Superiority of 3D transesophageal echocardiography in assessment of mechanical prosthetic valve dysfunction as compared to 2D transesophageal echocardiography
ORIGINAL RESEARCH ARTICLE
Superiority of 3D transesophageal echocardiography in assessment of mechanical prosthetic valve dysfunction as compared to 2D transesophageal echocardiography
Article Summary
- DOI: 10.24969/hvt.2023.421
- Page(s): 312-320
- Electrocardiography
- Published: 14/09/2023
- Received: 25/07/2023
- Revised: 01/09/2023
- Accepted: 03/09/2023
- Views: 6143
- Downloads: 3774
- Keywords: 3D echocardiography, prosthetic valve, prosthetic valve dysfunction
Address for Correspondence: Kewal Kanabar, Department of Cardiology, U. N. Mehta Institute of Cardiology and Research Centre, Civil Hospital Campus, Asarwa, Ahmedabad-380016, Gujarat, India.
Email: kewal.kanabar14@gmail.com Mobile: +91-813-0160184, Fax: +91-079-22682092
Nilesh Chandak1, Hasit Joshi2a, Pooja Vyas2a, Kamal Sharma2a, Gajendra Dubey2a, Iva Patel2b, Kewal Kanabar2a
1Department of cardiology, PDMMC and Dayasagar hospital, Amravati, Maharashtra, India
2aDepartment of Cardiology and 2bDepartment of Research, U.N. Mehta Institute of Cardiology and Research Centre (UNMICRC), Civil Hospital Campus, Asarwa, Ahmedabad-380016, Gujarat, India
Abstract
Objective: The advent of 3D transesophageal echocardiography (TEE) was supposed to facilitate more accurate etiological diagnosis of prosthetic valve dysfunction as compared to 2D TEE; however, data to support the same is sparse especially in Asian Indians.
Methods: This was a prospective, open- label study of 50 consecutive patients with prosthetic valve dysfunction who were eligible for the study. All patients underwent both 2D and 3D TEE for assessment of prosthetic valve dysfunction apart from their demographics, clinical evaluation, laboratory assessment and fluoroscopy.
Results: Of 50 patients, 12 had aortic valve prosthetic dysfunction and 38 patients had mitral valve prosthesis dysfunction. Of these, 10 were male and remaining 40 were female patients. Overall, 41 (82%) patients had subtherapeutic prothrombin time at the time of presentation. Thrombus in situ was visualized in 34% of patients with 3D TEE as compared to 2D TEE (4%) (p=0.004). Pannus formation was observed in 20% of patients with 3D TEE as compared to 2D TEE, which could not identify pannus formation in any patient (p=0.03). In 34% of patients, normal motion of leaflets could be seen using 3D TEE as compared to 30% of patients by 2D TEE.
Conclusion: Compared to 2D TEE, 3D TEE imaging offers superior capabilities in assessing prosthetic valve dysfunction, particularly when evaluating thrombus and pannus formation. Additionally, both modalities show nearly similar effectiveness in assessing leaflet motion.
Key words: 3D echocardiography, prosthetic valve, prosthetic valve dysfunction
Introduction
Over the past 40 years, a huge range of prosthetic valves has been developed with intention of improving hemodynamics, increasing durability, and reducing complications (1). However, there is no perfect valve, and all prosthetic valves are susceptible to dysfunction. Although numerous advances have been made for the development of better prostheses, several problems related to their use as thrombosis, pannus formation, thromboembolism, hemolysis, tissue overgrowth, regurgitation and leaflet motion remain a challenge (2). The echocardiographic evaluation of prosthetic heart valves presents significant challenges, primarily related to differentiating the degree of obstruction based on valve type and size. This becomes particularly problematic when attempting to distinguish between mild obstructions resulting from pathological changes and those caused by prosthetic-patient mismatch (PPM). Moreover, the presence of shielding and various artifacts further complicates insonation, making the assessment of the valve and regurgitant jets associated with it even more difficult (3-6).
Transesophageal echocardiography (TEE) has evolved from one-dimensional imaging using a probe with a single-crystal, M-mode transducer to two-dimensional (2D) imaging with phased-array transducers and now three-dimensional (3D) imaging with matrix array transducers is also available. Recent probes of 3D imaging are extremely superior to the previous multi-plane TEE imaging technique to reconstruct 3D images (7). For non-invasive evaluation of prosthetic valve dysfunction, echocardiography is the one of the best available technology. Echocardiography of prosthetic valve is often challenging to do but helps to evaluate almost all aspects of prosthesis function after valve replacement as compared with native valves evaluation (8).
The utility of 3D TEE in prosthetic heart valve assessment has significantly improved with the current advancements in real-time 3D (RT3D) TEE technology. The introduction of the matrix array in TEE probes allows for seamless real-time acquisition and on-line display of 3D TEE images. This eliminates the need for sequential multi-plane acquisition or off-line reconstruction, making the evaluation of prosthetic heart valves much more efficient and convenient in clinical practice. These advancements have enhanced the accuracy and effectiveness of 3D TEE imaging, enabling better visualization and assessment of prosthetic heart valves, thus contributing to improved patient care and management (7-9). RT3D TEE has significantly improved the visualization and assessment of prosthetic valves by providing unique orientations and enhanced longitudinal connection sequences, which were not possible with conventional 2D echocardiography. These advanced images enable better identification of valvular anatomy and offer a more comprehensive understanding of the prosthetic valve's structure and function. The enhanced capabilities of RT3D TEE have revolutionized the way we evaluate prosthetic heart valves, leading to more accurate diagnoses and better patient outcomes.
The present study aimed to assess whether 3D TEE can provide an incremental diagnostic and descriptive values as compared to 2D TEE in assessment of various prosthetic valve dysfunction.
Methods
Study design
The present prospective, observational study was conducted at a tertiary care cardiology institute in Western India after approval by the Institutional Ethics Committee. Patients who fulfilled the inclusion criteria had given the consent to undergo clinical and echocardiographic evaluation. Inclusion criteria for the study: age of >18 years, NYHA class I-III and suspected prosthetic valve malfunction. Exclusion criteria for the study: patients who had presented with prothrombin time with INR >3, NYHA class IV, history of upper gastrointestinal tract bleeding, diaphragmatic hernia, high risk of bleeding, esophageal stricture, perforation, spasm, and diverticula. Patients having prosthetic valve dysfunction with hemodynamic instability were excluded.
Clinical examinations
All patients underwent detailed clinical and laboratory evaluation, and fluoroscopy.
Echocardiography
All patients underwent, 2D transthoracic (TTE), 2D TEE AND 3D TEE echocardiographic examinations by two experienced echocardiographers to avoid interpersonal and intrapersonal differences.
2D&3D TTE probe ( X 5- 1 ), 2D & 3D TEE probe (X 7-2t) with echocardiography machine Philip VIVID-I 33 echocardiography machine were used for imaging in the study. The paravalvular leak, leaflet movement abnormality, pannus, thrombus and valve dehiscence were defined according to the literature (10-13).
To acquire 3D images, standard 2D images were first acquired to locate the best plane or imaging the prosthetic valve. Gain settings were optimized using narrow-angled acquisition mode, which allowed real-time 3D imaging without the need for electrocardiographic gating. Subsequently, 3D zoom mode, with the biplane image feature was used to focus on the prosthetic valve and acquire images. Color, full-volume acquisition were performed. This mode requires ECG gating because the data set is compiled by merging narrower pyramidal scans that were obtained over 4–7 heartbeats. Once the 3D data sets were acquired, they were cropped to optimally visualize the cardiac structures (Tables 1 and 2).
Both the modalities were used to compare for presence or absence of thrombus, pannus and leaflet mobility abnormality.
Essential parameters we used in the comprehensive evaluation of prosthetic valve (14) are presented in Table 1.
Prosthetic valve malfunction was suspected on the basis of following 2D echocardiographic criteria (Table 2) (2, 15).
Statistical analysis
All statistical studies were carried out using SPSS program version 20 (IBM, New York, USA). Quantitative variables are expressed as the mean (standard deviation) and qualitative variables were expressed as percentage (%). A comparison of parametric values between two groups was performed using the independent sample t test. Categorical variables were compared using the Chi-square test. A nominal significance was taken as a two tailed p value<0.05.
|
Table 1. Essential parameters in the comprehensive evaluation of prosthetic valve (14) |
||
|
N |
Examination |
Parameter |
|
1 |
Clinical information |
Date of valve replacement Type and size of the prosthetic valve Height, weight, body surface area Symptoms and related clinical findings Blood pressure and heart rate |
|
2 |
Imaging of the valve |
Motion of leaflets Presence of calcification on the leaflets or abnormal echo densities on the various components of the prosthesis Valve sewing ring integrity and motion |
|
3 |
Doppler echocardiography of the valve |
Contour of the jet velocity signal Peak velocity and gradient Mean pressure gradient VTI of the jet DVI Pressure half-time in MV and TV EOA Presence, location, and severity of regurgitation |
|
4 |
Other echocardiographic data |
LV and RV size, function, and hypertrophy LA and right atrial size Concomitant valvular disease Estimation of pulmonary artery pressure |
|
5 |
Previous postoperative studies, when available |
Comparison of above parameters is particularly helpful in suspected prosthetic valvular dysfunction |
|
Reproduced from reference 10 under CC-BY license DVI – Doppler velocity index, EOA – effective valvular orifice area, LA - left atrium, LV – left ventricle, MV – mitral valve, RV –right ventricle, TV- tricuspid valve, VTI – velocity time integral |
||
|
Table 2. 2D echocardiographic parameters for suspecting mechanical prosthetic valve dysfunction (2, 15) |
|
|
Mitral valve prosthesis |
Aortic valve prosthesis |
|
Peak velocity>1.9 m/s |
Peak velocity>3 m/s |
|
Mean gradient>6 mm of Hg |
Mean gradient>20 mm of Hg |
|
VTI pr/VTI lvot>2.2 |
DVI< 0.3 |
|
EOA(cm2) <2 |
EOA<1.2 cm2 |
|
PHT>130 m/s |
AT>80 ms |
|
Presence of para valvular regurgitation |
Presence of paravalvular regurgitation |
|
Abnormal valve motion |
Abnormal valve motion |
|
AT – acceleration time, DVI - Doppler velocity index, EOA - effective valvular orifice area, lvot- left ventricular outflow tract, PHT - pulmonary hypertension, pr- pulmonary regurgitation, VTI - velocity time integral |
|
Results
Between June 2015 to March 2018, 50 patients were admitted in our institute with prosthetic heart valve dysfunction who met the eligibility criteria for the study. These patients were evaluated by 3D TEE in addition to 2D TEE for the prosthetic valve dysfunction. In our study, total male to female ratio was 1:4. The mean age group of the population was 45.26 (13.47) years.
Table 3 presents baseline clinical characteristics and their distribution by sex and age. Of 50 patients, 12 had aortic valve prosthetic dysfunction and 38 patients had mitral valve prosthesis dysfunction. The majority had bilealeft prosthetic valve. Left ventricular dysfunction was recorded in ¼th of patients and pulmonary hypertension was detected in 84% of patients.
Overall, 41 (82%) patients had subtherapeutic prothrombin time and 9 (18%) patients had therapeutic range of prothrombin time.
Out of total 50 patients, 19 patients were between age of 20 to 40 years of age of whom 2 were males (10.53%) and 15 - females (89.47%). Other group consisting of >40 years age, had 31 patients, with 8 (25.81%) males and 23 (74.19%) females.
|
Table 3. Baseline clinical characterizes of patients: distribution by sex |
|||
|
Variables |
Total |
Male (N=10) |
Female (N=40) |
|
Age group <40 years |
19 (38%) |
2 (10.53%) |
17 (89.47%) |
|
Age group >40 years |
31 (62%) |
8 (25.81%) |
23 (74.19%) |
|
Clinical presentation |
|||
|
NYHA class II dyspnea |
21 (42%) |
6 (60%) |
15 (37.5%) |
|
NYHA class III dyspnea |
26 (52%) |
4 (40%) |
22 (55%) |
|
Palpitations |
3 (6%) |
0 |
3 (7.5%) |
|
Location of dysfunctional prosthetic valve |
|||
|
Aortic valve prosthesis |
12 (24%) |
3 (30%) |
9 (22.5%) |
|
Mitral valve prosthesis |
38 (76%) |
7 (70%) |
31 (77.5%) |
|
Type of mechanical prosthetic valve |
|||
|
Bileaflet prosthetic valve |
41 (82%) |
8 (80%) |
33 (82.5%) |
|
Tilting disc prosthetic valve |
9 (18%) |
2 (20%) |
7 (17.5%) |
|
Left ventricular dysfunction (LVEF<50%) |
12 (24%) |
3 (30%) |
9 (22.5%) |
|
Pulmonary artery systolic pressure > 50 mm of Hg |
42 (84%) |
9 (90%) |
33 (82.5%) |
|
Sub-therapeutic Range of INR |
41 (100%) |
9 (18%) |
32 (64%) |
|
Therapeutic Range of INR |
9 (100%) |
1 (2%) |
8 (16%) |
|
INR – international normalized ratio, LVEF – left ventricular ejection fraction |
|||
3D TEE evaluation of prosthetic valve dysfunction
Table 4 shows that 3D TEE had better diagnostic yield than 2D TEE in evaluation of prosthetic valve dysfunction. On 2D TEE, thrombus was visualized in only 2 (4%) patients, whereas 3D TEE showed thrombus in 17 (34%) patients. Pannus could not be diagnosed in any patient on 2D TEE, whereas 3D TEE detected pannus in 10 (20%) patients. Normal motion of leaflets was visualized in 15 (30%) patients on 2D TEE and 17 (34%) on 3D TEE, and restricted mobility was observed in 35 (70%) patients and 33 (66%) patients on 2D TEE and 3D TEE, respectively.
|
Table 4. Comparison of 2D and 3D echocardiography in mechanical prosthetic valve evaluation |
|||
|
Variables |
2D TEE |
3D TEE |
p |
|
Presence of thrombus, n(%) |
2 (4) |
17 (34) |
0.0004* |
|
Pannus formation, n(%) |
0 (0) |
10 (20) |
0.027 |
|
Prosthetic valve dehiscence |
1(2) |
2(4) |
1.000 |
|
Paravalvular leak, n(%) |
2(4) |
2(4) |
1.000 |
|
Normal motion of leaflets, n(%) |
15 (30) |
17 (34) |
0.8303 |
|
Restricted mobility of leaflets, n(%) |
35 (70) |
33 (66) |
0.8303 |
Figure 1 shows 2D TEE image of stuck aortic prosthetic valve showing no obvious clot or pannus. Figure 2 presents aortic prosthesis from aortic aspect showing pannus in growth with restricted mobility of one leaflet by 3D TEE. Figure 3A presents 2D TEE image of small thrombus on mitral prosthesis and figure 3B shows 3D TEE image of clot on left atrial aspect of mitral prosthesis. Figure 4 displays comparison of 2D and 3D mitral prosthesis of mitral valve prosthesis thrombus. Figure 5 presents the study one stuck leaflet through cine fluoroscopy.
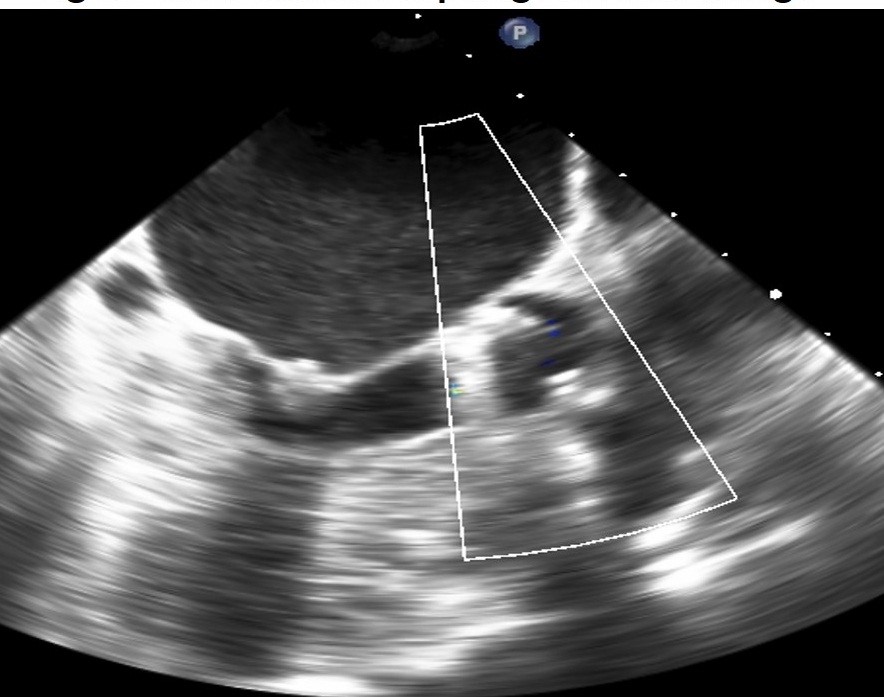
Figure 1. 2D transesophageal echocardiographic image of stuck aortic prosthetic valve withut signs of obvious thrombus or pannus
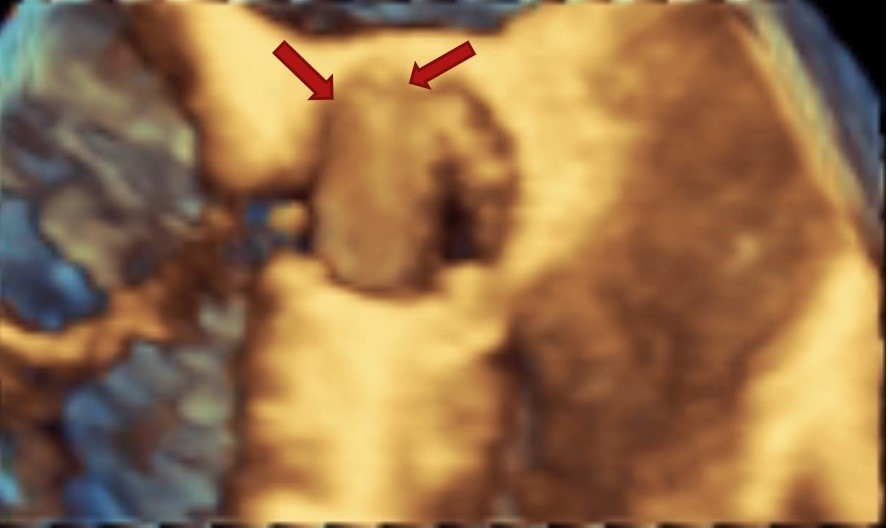
Figure 2. 3D transesophageal echocardiographic image from aortic aspect showing pannus ingrowth with restricted mobility of one leaflet
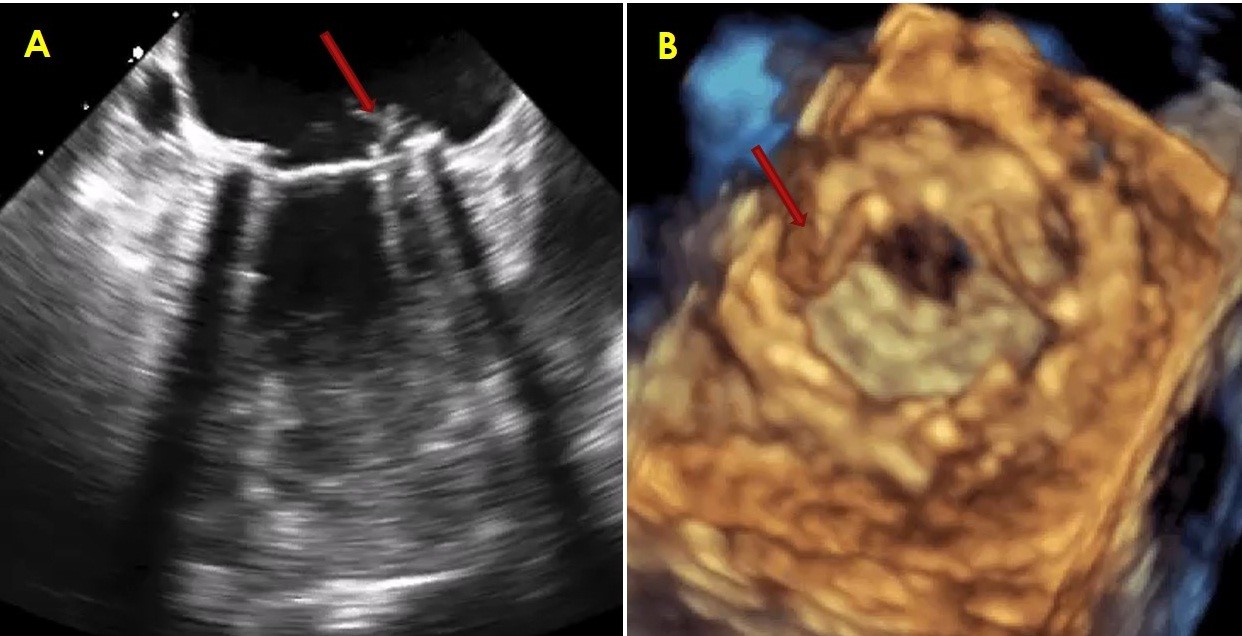
Figure 3. 2D transesophageal image (left) of small thrombus on mitral valve prosthesis and 3D transesophageal image (right) of small thrombus on left atrial aspect of mitral valve prosthesis
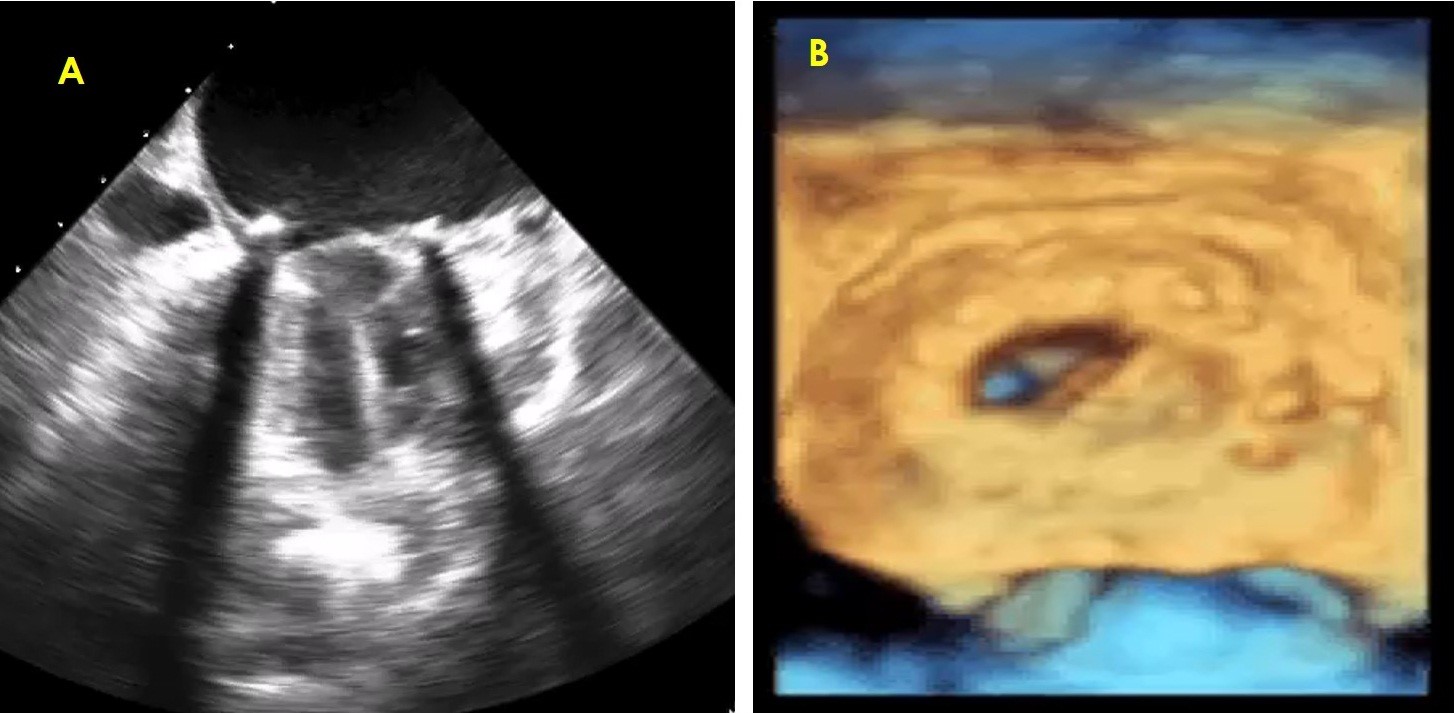
Figure 4. Comparison between 2D (left) and 3D (right) transesophageal images of bileaflet mitral prosthesis with thrombus on one leaflet with restricted mobility
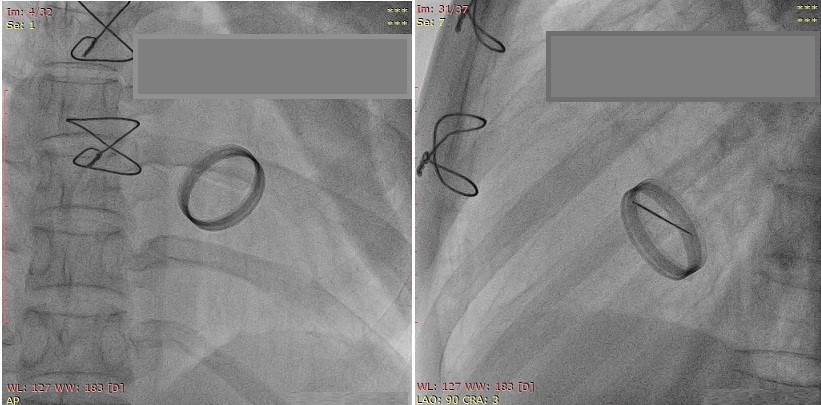
Figure 5. Cine-fluoroscopy image shows stuck one leaflet
Discussion
RT3D imaging permits clinically valuable imaging of prosthetic valve components such as the leaflets, rings and struts of all prosthetic valves irrespective of position. This is particularly beneficial for the assessment of mechanical mitral and aortic valves and visualization of ventricular side of mitral prosthetic valves where 2D images are often of low quality due to acoustic shadowing. RT3D does not substantially lengthen procedural time. Acquisition times for RT3D imaging routinely requires approximately 10 additional minutes.
It is essential to differentiate between thrombus and pannus as the underlying etiology of obstruction, if thrombolytic therapy is contemplated. TEE along with other clinical factors can help to distinguish the two entities (16). Thrombi in general are larger and have a soft ultrasound density, similar to that of the myocardium. Detection of abnormal prosthetic valve motion by TEE is more common in valves with thrombus. In 30% of cases, pannus formation includes small dense mass though their specific features may not be distinctly visualized. Pannus formation was more common in aortic position as shown in figures 1 and 2. As compared to pannus formation, obstruction due to thrombus is often associated with a shorter duration of symptoms and with a history of inadequate anticoagulation (17). The combination of findings of a soft density on the prosthesis and an inadequate international normalized ratio has reported positive and negative predictive values of 87% and 89%, respectively, for thrombus formation (16). Thrombus formation may interfere with the mechanism of valve motion and cause significant obstruction that may be catastrophic (Fig. 3 and 4).
Recently, fibrinolytic therapy has emerged as an alternative to surgical treatment for obstructed left-sided prosthetic valves and is considered the treatment of choice for tricuspid valve thrombosis (18-20). If thrombolysis is anticipated, TEE should be performed for opinion and risk stratification. A thrombus area on TEE of <0.85 cm2 poses a lower risk for embolic phenomena or death associated with thrombolysis (18). Doppler echocardiography is the preferred modality to assess serially the hemodynamic success of thrombolysis (18). It is important to remember that pannus and thrombus may both be present in some cases.
Patient had also undergone cinefluoroscopy, which suggested bileaflet prosthetic valve was stuck for one of the leaflets (Fig. 5). Patients in whom 3D TEE evaluation identified pannus on prosthetic valve causing restricted mobility of the leaflet, perioperative findings confirmed presence of pannus. Images of one patient are displayed showing superiority of 3D echocardiographic over 2D echocardiographic evaluation in prosthetic valve dysfunction.
In our study, 3d TEE was found of similar accuracy to 2d TEE in diagnosing prosthetic valve dehiscence, paravalvular leak and leaflet motion abnormality. Sordelli et al. (2) showed superiority of 3d TEE in the assessment of paravalvular leak by improved localization and providing accurate size and shape of paravalvular leak.
Study limitations
We had excluded patients who presented with prosthetic valve dysfunction with hemodynamic instability. Our study population is small, so a larger sample study is advisable. Patients with valvular heart disease who presented with arrhythmia at the time of imaging, had suboptimal images with artifacts due to poor ECG gating during image acquisition.
Conclusion
In conclusion, our study suggests that 3D TEE is superior to 2D TEE in the assessment of mechanical prosthetic valve dysfunction. Its enhanced diagnostic accuracy makes it a valuable tool for clinicians in the evaluation of patients with mechanical prosthetic heart valves. Incorporating 3D TEE into routine clinical practice for these cases can lead to better patient outcomes and management decisions.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: N.C., H.J., P.V., K.S., G.D., I.P., and K.K. equally contributed to the study and preparation of manuscript and fulfilled authorship criteria.
Acknowledgement and funding: This work was supported by U.N. Mehta Institute of Cardiology and Research Center itself and received no specific grant from any funding agency, commercial or not-for-profit sectors
References
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

