Predictive role of fragmented QRS in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention
ORIGINAL RESEARCH ARTICLE
Predictive role of fragmented QRS in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention
Article Summary
- DOI: 10.24969/hvt.2023.395
- Cardiac Surgery
- Published: 09/06/2023
- Received: 11/04/2023
- Revised: 27/05/2023
- Accepted: 29/05/2023
- Views: 6444
- Downloads: 4364
- Keywords: ST-elevation myocardial infarction, fragmented QRS, heart failure, NT-proBNP, primary percutaneous coronary intervention
Address for Correspondence*: Pooja Vyas, Department of Cardiology, UNMICRC, Civil Hospital Campus, Asarwa, Ahmedabad-380016,Gujarat, India Email: poojavyaskothari@gmail.com Mobile: +91-9925004922,
Fax: +91-079-2682092
Sunil Bobade1a, Kewal Kanabar1b, Hasit Joshi1b, Pooja Vyas1b*, Iva Patel1c, Kunal Parwani2, Mit Chaudhary, Prarthi Shah, Tanmay Boob, Poojan Prajapati
1aInterventional Cardiology, 1bDepartment of Cardiology; 1cDepartment of Research, U. N. Mehta Institute of Cardiology and Research Centre (UNMICRC), Civil Hospital Campus, Asarwa, Ahmedabad-380016, Gujarat, India
2B.J.Medical college, Ahmedabad, Gujarat, India
Abstract
Objective: Fragmented QRS (fQRS), as defined by additional spikes in the QRS complex of a 12-lead electrocardiogram (ECG), is a marker of scarred myocardium. In patients with coronary artery disease (CAD), fQRS is a predictor of heart failure (HF) and other major adverse cardiac events (MACE). The study was aimed to evaluate the role of fQRS in prediction of HF in patients with ST-elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI).
Methods: In a prospective, non-randomized, small observational study, we enrolled 188 consecutive patients with STEMI undergoing primary PCI. Patients were grouped according to the presence or absence of fQRS and their in-hospital, 1 and 6-month MACE outcomes were assessed.
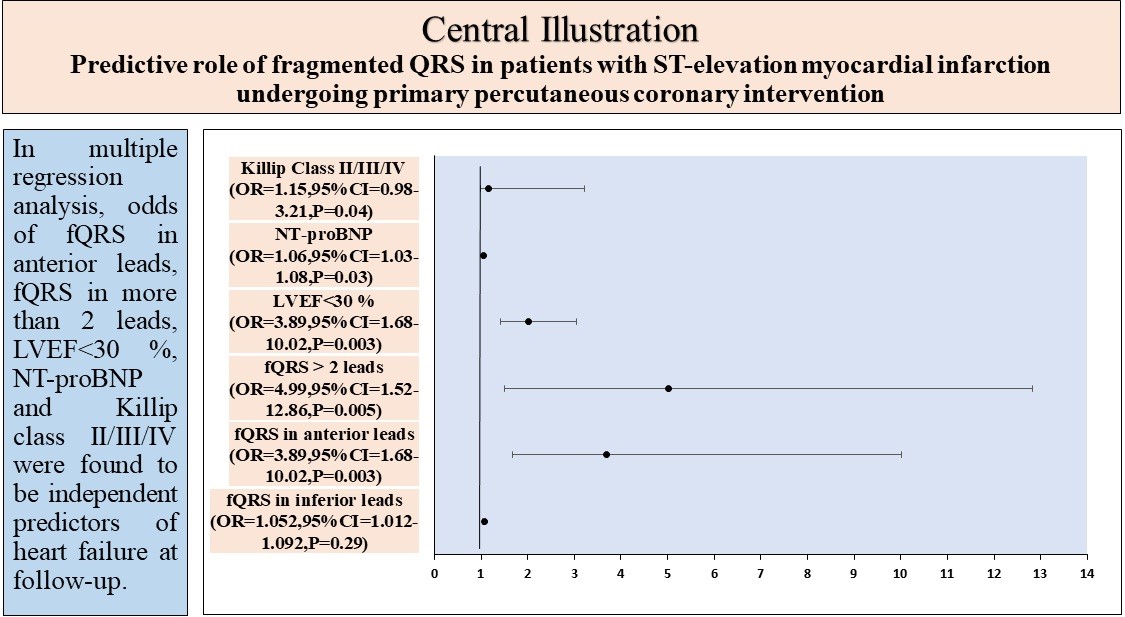
Results: Of the 188 patients, fQRS were noted in 92 (48.94%) patients. Patients with fQRS were more likely to have Killip class II/III/IV. Patients with fQRS had a significantly higher corrected QT interval, lower left ventricular ejection fraction (LVEF), and higher N-terminal pro brain natriuretic peptide (NT-pro BNP) at 24 hours and 48 hours compared to patients without fQRS. The in-hospital (P=0.001), 30-day (P=0.03) and 6-month (p=0.01) MACE were higher in patients with fQRS. On logistic multiple analysis, fQRS in anterior leads (OR=3.70, CI=1.68-10.02, p=0.001), fQRS in more than 2 leads (OR=5.20, CI=1.51-12.83, p=0.01), NT-proBNP (OR=1.05, CI=1.03-1.08, p=0.02) and Killip class II/III/IV were found to be significant predictors for HF hospitalization.
Conclusion: Our findings suggest that fQRS can be a predictor for HF in patients with STEMI and provide a simple and readily available technique for predicting prognosis. Larger studies are required to validate these findings.
Key words: ST-elevation myocardial infarction, fragmented QRS, heart failure, NT-proBNP, primary percutaneous coronary intervention
Abbreviations:
CAD - coronary artery disease
CK-MB - creatine kinase-MB
ECG - electrocardiogram
fQRS - fragmented QRS
LVEF - left ventricular ejection fraction
MACE - major adverse cardiac events
NT-pro BNP - N-terminal pro brain natriuretic peptide
NYHA - New York Heart Association
pPCI - primary percutaneous coronary intervention
STEMI - ST-elevation myocardial infraction
TVR - target vessel revascularization
Introduction
ST-elevation myocardial infarction (STEMI) is a common cause of acute coronary syndrome (ACS), with an estimated yearly incidence of 3 million cases from India (1). Despite advances in the management, STEMI remains a major cause of morbidity, mortality, and heart failure (HF) (2). Current diagnostic tools for predicting HF after STEMI, such as transthoracic 2-D echocardiogram (TTE) and biomarkers are extremely useful but may have certain have limitations in terms of their cost and availability in resource-limited settings (3). Fragmented QRS (fQRS), as defined by additional spikes on the QRS complex of a 12-lead electrocardiogram (ECG), is a relatively newer ECG finding that has been proposed as a potential predictor of myocardial damage and fibrosis (4). The presence of fQRS suggests abnormalities in the cardiac conduction system (5-6). Several studies have suggested that fQRS may be associated with adverse cardiovascular outcomes, including HF (7-8).
Earlier detection and prediction of HF after STEMI is crucial for improving patient outcomes. The use of fQRS as a predictor of HF post-STEMI may provide a valuable tool to identify patients at high risk of developing HF, thus allowing for appropriate and timely medical intervention.
We aimed to explore the potential role of fQRS as a predictor of heart failure post-STEMI undergoing primary percutaneous coronary angioplasty.
Methods
Study design and patient selection
This was a prospective, single-center, non-randomized observational study carried out at the U.N. Mehta Institute of Cardiology and Research Center, Ahmedabad, India, which is a tertiary level cardiac referral center. The study protocol conforms to the ethical guidelines of the Declaration of Helsinki and was reviewed and cleared by the Ethics Committee of the institute (UNMICRC/CARDIO/2018/09). Informed written consent was obtained from all patients or appropriate legally authorized representatives.
The study enrolled patients presenting with STEMI undergoing pPCI from December 2018 to June 2020. Patients presenting within 12 hours from symptom onset, defined as typical chest pain lasting for > 30 min, ST-segment elevation ≥ 1 mm in two contiguous ECG leads, and undergoing pPCI including balloon angioplasty/thrombus aspiration, and/or stent implantation were included. The patients with QRS duration >120 ms, bundle branch blocks, Wolff-Parkinson-White syndrome, Brugada syndrome, and those with prior permanent pacemaker insertion were excluded.
Clinical data
The data regarding baseline demographic characteristics and risk factors like age, sex, diabetes, hypertension, smoking, dyslipidemia, and prior heart disease, ECG, echocardiogram, coronary angiogram, laboratory investigations, and in-hospital complications were recorded. Heart failure was defined by the New York Heart Association (NYHA) functional classification of ≥3 during index hospital stay or NYHA class ≥3 which required re-hospitalization on follow-up.
Biomarkers
Several biomarkers such as N-terminal-pro brain natriuretic peptide (NT-proBNP), creatine kinase-MB (CK-MB), and troponin I were measured on admission and 24 and 48 hours later. NT-proBNP was measured using an Elecsys® NT-proBNP analyzer. Blood sample was collected at the admission time for assessment of NT pro-BNP. The normal value for NT pro-BNP was <125 pg/ml for <75 years old and <450pg/ml for >75 years old.
fQRS
A 12-lead ECG was recorded at hospital admission, and 1, 24, and 48 hours following pPCI. The QRS duration was measured using the longest QRS complex in any lead by manual measurements. STEMI was defined as per the standard diagnostic criteria (9-10). The fQRS included various morphologies of the QRS such as an additional R wave or notching in the nadir of the S wave, or > 1R (fragmentation) in two contiguous leads corresponding to a major coronary artery territory (11).
Echocardiography
Transthoracic echocardiogram was performed with commercially available system iE 33 xMatrix (Philips Healthcare, Andover, MA, USA).
Primary percutaneous coronary intervention
Primary PCI was performed using the femoral/radial artery approach as per the operator’s discretion.
Follow up and outcome
Patients were followed up in-hospital and at 1 and 6 months after discharge. The major adverse cardiac events (MACE) included cardiovascular mortality, HF hospitalization, cerebrovascular stroke, re-myocardial infarction, and target vessel revascularization (TVR).
Statistical analysis
All data were prospectively collected by trained physicians (four authors of the study) and entered into a spreadsheet (Microsoft Excel 2016TM; Microsoft Corporation, USA). Statistical analysis was performed using the Statistical package for social sciences (SPSS Inc., version 23.0TM; IBM Corporation, Chicago, USA). All continuous variables were summarized as mean (standard deviation). Categorical variables were described as proportions and frequencies (%). The comparison between two groups for continuous variables was performed by using the Student's t-test. The comparison between two categorical variables was performed by using the Chi-square test or Fisher exact test. Subsequently, variables with p < 0.10 on univariate analysis were included in a multiple regression analysis to identify independent predictors of HF at 6-month follow-up. All p values are two-tailed and set at a statistical significance of 0.05.
Results
Of the 203 patients screened, 188 patients were included and divided into two groups according to the presence or absence of fQRS after pPCI.
There was no significant intergroup difference in the age, gender, and cardiovascular risk factors. Patients with fQRS were more likely to be in a higher Killip class at presentation (p<0.05). Patients with fQRS had a lower left ventricular ejection fraction (LVEF) (p=0.02) and higher corrected QT interval (p=0.001) (Table 1).
The mean NT-pro-BNP at 24 and 48 hours was higher in patients with fQRS (p=0.01 and p=0.0001) and HF was more frequent in fQRS group (p=0.003). In-hospital MACE (p<0.0001), 30-day and 6-month HF hospitalizations (p=0.01 for both) were significantly higher in patients with fQRS (Table 2).
In univariate analysis (Table 3), fQRS in anterior leads [Odds ratio (OR) 10.78; 95 confidence interval (CI) 2.64-42.94;], fQRS > 2 leads [OR 19.87; CI 6.29-60.77]; LVEF < 30% [OR 4.20; CI 0.810-21.76], NT-ProBNP [OR 1.10; CI, 1.01-1.21] were found significant predictors for HF at follow-up. In multiple regression analysis, fQRS in anterior leads, fQRS in more than 2 leads, LVEF<30 %, NT-proBNP> 900 pg/ml, and Killip class II/III/IV were found to be independent predictors of HF at follow-up.
Discussion
In a single-center prospective observational study, we found that fQRS is associated with a higher rate of MACE in patients with STEMI undergoing pPCI. In multiple regression analysis, fQRS in anterior leads, fQRS in more than 2 leads, NT-proBNP, and Killip class were found to be independent predictors of HF at 6-month follow-up.
Despite considerable advances in the emergency management, STEMI remains a major cause of mortality, morbidity and adverse long-term outcomes. Several characteristics such as advanced age, prior MI, anterior MI, and lower LVEF have been associated with adverse outcomes. However, the traditional risk stratification tools in acute MI do not include newer ECG parameters which represent myocardial activity. fQRS indicates the presence of high frequency potentials inside the QRS complex.
The term was first coined following an experimental study of canine hearts where coronary occlusion provoked the appearance of fQRS (12). It can be provoked by any condition affecting myocardial depolarization such as ischemia, scar, fibrosis, myofiber disarray, inflammation, and microvascular abnormality.
Early studies suggested a good correlation between fQRS and the presence of myocardial scar detected by single photon emission tomography (6).
|
Table 1. Clinical characteristics |
|||
|
Variables |
No Fragmented QRS (-) n=96) |
Fragmented QRS (+) (n=92) |
p |
|
Age, years |
57.0 (11.06) |
57.0 (12.77) |
1 |
|
Sex, n (%) Male Female |
68 (70.8) 28 (29.2) |
76 (82.6) 16 (17.4) |
0.27 |
|
Risk factors, n (%) Diabetes Hypertension Smoker Dyslipidemia Known CAD Prior PCI Prior Stroke |
36 (37.5) 42 (43.8) 34 (35.4) 14 (14.6) 10 (10.4) 2 (2.08) 1 (0.10) |
36 (39.1) 50 (54.3) 40 (43.5) 18 (19.6) 08 (8.7) 1 (1.09) 0 |
0.87 0.30 0.42 0.52 0.78 0.97 0.98 |
|
Killip class, n (%) I II III IV |
76 (79.17) 10 (10.42) 6 (6.25) 4 (4.17) |
48 (52.17) 18 (19.56) 14 (15.22) 12 (13.04) |
0.05 |
|
Heart failure, n (%) |
10 (10.42) |
26 (28.32) |
0.003 |
|
LVEF, % |
35.83 (6.55) |
32.48 (6.66) |
0.02 |
|
Mitral regurgitation, n (%) Mild Moderate Severe |
56 (16.7) 20 (20.8) 4 (4.2) |
44 (47.8) 10 (19.6) 4 (4.3) |
0.59 |
|
Door to balloon time, min |
46.50 (18.16) |
41.63 (17.16) |
0.185 |
|
Anterior MI, n (%) |
52 (54.2) |
44 (47.8) |
0.68 |
|
PR interval, ms |
148.33 (41.40) |
149.13 (49.40) |
0.93 |
|
QRS duration, ms |
97.79 (12.82) |
103.22 (16.63) |
0.07 |
|
QT interval, ms |
406.79 (34.50) |
427.63 (37.89) |
0.001 |
|
NT-proBNP, pg/ml At 24 hours At 48 hours |
957.21 (1252.82) 1023.96 (1252.82) |
2175.04 (1667.25) 5123.06 (11143.13) |
0.0001 0.01 |
|
Troponin I, ng/mL |
28783.69 (19999.151) |
29828.13 (19382.14) |
0.193 |
|
LVEF- left ventricular ejection fraction, MI – myocardial infarction, NT-proBNP - N-terminal pro-brain natriuretic peptide |
|||
|
Table 2. In hospital, 1-month and 6-month major adverse cardiac events |
|||
|
Variables |
No Fragmented QRS (-) (n=96) |
Fragmented QRS (+) (n=92) |
p |
|
In hospital MACE, n (%) Cardiovascular mortality Heart failure Stroke Re-infarction
MACE |
02 (2.08%) 14 (14.58%) 0 2 (2.08%) 2 (2.08%) 20 (20.83%) |
10 (10.87%) 32 (34.78%) 2 (2.17%) 2 (2.17%) 2 (2.17%) 48 (52.2%) |
0.03 0.002 0.45 0.64 0.64 <0.0001 |
|
30 days MACE, n (%) |
|||
|
Heart failure hospitalization |
02 (2.08%) |
14 (15.22%) |
0.01 |
|
6 months MACE, n (%) |
|||
|
Heart failure hospitalization |
02 (2.08%) |
18 (19.6%) |
0.01 |
|
Cardiovascular mortality |
0 |
2 (2.17%) |
0.45 |
|
Total MACE numbers |
24 |
82 |
|
|
MACE - major adverse cardiac events, TVR - target vessel revascularization |
|||
|
Table 3. Univariate and multiple logistic regression analysis for predictors of heart failure |
||||
|
Variables |
Univariate |
Multivariate |
||
|
OR (95%CI) |
p |
OR (95%CI) |
p |
|
|
fQRS in inferior leads |
1.83 (1.09-3.92) |
0.04 |
1.05 (0.98-1.092) |
0.29 |
|
fQRS in anterior leads |
10.78 (2.64-42.94) |
< 0.001 |
3.89 (1.68-10.02) |
0.003 |
|
fQRS > 2 leads |
11.87 (6.29-40.77) |
< 0.001 |
4.99(1.52--12.86) |
0.005 |
|
LVEF<30 % |
4.20(0.810-21.76) |
0.043 |
2.03 (1.42-3.05) |
0.05 |
|
NT-proBNP |
1.10 (1.01-1.21) |
< 0.001 |
1.06 (1.03-1.08) |
0.03 |
|
Killip class II/III/IV |
1.68 (1.01-3.99) |
0.02 |
1.15 (0.98-3.21 ) |
0.04 |
|
CI – confidence interval, fQRS – fragmented QRS, LVEF - left ventricular ejection fraction, NT-proBNP - N-terminal pro-brain natriuretic peptide, OR – odds ratio |
||||
Several studies and meta-analyses have found fQRS to be associated with adverse short and long-term outcomes including HF, TVR, and mortality. In our study, fQRS on ECG was negatively associated with a LVEF, similar to previously reported data (13), but contrary to data by Mehmat et al. (14) and Sukru et al. (15). Likewise, fQRS was associated with higher NT-proBNP at 24 hours and 48 hours, similar to the previously reported data (15). These findings corroborate with the fact that fQRS is associated with myocardial scar.
Previous studies have demonstrated a significant association of fQRS with in hospital cardiovascular complications in STEMI as well as adverse outcomes in microvascular angina, idiopathic dilated cardiomyopathy (5, 13, 16-18). Fragmented QRS, NT-proBNP, and Killip class were the only predictors of HF at follow-up in multiple regression analysis. Several studies and a meta-analysis has reported fragmented QRS to be associated with in hospital and long-term mortality and MACE in STEMI (19). The current study adds to the existing literature on the predictive value of fQRS in STEMI.
The much higher rate of HF at presentation and very low LVEF in our study could be explained due to the fact that anterior STEMI accounted for 50% of cases. Additionally, the time to presentation in our center is typically longer than the traditionally reported data from western literature. The median time to presentation was 4.5 hours.
Study limitations
This is the single-center study with a small sample size and a larger, multi-center study with a longer clinical follow-up may help clarify the role of fQRS in assessment of long-term prognosis. We did not record the baseline and follow-up pharmacological therapy including dose of diuretics, renin-angiotensin-aldosterone-system inhibitors and beta- blockers. We did not prospectively assess the baseline renal function of the study population. Baseline renal function and at follow-up may have helped to predict future HF.
Conclusions
The current study has shown that fQRS is a reliable predictor of heart failure post-STEMI, independent of other clinical and demographic factors. The use of fQRS as a predictor of heart failure post-STEMI has several advantages, including its simplicity, non-invasiveness and low cost. Earlier detection and prediction of heart failure after STEMI is crucial for improving patient outcomes and ability to use fQRS as a reliable predictor of heart failure post-STEMI may provide a valuable tool for clinicians to identify patients at high risk of developing heart failure.
Ethics: Informed consent was obtained from patients before all procedures. The study protocol conforms to the ethical guidelines of the Declaration of Helsinki and was reviewed and cleared by the Ethics Committee of the institute (UNMICRC/CARDIO/2018/09).
Peer-review: External and internal
Conflicts of interest: None to declare
Authorship: S.B., K.K., H.J., P.V., I.P., K.P., M.C., P.S., T.B., P.P. equally contributed to study and manuscript preparation.
Acknowledgement and funding: None to declare.
References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

