Study of usefulness of speckle-tracking echocardiography in detecting left ventricular dysfunction among adult cancer patients undergoing chemotherapy
ORIGINAL RESEARCH ARTICLE
Study of usefulness of speckle-tracking echocardiography in detecting left ventricular dysfunction among adult cancer patients undergoing chemotherapy
Article Summary
- DOI: 10.24969/hvt.2023.401
- Page(s): 282-289
- CARDIOVASCULAR DISEASES
- Published: 14/07/2023
- Received: 03/05/2023
- Revised: 18/06/2023
- Accepted: 19/06/2023
- Views: 5632
- Downloads: 4057
- Keywords: cardiotoxicity, left ventricular dysfunction, strain imaging, chemotherapy, accuracy, prognosis
Address for Correspondence*: Vishal Sharma, Department of Cardiology, U. N. Mehta Institute of Cardiology and Research Centre, Civil hospital campus, Asarva, Ahmedabad-380016, Gujarat, India
E-mail: dr.vishal88@gmail.com Mobile:+ 91-909-9989940 Fax: +91-079-22682092
Chandrakant Usendia1, Anand Shukla2a, Mithilesh Kulkarni2a, Vishal Sharma2a*, Karthik Natrajan2a, Kewal Kanabar2a, Dinesh Joshi2a, Riyaz Charaniya2a, Pratik Raval2a, Krutika Patel2b
1 Pulse Hospital, Bhavnagar-364002, Gujarat, India
2aDepartment of cardiology and 2bDepartment of Research, U. N. Mehta Institute of Cardiology and Research Centre (UNMICRC), Civil Hospital Campus, Asarwa, Ahmedabad-380016, Gujarat, India
Abstract:
Objective: Cancer treatment-related cardiac dysfunction (CTRCD) is a significant concern for patients undergoing chemotherapy. The aim of the present study was to study the accuracy and value of longitudinal strain in prediction of left ventricular dysfunction (LVD) in cancer patients undergoing cancer therapy.
Methods: This was a prospective observational study conducted among 183 adult patients undergoing chemotherapy between 2018 and 2020. Patients with congenital or acquired valvular disease, prior myocardial infarction, coronary revascularization, or cardiac surgery were excluded. The patients were evaluated using a detailed history, clinical examination and echocardiography at baseline, 1 month, 3 months, and 6 months after chemotherapy. Speckle-tracking strain analysis was used to evaluate left ventricular (LV) global longitudinal strain (GLS), circumferential strain (GCS), and radial strain (GRS). LVD was defined as >15% decrease in GLS, GCS, or GRS from baseline to 6 months. Accuracy of longitudinal strain in prediction of LVD was studied using ROC analysis.
Results: Of the 183 patients, 59% were male, and 54.1% were between 46-60 years of age. Breast cancer was the most common malignancy (10.9%). The most common chemotherapy regimen was doxorubicin + paclitaxel (9.9%). At baseline, the mean GLS, GCS, and GRS were -18.6 (1.03)%, -20.4 (1.11)%, and 39.9 (6.09)%, respectively. At the 6-month follow-up, 27 (14.8%) patients had LVD. The incidence of LVD was higher (51.48%) in patients who received doxorubicin-containing regimens compared to non-doxorubicin-containing regimens (P <0.0001). GLS has good accuracy in prediction of LVD at 6 months of follow-up (88.37%).
Conclusions: The incidence of LV dysfunction was higher (51.48%) in patients who received doxorubicin-containing regimens. GLS is different in LVD vs non LVD and the accuracy of GLS is more in prediction of LVD development during 6-month follow-up (88.37%).
Abbreviations
CTRCD Cancer treatment-related cardiac dysfunction
LVD Left ventricular dysfunction
LV Left ventricular
GLS Global longitudinal strain
GCS Global circumferential strain
GRS Global radial strain
LVEF Left ventricular ejection fraction
2DE 2D echocardiography
HTN Hypertension
DM-II Diabetes mellitus-II
RT Renal transplant
PPV Positive predictive value
AUC Area under the curve
Introduction
Cancer therapy has been a crucial element in the fight against cancer. However, it can cause several complications, and one of the most significant is cardiac dysfunction resulting from exposure to cancer treatment (1). The recognition of this complication dates back to the 1960s with the use of anthracyclines in cancer therapy. Anthracycline-associated heart failure has been identified as an important side effect, leading physicians to reduce their doses to avoid cardiac dysfunction (2, 3).
Several strategies have been used to detect this dysfunction, including monitoring of the left ventricular ejection fraction (LVEF) by cardiac imaging and endomyocardial biopsies. Over time, noninvasive evaluation of LVEF has become the most widely used strategy for monitoring cardiac function (1, 3).
Cancer treatment-related cardiac dysfunction (CTRCD) is characterized by a decrease in LVEF of more than 10%, reaching a value of less than 53%. LVEF measurement is crucial in the evaluation of left ventricular (LV) function, but it has low sensitivity to detect small changes in LV contractility. Hence, the noninvasive evaluation of LVEF may have some limitations, and further strategies for the detection of CTRCD are needed (4).
In addition to the limitations of conventional 2D echocardiography (2DE), strain imaging has emerged as a promising tool for the early detection of CTRCD. Strain imaging is a more sensitive measure of myocardial function and detects changes in contractility before the LVEF decreases. Strain is defined as the percentage change in length of a myocardial segment during systole relative to its length at end-diastole. Global longitudinal strain (GLS), which measures the deformation of the left ventricle in the longitudinal direction, is the most commonly used strain parameter for the detection of CTRCD (5, 6).
Studies have shown that GLS is more sensitive than LVEF for detecting subclinical changes in cardiac function caused by cancer treatment (7, 8).
Furthermore, patients with a GLS decrease of more than 15% had a higher risk of developing clinical heart failure compared to those with a smaller decrease in GLS (9). Strain imaging has also been used to differentiate between reversible and irreversible CTRCD and hence can be used to monitor the response to cardioprotective interventions and guide the management of CTRCD (10).
In summary, strain imaging is a promising tool for the early detection of CTRCD and has the potential to improve the management and outcomes of cancer patients receiving potentially cardiotoxic treatments (4).
The aim of the present study was to study the accuracy and value of longitudinal strain in prediction of left ventricular dysfunction (LVD) in cancer patients undergoing cancer therapy.
Methods
Study design and population
A prospective observational study was conducted at tertiary cardiac care hospital to assess the longitudinal subclinical LVD using speckle-tracking strain analysis in adult patients undergoing chemotherapy. The study population consisted of 183 adult patients undergoing chemotherapy, who were referred to the institution for echocardiography between 2018 and 2020.
Inclusion criteria: patients undergoing chemotherapy, with at least one month and three months of echocardiographic follow-up and willing to participate with a signed consent form, were included in the study.
Exclusion criteria: patients with associated congenital or acquired significant valvular disease, prior myocardial infarction, coronary revascularization, or cardiac surgery, as well as those without baseline and follow-up echocardiography, were excluded from the study.
The written informed consent was obtained from patients before all procedures The design of the work has been approved by institutional ethics committee (UNMICRC/CARDIO/2018/12).
Baseline variables
The patients were categorized based on their age, gender, symptoms, malignancy, and chemotherapy, including the dose to be received. A detailed clinical history and past history of all patients were recorded.
Echocardiographic evaluation
Transthoracic echocardiography was performed with commercially available systems Vivid 7 and Vivid IQ, GE). The images were obtained with a 3.5 MHz transducer and digitally stored for off-line analysis. Baseline echocardiography was performed before starting chemotherapy and follow-up echocardiography was performed just after finishing all scheduled cycles of the chemotherapy regimen. In cases of symptoms or signs of heart failure, however, follow-up echocardiography was performed as soon as possible, even though all the scheduled cycles of chemotherapy were not completed. According to the current recommendations, LV end-diastolic and end-systolic volumes were calculated from the two- and four-chamber apical views, by using Simpson's biplane method and were indexed for body surface area; LVEF was subsequently calculated.
Speckle-tracking strain analysis
Speckle-tracking was performed with Vivid 7 and Vivid IQ Automated Function Imaging (AFI) 2.04. This second generation parametric imaging tool which gives quantitative data for global and segmental strain. Longitudinal strain was measured from the parasternal short axis view at the mid-papillary level and with apical 4-chamber, apical 3 chamber, and apical 2-chamber views. Strain measurement was performed using commercially available software. LV endocardial border was manually traced in the end-systolic frame, and the software automatically extracted a strain curve from the gray-scale images. Global longitudinal and circumferential strains were measured as changes of the whole myocardium, not an averaged value of each segmental strain. Peak strain was defined as the peak negative value on the strain curve during the entire cardiac cycle. GLS, systolic circumferential strain (GCS), and radial systolic strain (GRS) decreased by >15% from baseline to six months were considered LV dysfunction (9).
In healthy individuals, the average peak systolic LV GLS assessed by speckle-tracking technique is in the range of -18 to -20%, GCS is 20 to -22%, and GRS > +40%, respectively (11).
Chemotherapy Protocol:
Patients undergoing chemotherapy are preloaded with anti-emetic, i.v. ondensetron (4-8mg) + i.v. dexamethasone (2-8mg) in 100 ml normal saline (0.9N) over 30 minutes. After waiting for 30 min chemotherapy drug is given intravenously in 500ml dextrose 5%/ normal saline 500ml. Drug doses used for study protocol are paclitaxel 250 mg, carboplastine 450/300 mg, trastuzumab 300 mg, cyclophosphamide 950/600 mg, doxorubicin 25 mg, gemcitabine 150 mg, docetaxel 110 mg, bortezomib 2 mg, thalidomide 100 mg, cisplatin 50 mg /75 mg + injection of mannitol (20%), bleomycin 10 mg, vinblastine 6 mg, oxaliplatin 200 mg, capecitabine 500 mg, 5 fluorouracil (5 fu) 1750 mg, pemetrexed 500 mg, mitoxantrone 12 mg, pentostatine 4 mg, topotecan 1.5mg, dacarbazine 375 mg.
Follow up
The same echocardiographic evaluation was repeated in all patients at follow-up at 1, 3, and 6 months after chemotherapy to assess the incidence of subclinical LVD.
Statistical analysis
Statistical analysis was performed using Statistical Package for Social Sciences (SPSS vs. 26.0). The continuous data are presented as mean (SD) and the categorical variables were presented as frequencies and percentage. Independent samples t test was used to compare continuous variables and Ch-square test – categorical variables. Receiver operating characteristic curve (ROC) was used to find out cut off value of GLS, GCS to predict sub clinical LVD. Multiple regression model was used to find out the predictors of post chemotherapy LVD. A one-way repeated measures ANOVA was conducted to order identify whether there are significant differences in the longitudinal strains (GLS, GCS and GRS) means at baseline to 6 month follow up. Medcalc® version 12.2.1.0 software was used to calculate longitudinal strain’s accuracy. A P value < 0.05 was considered statistically significant.
Results
The study included patients with various types of cancer who received chemotherapy. Over all incidence of LVD in 17.6% (27 patients).
More than half (54.1%) of the patients were aged between 46 and 60 years, and 59.0% of the patients were male (Table 1). Lung cancer was the most common cancer type (18.6%), followed by breast cancer (11.5%), carcinoma of cervix (7.1%), oral cancer (6.0%), and gastrointestinal malignancy (5.5%).
|
Table 1. Clinical characteristics |
||||
|
Variables |
All patients (n=183) |
Non LVD group (n=156) |
LVD group (n=27) |
p |
|
Age, years |
55.16 (12.2) |
55.08 (12.52) |
55.67 (10.28) |
0.79 |
|
Male, n(%) |
108(59) |
98(62.8) |
10(37) |
0.02 |
|
Female, n(%) |
75(41) |
58(37.2) |
17(63) |
|
|
Hypertension, n(%) |
78(42.6) |
63(40.4) |
15(55.6) |
0.21 |
|
Diabetes mellitus, n(%) |
29(15.8) |
24(15.4) |
5(18.5) |
0.90 |
|
Dyslipidemia, n(%) |
7(3.8) |
5(3.2) |
2(7.4) |
0.61 |
|
Radiation therapy, n(%) |
66(36.1) |
55(35.3) |
11(40.7) |
0.74 |
|
Family history, n(%) |
9(4.9) |
7(4.4) |
2(7.4) |
0.87 |
|
Clinical presentation, n(%) |
|
|
|
|
|
Abdominal pain |
44(24) |
40(25.6) |
4(14.8) |
0.33 |
|
Hemoptysis |
39(21.3) |
33(21.2) |
6(22.2) |
0.90 |
|
Bleeding |
28(15.3) |
22(14.1) |
6(22.2) |
0.43 |
|
Breast lump |
20(10.9) |
16(10.3) |
4(14.8) |
0.71 |
|
Ulcer on lip |
12(6.6) |
11(7.1) |
1(3.7) |
0.82 |
|
Ulcer on cheek |
14(7.7) |
10(6.4) |
4(14.8) |
0.26 |
|
Difficulty in swallowing |
10(5.5) |
9(5.8) |
1(3.7) |
0.98 |
|
Oral ulcer |
2(1.1) |
2(1.3) |
0 |
0.68 |
|
Hematuria |
5(2.7) |
5(3.2) |
0 |
0.76 |
|
Throat pain |
9(4.9) |
8(5.1) |
1(3.7) |
0.87 |
|
Treatment, n (%) |
|
|
|
|
|
Doxorubicine + paclitaxel |
17(9.3) |
2(1.3) |
14(51.8) |
<0.0001 |
|
5 FU |
16(8.7) |
14(9.0) |
2(7.4) |
0.92 |
|
Trastuzumab |
10(5.5) |
7(4.5) |
3(11.1) |
0.35 |
|
Docetaxel |
10(5.5) |
8(5.1) |
2(7.4) |
0.98 |
|
Cisplastin+5 FU |
9(4.9) |
9(5.8) |
0 |
0.42 |
|
Data are presented as mean (SD) and n(%), Chi-square and t test for independent samples FU – fluorouracil, LVD – left ventricular dysfunction |
||||
Treatment and clinical signs of patients are mentioned in Table 1. Doxorubicine + paclitaxel was the most commonly used chemotherapy drug (9.3%). Out of 27(17.6%) patients 14 (51.85%) patients had LVD in Doxorubicine containing regime used (p <0.0001).
The majority of patients (95.1%) had no family history of diseases. Most common comorbidities were hypertension (42.6%), 36.1% had radiation therapy (RT) followed by 15.8% with diabetes mellitus type 2 (DM) and 3.8% patients had dyslipidemia.
Changes in LV strain before and after chemotherapy
The study found that there was no significant difference in LVEF at different time points (baseline, 1 month, 3 months, and 6 months). However, there was a significant reduction in LV GLS and LV GCS from baseline to 1 month, 3 months, and 6 months (Table 2). The average GLS decreased from -18.59 (1.03)% at baseline to -17.95 (1.59)% at 6 months (p=0.001). The average GCS decreased from -20.24 (1.11)% at baseline to -19.40 (3.75)% at 6 months (p=0.003). GRS decreased nonsignificantly from baseline to 39.90 (6.09) to 39.15 (3.7) at 6 months (p=0.07).
The mean difference of the all LV strains in normal LV function and LVD at baseline to 6-month follow-up are mentioned in Table 3.
|
Table 2. Changes of LV longitudinal, circumferential and radial strain before and after chemotherapy during follow-up |
||||||||
|
Variables |
At baseline |
At 1 month |
At 3 months |
At 6 months |
Greenhouse-Geisser df |
F |
Ƞ2 |
p |
|
GLS |
-18.59 (1.03) |
-18.39 (1.20) |
-18.01 (1.43) |
17.95 (1.59) |
1.31 |
23.91 |
0.14 |
0.001 |
|
GCS |
-20.41 (1.11) |
-20.20 (1.93) |
-19.47 (3.77) |
-19.40 (3.75) |
1.03 |
8.37 |
0.05 |
0.003 |
|
GRS |
39.9 (6.09) |
39.36 (5.76) |
38.22 8.02) |
39.15 (3.69) |
1.35 |
20.78 |
0.12 |
0.07 |
|
Data are presented as mean (SD), One-way ANOVA for repeated measures GLS- global longitudinal strain, GCS- global circumferential strain, GRS-global radial systolic strain |
||||||||
|
Table 3. LV strain mean difference |
|||||
|
|
Total (n=183) |
F |
Non LVD (n=156) |
LVD (n=27) |
p |
|
GLS |
|
|
|
|
|
|
Baseline |
-18.59 ± 1.03 |
0.62 |
-18.60 ± 1.04 |
-18.52 ± 0.98 |
0.71 |
|
1 Month |
-18.39 ±1.20 |
26.65 |
-18.50 ± 1.06 |
-18.50 ± 1.06 |
0.65 |
|
3 Month |
-18.01 ±1.43 |
0.61 |
-18.31 ±1.19 |
-16.24 ± 1.48 |
<0.0001* |
|
6 Month |
-17.95 ±1.59 |
13.03 |
-18.28 ± 1.28 |
-15.95 ± 1.83 |
<0.0001* |
|
GCS |
|
|
|
|
|
|
Baseline |
-20.41 ±1.11 |
0.39 |
-20.44 ± 1.1 |
-20.19 ± 1.21 |
0.29 |
|
1 Month |
-20.20 ± 1.93 |
9.69 |
-20.32 ± 1.11 |
-19.43 ±1.43 |
<0.0001* |
|
3 Month |
-19.47 ± 3.77 |
10.88 |
-20.16 ± 1.31 |
-15.33 ±8.51 |
<0.0001* |
|
6 Month |
-19.40 ± 3.75 |
12.71 |
-20.09 ± 1.43 |
-15.19 ±8.30 |
<0.0001* |
|
GRS |
|
|
|
|
|
|
Baseline |
39.90 ± 6.09 |
0.005 |
39.92 ± 6.47 |
39.7 ± 3.18 |
0.86 |
|
1 Month |
39.36 ± 5.76 |
6.03 |
39.77 ± 5.79 |
36.96 ± 5.04 |
0.02* |
|
3 Month |
38.22 ± 8.02 |
8.60 |
39.38 ± 6.11 |
31.24 ± 13.25 |
<0.0001* |
|
6 Month |
39.15 ± 3.7 |
79.33 |
40.07 ± 2.28 |
33.57 ± 5.44 |
<0.0001* |
|
Data are presented as mean (SD), One-way ANOVA for repeated measures GLS- global longitudinal strain, GCS- global circumferential strain, GRS-global radial systolic strain, LV- left ventricular, LVD – left ventricular dysfunction
|
|||||
A one-way repeated measures ANOVA preliminary screening revealed a violation of the sphericity assumption by using the Mauchly’s test, which yielded for GLS, GCS and GRS- F(5)= 0.436, 0.345 and 0.448, p<0.0001 consecutively. The ANOVA test with the degree of freedom adjusted by the Greenhouse-Geisser estimate yielded marginally significant results for GLS; F(1.308)= 23.91, p<0.0001. The effect size as measured by partial ƞ2 was 0.14, which is a large effect. For GCS; F(1.034)= 8.373,p= 0.004. The effect size as measured by partial ƞ2 was 0.054, which is a medium effect. For GRS F(1.345)=20.782,p <0.0001. The effect size as measured by partial ƞ2 was 0.12, which is a large effect. The post-hoc tests (pairwise comparisons) revealed that there was significant difference between the baseline to 6 month follow up values. The mean difference for GLS 0.203 (95% CI 0.352 to 0.053, p=0.002), GCS 0.209(95% CI 0.33 to 0.088, p=0.0001) and GRS 0.392 (95% CI 0.03 to 0.754, p=0.03).
Accuracy of LV strain in prediction of LVD
The highest incidence of LVD was in the 46 to 60 years age group 13(48.1%) and lowest in the 18 to 30 years age group (0%).
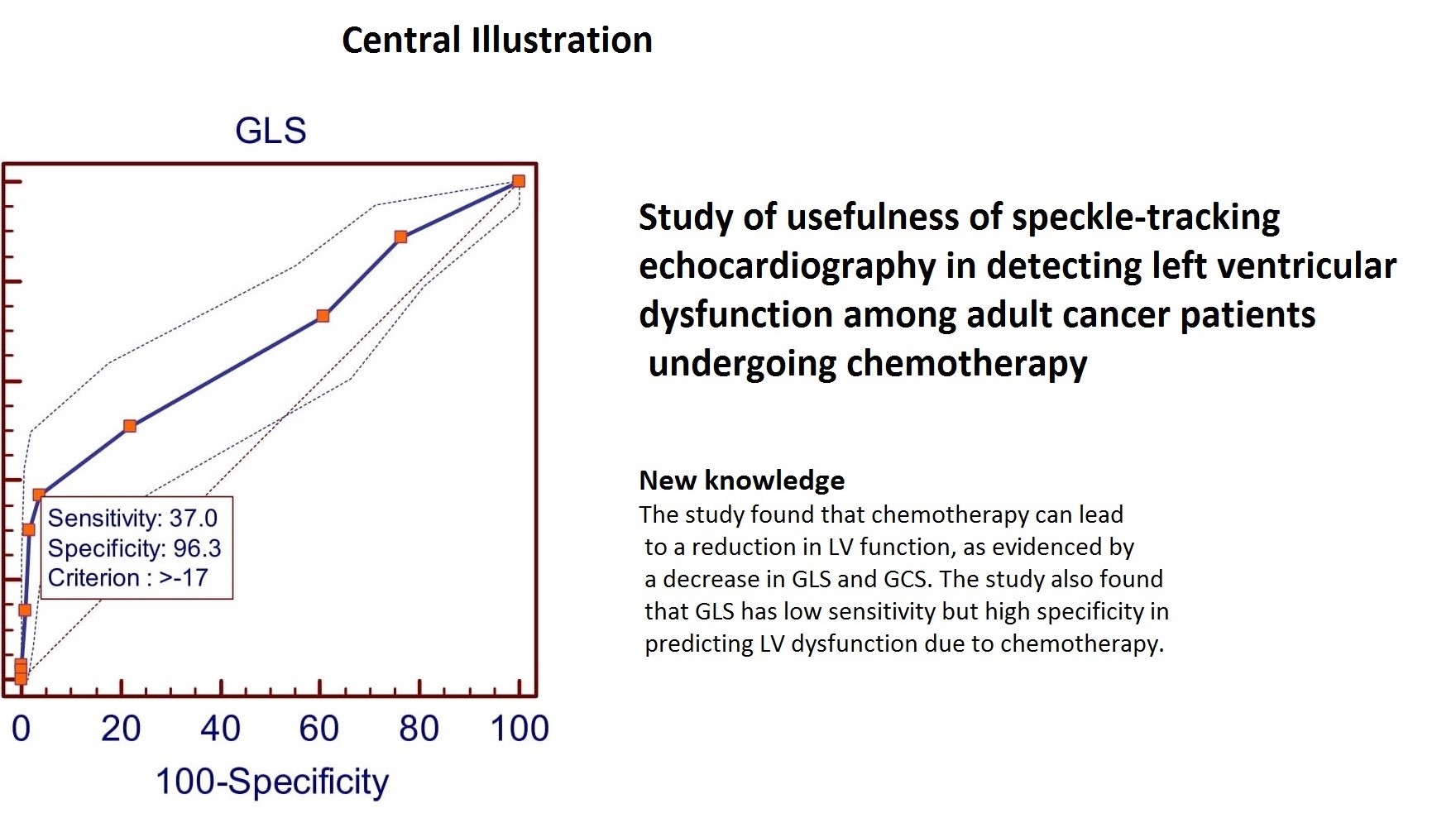
According to receiver operating characteristic curve (ROC curve) analysis the study found that GLS had a sensitivity of 37% and a specificity of 96.3% at a cut-off value of >-17 (Fig. 1). The positive predictive value (PPV) is 67.3%, and the negative predictive value (NPV) is 88.2%. The area under the curve (AUC) is 0.68 (95% CI 0.64 to 0.72; p<0.0001). GCS shows at the cut-off value of >-19 (Fig. 2) it gives 46% sensitivity and 94.9% specificity with 64.8% PPV and 89.6% NPV [AUC 0.73(95% CI 0.69 to 0.76; p<0.0001)]. GRS shows at the cut-off value of <=36 (Fig. 3) it gives 41% sensitivity and 97.8% specificity with 78.8% PPV and 89% NPV [AUC 0.73(95% CI 0.69 to 0.77; p<0.0001)].
In summary, the study found that chemotherapy can lead to a reduction in LV function, as evidenced by a decrease in GLS and GCS. According to ROC analysis reduction in GLS have less sensitivity and high specificity (37% sensitivity and 96.3% specificity) in predicting LV dysfunction.
Accuracy of GLS, GCS and GRS in prediction of LVD was 88.37% (95% CI 85.74 to 90.67%), 86.75% (95% CI 84.08 to 89.12%) and 86.20% (95% CI 83.49 to 88.62%) consecutively. GLS is different in LVD vs non LVD and the accuracy of GLS is more in prediction of LVD development during 6-month follow-up (88.37%).
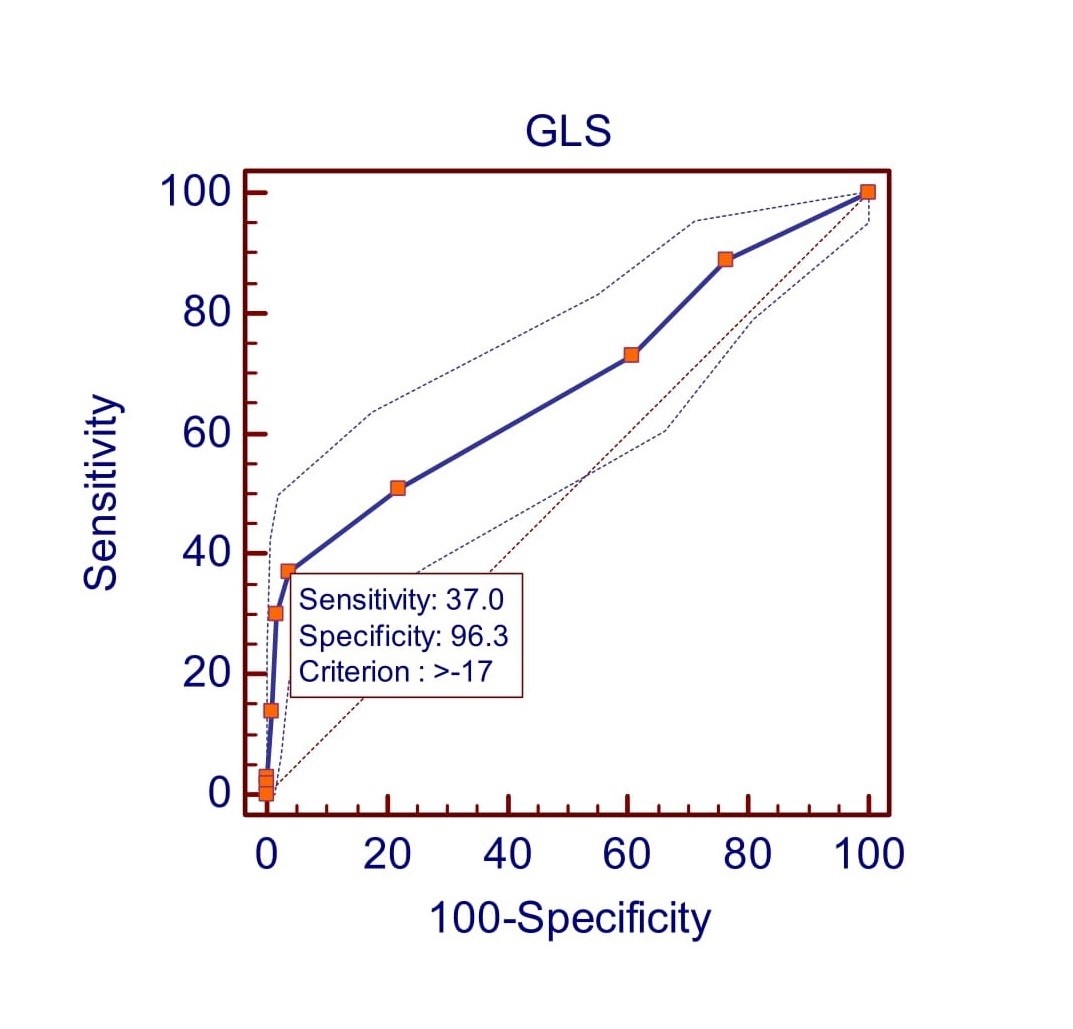
Figure 1. Receiver operating characteristic (ROC) curve predicted left ventricular dysfunction at >-17 by GLS.
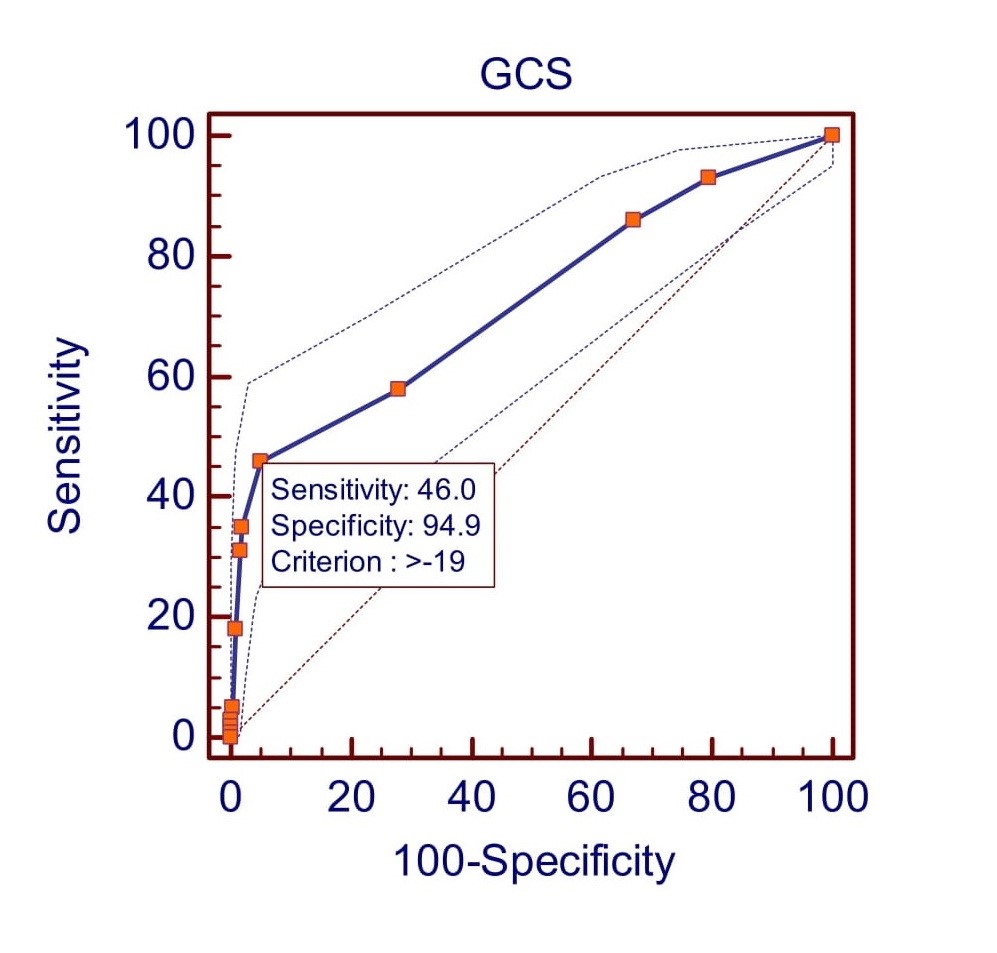
Figure 2. Receiver operating characteristic (ROC) curve predicted left ventricular dysfunction at >-19 by GCS.
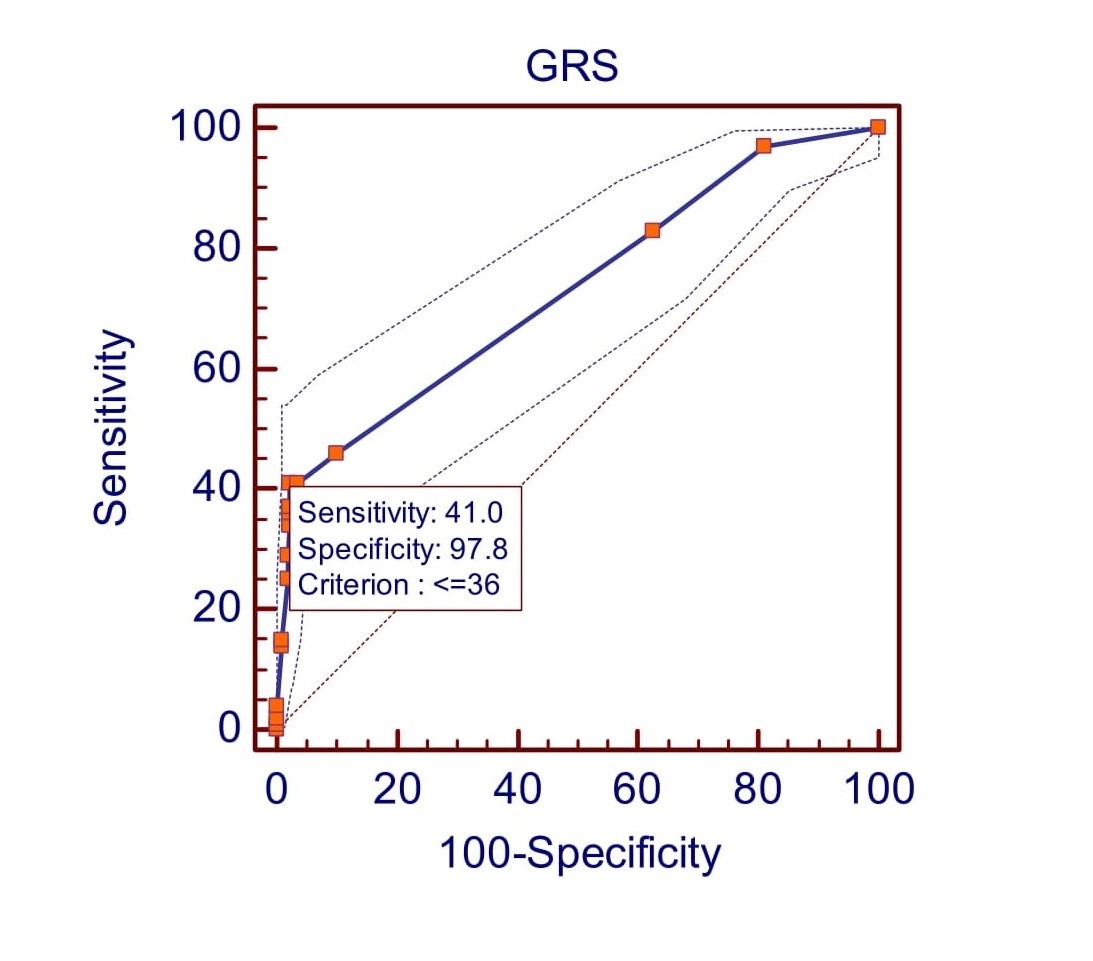
Figure 3: Receiver operating characteristic (ROC) curve predicted left ventricular dysfunction at ≤36 by GRS
Discussion
The present study aimed to evaluate the usefulness of speckle-tracking strain analysis in detecting subclinical LVD among adult cancer patients undergoing chemotherapy. We demonstrated that GLS and GCS decrease during chemotherapy and strain parameters have good accuracy in prediction of LVD development after chemotherapy in cancer patients.
In our study we show, that two-third (59.0%) of patients were male and 41.0% of patient were female. Kim et al. (12) reported mean age was 55 years in patients with LVD after cancer therapy. Singh et al. (13) found in his study 50% of male and 50% of female patients.
In our study, 15.8% of patients had DM, 42.6% of patients had hypertension, 4.9% % of patients had family history of risk factors. Kim et al. (12) revealed past history of hypertension in 17.1% patients, diabetes - in 8.5% patients and renal failure - in 3.7% of patients. Tang et al. (14) found that past history of hypertension was seen in 13.0% of patients, diabetes mellitus - in 6.0% of patients and hyperlipidemia was seen in 19.0% of patients (14). Arciniegas Calle et al. (15) found 6.1% of patient had family history of premature coronary artery disease and Tang et al. (14) reported family history of premature coronary artery disease was seen in 6.0% of patients.
Accuracy of GLS was 88.37% than GCS and GRS for predicting LV dysfunction at 6 months follow up after chemotherapy. The incidence of LVD was higher in our patients who received doxorubicin-containing regimens. Overall, strain imaging has the potential to improve the management and outcomes of cancer patients receiving potentially cardiotoxic treatments.
Our finding that speckle-tracking strain analysis is a useful tool for the early detection of subclinical LVD is consistent with previous studies that have shown that strain imaging is more sensitive than LVEF for detecting subtle changes in cardiac function (5). Furthermore, a decrease in GLS of more than 15% is associated with a higher risk of developing clinical heart failure (9). Laufer-Perl M et al. (16) reported reduction in GLS as an early marker for cardiac injury. Most of the studies reported that in patients during or after chemotherapy clinical value of GLS in predicting LVD appears to be more preferable than GCS and GRS (16-19). de Baat et al. (20) supported our findings that decrease in at the cut-off point of GLS >-17.5% suspecting LVD.
The higher incidence of LVD in patients who received doxorubicin-containing regimens is also consistent with previous studies that have identified anthracycline-containing regimens as a significant risk factor for CTRCD (4, 5). Present study reported 51.85% incidence of LVD (out of 27 patients 14 patients had LVD) in patients who received doxorubicin-containing regimens. Safaei et al. (21) reported 9% incidence of LVD in post chemotherapy after 2 week follow up. In our study incidence of LV dysfunction was 48.1% in 46 to 60 years of age group, 29.6% in 61 to 75 years of age group, 18.5% in 31 to 45 years of age group, 3.7% in 75 to 90 years of age group and no any case of LV dysfunction in 18 to 30 years of age group. In a Singh et al. (13) study LVD was more frequent in 43.73 (13.75) years of age group. These findings suggest that cancer patients who receive anthracycline-containing regimens require closer monitoring of their cardiac function using strain imaging.
EARLY-HEART study suggested a strong relationship between absorbed dose and the occurrence of subclinical LV dysfunction (22).
Study limitations
The study's limitations include its small sample size, limited follow-up period, and lack of a control group. Speckle tracking echocardiography strain evaluation mostly depends on the quality of the 2D echocardiographic images and it is Influenced by preloading conditions. Lack of long-term randomized clinical trials for assessing the ability of GLS to predict persistent reduced LVEF and lack of data as to the reproducibility of GLS in nonacademic centers or private hospitals are also limitations. The attraction of this approach is that there is a potential benefit for any patient, and those without dysfunction are not burdened by the treatment. The long-term clinical significance of subclinical LV dysfunction detected by strain imaging requires further evaluation.
Conclusion
The incidence of LVD was higher (51.48%) in patients with cancer who received doxorubicin-containing regimens. GLS is reduced in LVD and reduced GLS predicts LVD development during 6-month follow-up with accuracy of 88.37%.
Ethics: Informed consent was obtained from all individual participants. The study has been approved by institutional ethics committee (UNMICRC/CARDIO/2018/12).
Peer-review: External and internal
Authorship: C.U., A.S., M.K., V.S., K.N., K.K., D.J., R.C., P.R., and K.P. equally contributed to preparation of manuscript and fulfill authorship criteria.
Conflict of interest: None to declare
Acknowledgement and funding: This work was supported by U. N. Mehta Institute of Cardiology and Research Centre itself and received no specific grant from any funding agency, commercial or not for profit sectors.
References
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

