factors and without signs and/or symptoms of cardiac disease (e.g. chest pain, dyspnea, peripheral edema, etc) ECG and biomarkers (high sensitivity cardiac troponin I/T and/or BNP/NT-proBNP) should be considered prior to high-risk surgery and 24h and 48h afterwards. Otherwise, in patients aged >65 years, with CV risk factors or established CV disease, these exams are recommended also in case of intermediate-risk surgery. In case of abnormal findings on clinical examination or on ECG or laboratory tests, as well as in all symptomatic patients, regardless of risk category, transthoracic echocardiography (TTE) should be performed followed by further examinations if indicated (e.g. imaging or functional tests to rule-out CAD) before NCS.
Pre-operative assessment tools
Other crucial aspects to be considered while evaluating peri-operative CV risk, especially in elderly, are clinical risk score and functional capacity. ![]()
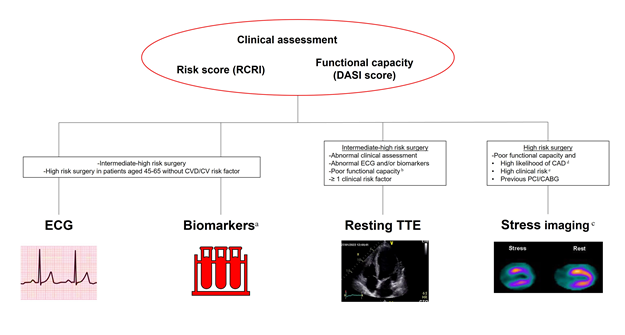
Figure 1. Overview of main pre-operative assessment tools
a high sensitivity cardiac troponin I/T and BNP/NT-proBNP
b DASI score < 34
c cardiac computed tomography angiography in patients unsuitable for non-invasive functional testing
d pre-test probability 15% based on age, sex, and nature of symptoms; or ≥2 CV risk factors among dyslipidemia, diabetes, hypertension, smoking, family history of CV disease; or resting ECG changes or left ventricular dysfunction suggestive of CAD.
e RCRI ≥ 1
CABG, coronary artery bypass graft surgery; CAD, coronary artery disease; CV, cardiovascular; CVD cardiovascular disease; DASI, Duke activity status index; RCRI, revised cardiac risk index; PCI, percutaneous coronary intervention; TTE, transthoracic echocardiography.
Several risk indices have been developed enclosing variables related to patient clinical status and surgery-related risk factors (3-5). Among them, the Revised Cardiac Risk Index (RCRI) is the most widely used being very easy and quick to assess (6). It estimates 30-day mortality, MI or cardiac arrest based on six variables.
Other indices such as The American College of Surgery National Surgical Quality Improvement Program are less accessible than RCRI but offers procedure-specific absolute risk estimation, which may help in patient-guided decision-making (5). The new guidelines suggest the use of the Duke Activity Score Index (DASI score) for the assessment of functional capacity being metabolic equivalents (METS) <4, which have long been considered markers of poor functional status, based on subjective interviewing and lacking proven value (7). A DASI score <34 denotes an increased odds of 30-day mortality or MI (8).
To recognize poor functional capacity in patients undergoing high-risk surgery is of great clinical concern, being some patients at risk of developing periprocedural MI due to significant despite still asymptomatic underlying CAD. In all patients with poor functional capacity prior to high-risk surgery, current guidelines recommend performing TTE and to further stratify their perioperative risk with a stress imaging test in case of previous PCI or coronary artery bypass graft (CABG) or in case of high likelihood of CAD (pre-test probability 15% based on age, sex, and nature of symptoms; or ≥2 CV risk factors among dyslipidemia, diabetes, hypertension, smoking, family history of CV disease; or resting ECG changes or left ventricular dysfunction suggestive of CAD) or high clinical risk (RCRI ≥1) (9-11).
If functional stress test is indicated, exercise stress test should be preferred. Alternatively, pharmacological stress imaging or myocardial scintigraphy should be performed whenever possible.
Coronary artery assessment by computed tomography is another emergent opportunity especially if not only morphological evaluation of coronary plaques and stenosis is feasible but also functional evaluation of coronary epicardial arteries and microcirculation is available. An overview of main pre-operative assessment tools is summarized in Figure 1.
General risk reduction strategies
One of the leading challenges for clinicians evaluating patients undergoing NCS is the management of antithrombotic agents. An interdisciplinary, thorough, risk assessment of both ischemic and bleeding risk (concerning both patient and surgical procedure) is mandatory in order to prevent peri-operative major bleeding, thrombotic events and mortality. Based on hemorrhagic risk, surgical procedures are labeled by guidelines into minor, low, or high-risk.
Single antiplatelet therapy with aspirin for primary prevention may be safely interrupted before NCS as the risk of ischemic events in these patients is low. In case of previous PCI, ischemic benefit of peri-operative aspirin use outweighs bleeding risk, thus it should not be discontinued except for surgery at very high bleeding risk (spinal surgery or certain neurosurgical or ophthalmological operations) on a case-by-case basis. In the latter situation, aspirin should be withdrawn for at least 7 days.
In patients taking clopidogrel monotherapy in secondary prevention, a short interruption in chronic coronary syndrome (CCS) patients is advisable, while several strategies may be adopted on individual basis in case of a recent stroke, peripheral artery disease (PAD) or aspirin intolerance (surgery under P2Y12 monotherapy, switching to aspirin, short interruption, or bridging in the peri-operative phase) (12-14).
In patients taking dual antiplatelet therapy (DAPT), the peri-operative management of antithrombotic therapy prior to high bleeding risk surgery strictly depends on the timing of last PCI or acute coronary syndrome (ACS). Patients with recent PCI <1 month, ACS <3 months or high risk of stent thrombosis [defined as by at least one of the following: history of stent thrombosis under antiplatelet therapy, left ventricular ejection fraction <40%, poorly controlled diabetes, severely impaired renal function/hemodialysis, recent complex PCI (i.e. severely calcified lesion, left main PCI, chronic total occlusion, bifurcational/crush technique, bypass graft PCI), or stent malapposition/residual dissection] are all considered at high thrombotic risk and surgery, whenever possible, should be defer. In rare case of time-sensitive undeferrable NCS and both high bleeding and thrombotic risk, interruption of DAPT and bridging with cangrelor or glycoprotein IIb/IIIa inhibitors (GPI) might be applicable (15).
In case of high bleeding risk surgery without high thrombotic risk, P2Y12 inhibitor should be interrupted (clopidogrel 5 days, ticagrelor 3-5 days, prasugrel 7 days) prior to NCS and resumed within 48 hours, while continuation of aspirin is recommended.
As regard to oral anticoagulants (OACs), prior to non-high bleeding risk surgery vitamin-k antagonists (VKA) should be continued with International Normalized Ratio (INR) at lower levels or shortly interrupted, while direct oral anticoagulants (DOACs) should be temporarily withdrawn. If bleeding risk concerns, anticoagulation therapy should be always interrupted. In case of undeferrable NCS at both high bleeding and thrombotic risk (e.g. mechanical heart valves, recent stroke <3 months, high risk of venous thromboembolism recurrences, left ventricular apex thrombus, atrial fibrillation with CHA2DS2-VASc >6), anticoagulants should be interrupted and bridging therapy with low molecular weight heparin (LMWH) or unfractionated heparin (UFH) should be considered. In particular, bridging therapy under DOACs is suggested only in very few cases, such as: recent thromboembolic event within 3 months; patients who experienced a thromboembolic event during previous interruption of DOAC therapy.
The best timing for discontinuation and resumption of therapy depends on the surgery-related bleeding risk, the type of OAC, and patient clinical characteristics. VKA should be discontinued 3-5 days before surgery and restart after at least 12-24 hours, in case of well controlled bleeding. VKA should be resumed together with LMWH or UFH in case of pre-operative bridging and the restarting dose should be equivalent to the maintenance dose plus a boosting dose of 50% for 2 days. In case of high bleeding risk surgery, anticoagulation should be postponed to 48-72 hours after hemostasis has been secured.
In patients with normal renal function DOACs should be withdrawn the day of surgery in case of minor bleeding risk (or the evening before surgery in case of apixaban and dabigatran), 24 hours before surgery in case of low bleeding risk and 48 hours before surgery when high bleeding risk subsists.
Specific discontinuation times should be observed in the case of renal dysfunction, especially for dabigatran, for which at least 96 hours of suspension is required prior to high bleeding risk surgery if estimated glomerular filtration rate (eGFR) is below 50 ml/min (1). Resumption of DOACs is generally recommended 6-8 hours after surgery with rapid and complete hemostasis. After high bleeding risk procedures, DOACs should be restarted after 48-72 hours considering prophylactic heparin until full dose DOAC is deemed safe.
For interventions considered at very high risk of bleeding (e.g. spinal or epidural anesthesia) interruption of DOACs for up to five half-lives and resumption after 24 hours should be considered. In case of unplanned, non-deferrable surgery, anticoagulant therapy should be stopped immediately, and the perioperative bleeding risk profile should be carefully assessed through blood coagulation tests and measurements of DOACs plasma levels, if available.
Reversal of VKA may be managed with vitamin K, prothrombin complex concentrates (PCC) or plasma administration. Vitamin K (2-10 mg) can be used orally (achieving a reduction in INR in 18-24 hours) or i.v., for a more rapid reversal of anticoagulation (4-6 hours). Otherwise, if reversal is required prior to emergency surgery, four-factor PCC is the preferred strategy. Three-factor PCC or plasma may be used as second option. Reversal of DOACs prior to undeferrable urgent/emergent NCS maybe indicated in case of last dose intake < 12 hours (< 24 hours if eGFR < 50 ml/min) and depends on surgery-related bleeding risk and specific reversal agents availability.
It should be emphasized that whereas the open-label prospective trial testing idarucizumab as specific reversal agent in patients on dabigatran enrolled participants undergoing urgent NCS, the trial testing the reversal agent andexanet alpha for FXa inhibitors included only patients with acute major bleeding under therapy (16, 17). Thus, andexanet alpha may be used off-label in life-threatening situations requiring an immediate intervention. If specific reversal agents are not available, non-specific agents (PCC or activated PCC) should be used instead.
Perioperative cardiovascular complications
Several factors related to the perioperative clinical status of patients (e.g. anesthesia, analgesia, post-operative pains, surgical wounds) may all mask typical symptoms related to acute cardiac disorders, thus leading to a dangerous underdiagnosis of CV complications following NCS. An active surveillance during perioperative phase is warranted in selected high-risk patients prior to intermediate-to-high risk surgery. In such clinical scenario, perioperative myocardial infarction/injury (PMI), defined as postoperative increase of high sensitivity cardiac troponin I/T (hs cTn I/T) above 99th percentile upper reference limit with or without accompanying symptoms, ECG changes or imaging evidence of acute myocardial ischemia, may often be exclusively detected via hs cTn I/T assessment before and after 24 hours and 48 hours post-operatively. After detection of PMI, recognizing whether it is related to a non-cardiac (e.g. severe sepsis, pulmonary embolism, stroke) or cardiac cause (type I or II MI, tachyarrhythmia, acute heart failure) is crucial in promptly providing the adequate therapeutic strategy. TTE should be the first diagnostic tool during initial work-up and may often orient toward a specific diagnosis. However, in about 50% of cases the pathophysiology underlying the PMI cannot be clearly identified and is assumed to be likely type II MI (due to undocumented or relative hypotension) or missed type I MI. In the latter cases, non-invasive or invasive assessment of CAD (based on pre-test clinical probability and patient characteristics), along aspirin and statin, is the suggested managing strategy. When PMI is accompanied by ST segment elevation /depression or typical symptoms, in absence of severe anemia or active bleeding suggesting type II MI, the therapeutic approach should be the same as for type I MI, including invasive coronary angiography, antiplatelet therapy and statin.
Peer-review: Internal
Conflict of interest: None to declare
Authorship: G.I., R.C. equally contribute to the preparation of manuscript
Acknowledgement and funding: None to declare
References
- 1.Halvorsen S, Mehilli J, Cassese S, Hall TS, Abdelhamid M, Barbato E, et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur Heart J 2022; 43: 3826-924.
- 2. Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, Hert SD, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35: 2383-431.
- 3.Duceppe E, Parlow J, MacDonald P, Lyons K, McMullen M, Srinathan S, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017; 33: 17-32.
- 4. Dakik HA, Chehab O, Eldirani M, Sbeity E, Karam C, Abou Hassan O, et al. A new index for pre-operative cardiovascular evaluation. J Am Coll Cardiol 2019; 73: 3067-78.
- 5.Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217: 833-42.e1-3.
- 6.Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med 2010; 152: 26-35.
- 7.Wijeysundera DN, Pearse RM, Shulman MA, Abbott TEF, Torres E, Ambosta A, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391: 2631-40.
- 8.Wijeysundera DN, Beattie WS, Hillis GS, Abbott TEF, Shulman MA, Ackland GL, et al. Integration of the Duke Activity Status Index into preoperative risk evaluation: a multicentre prospective cohort study. Br J Anaesth 2020; 124: 261-70.
- 9.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020; 41: 407-77.
- 10.Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014; 63: 380-406.
- 11.Cullen MW, McCully RB, Widmer RJ, Schroeder DR, Salonen BR, Raslau D, et al. Preoperative dobutamine stress echocardiography and clinical factors for assessment of cardiac risk after noncardiac surgery. J Am Soc Echocardiogr 2020; 33: 423-32.
- 12.Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N Engl J Med 2019; 381: 2032-42.
- 13.Vranckx P, Valgimigli M, Jüni P, Hamm C, Steg PG, Heg D, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet 2018; 392: 940-9.
- 14.Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, et al. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: The STOPDAPT-2 Randomized Clinical Trial. JAMA 2019; 321: 2414-27.
- 15.Sullivan AE, Nanna MG, Wang TY, Bhatt DL, Angiolillo DJ, Mehran R, et al. Bridging antiplatelet therapy after percutaneous coronary intervention: JACC Review Topic of the Week. J Am Coll Cardiol 2021; 78: 1550-63.
- 16.Pollack CV, Jr., Reilly PA, van Ryn J, Eikelboom JW, Glund S, Bernstein RA, et al. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med 2017; 377: 431-41.
- 17.Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med 2019; 380: 1326-35.
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

