Left atrial appendage thrombus formation despite oral anticoagulation: what can we do when there is nothing to offer?
ORIGINAL RESEARCH ARTICLE
Left atrial appendage thrombus formation despite oral anticoagulation: what can we do when there is nothing to offer?
Article Summary
- DOI: 10.24969/hvt.2023.426
- Page(s): 338-346
- CARDIOVASCULAR DISEASES
- Published: 11/10/2023
- Received: 23/08/2023
- Revised: 30/09/2023
- Accepted: 01/10/2023
- Views: 6073
- Downloads: 3911
- Keywords: oral anticoagulation failure, thrombus, left atrial appendage, stroke. Devices, atrial fibrillation
Address for Correspondence: Luis Antonio Arabia, Arrhythmia Unit, Instituto Oulton, COrdoba. Argentina
E-mail: luisarabi@hotmail.com ORCID: 0000-0003-0673-0234
Luis Antonio Arabia, Juan Jose Luciano
1Arrhythmia Unit, Instituto Oulton, Cordoba, Argentina.
2Catheterization Laboratory, Hospital Nacional de Clinicas, Cordoba, Argentina
Abstract
Objective: Atrial fibrillation (AF) is the most common sustained arrhythmia in the general population and the main common cause of embolism of cardiac origin, both brain and systemic. In about 90% of nonvalvular cases the source is the left atrial appendage (LAA), and the usual prevention approach is oral anticoagulation, but sometimes it fails. LAA thrombus is a classical contraindication to perform procedures in the left atrium. The aim of the study was to share experience with closure of LAA in patients with AF and with left atrial thrombus.
Methods: We present our prospective experience from January 2021 to January 2023 with 11 patients of 275 patients referred for AF ablation, who presented thrombus in the computed tomography scan previous the procedure. Modifications of the usual LAA occlusion technique (no touch technique and brain protection) was used.
Results: All patients were subjected to LAA occlusion with good success, without complications as leaks, device related thrombus, stroke or systemic embolism. Only one vascular access complication was seen. Different therapeutic strategies are discussed, including intensification of anticoagulation.
Conclusions: In patients with nonvalvular AF and failure of anticoagulation, primary percutaneous LAA closure is a possible therapy using specific techniques of image analysis, programming and performance of the procedure. More studies are needed in order to validate this therapy.
Key Words: oral anticoagulation failure, thrombus, left atrial appendage, stroke. Devices, atrial fibrillation
Introduction
Atrial fibrillation (AF) is the most prevalent sustained cardiac arrhythmia and it is considered the major cause of cardiac thromboembolism, both systemic and encephalic (1).
In patients with nonvalvular AF, the thrombus is usually originated in an anatomical structure called left atrial appendage (LAA) (2, 3). Left atrial dysfunction (atrial myopathy) has recently been linked to these phenomena, even in sinus rhythm, and LAA exclusion is an accepted therapy for decades and is associated with a decrease in stroke events(4, 5)
The classic thromboembolic prevention in high-risk patients is oral anticoagulation (OAC) (5), but unfortunately we are observing a growing number of patients who present LAA thrombus formation despite being under this treatment, which is linked to event risk and a false sense of protection to the patient and the treating physician (6-14).
The thrombus of the LAA is classically considered a contraindication to performing procedures in the left atrium and especially in the LAA (15-21), but is there something to offer to these patients in whom the OAC has failed?
The aim of the study was to share experience with closure of LAA in patients with AF and with left atrial thrombus.
Methods
In this paper, we present our prospective experience with 11 patients with LAA thrombus of 275 patients referred for ablation of nonvalvular AF, enrolled from January 2021 to January 2023. All of the patients gave their informed consent and this study was approved by the Ethical Committee.
All patients underwent clinical, laboratory, electrocardiographic and echocardiographic examinations. Computed tomography (CT) was used to confirm presence of LAA thrombus or sludge. Transesophageal echocardiography (TEE) was used to guide LAA closure
We assessed demographic variables, risk factors for thromboembolic events, CHADS-VASc2 score, presence of heart failure, complications as leak, stroke, bleeding, and pseudoaneurysm, and OAC.
Left atrial appendage closure was performed in all patients – see results section for details of procedure.
Minimum follow-up was 7 months and maximum 31 months.
Results
Overall 11 patients of 275 patients referred for ablation of nonvalvular AF, underwent LAA closure.
Nine of them had persistent AF, all of them were under OAC. LAA occupying lesion (thrombus or sludge) was detected in all patients on CT performed as part of the pre-ablation protocol.
As can be seen from Table 1, all patients were male, average age 59 years (41-71), CHADS-VASC2 1.4 (0-3), being 1 the most frequent and 3 in only one patient. One of patents was under treatment with apixaban 5 mg twice a day and the rest- under rivaroxaban 20 mg in single doses per day.
The most common item of the CHADS VASC2 score found in our population was hypertension and the second one was heart failure.
Left atrial appendage closure was feasible in all of them, with neither leak nor complications (stroke, bleeding), except a right femoral artery pseudoaneurysm.
Procedure planning
Procedure planning consisted of:
A) image analysis
B) management of prior anticoagulation
C) choice of the device
D) implant technique
E) subsequent pharmacological management
A) Image analysis
We based the image analysis on 80 slices cardiac CT scan with three-dimensional volumetric reconstruction, initially with determination of the presence or absence of lack of contrasted filling in the LAA. In case of filling defect on the arterial (first sequence), our protocol included delayed acquisition and prone position sequence (22). This helps to differentiate thrombus versus sludge or slow circulation.
Other very important points to define are the location, proximal extension of the thrombus and LAA morphology. In the case of ``chicken wing`` morphology, we do not base the differentiation of proximal or distal on 50% of the extension of the appendage but from where it presents the most pronounced curve, from the ostium to the bending we consider proximal and from it towards the tip as distal.
|
Table 1. Clinical characteristics of patients |
|||||
|
|
Age |
Sex |
Heart Failure |
Chads -Vasc2 score |
OAC |
|
1 |
41 |
Male |
Yes |
1 |
Apixaban 5 mg T/D |
|
2 |
59 |
Male |
No |
0 |
Rivaroxaban 20 mg O/D |
|
3 |
58 |
Male |
No |
2 |
Rivaroxaban 20 mg O/D |
|
4 |
61 |
Male |
No |
1 |
Rivaroxaban 20 mg O/D |
|
5 |
63 |
Male |
No |
1 |
Rivaroxaban 20 mg O/D |
|
6 |
71 |
Male |
Yes |
3 |
Rivaroxaban 20 mg O/D |
|
7 |
54 |
Male |
No |
1 |
Rivaroxaban 20 mg O/D |
|
8 |
63 |
Male |
No |
1 |
Rivaroxaban 20 mg O/D |
|
9 |
67 |
Male |
Yes |
2 |
Rivaroxaban 20 mg O/D |
|
10 |
60 |
Male |
No |
2 |
Rivaroxaban 20 mg O/D |
|
11 |
57 |
Male |
No |
1 |
Rivaroxaban 20 mg O/D |
|
OAC – oral anticoagulation, O/D – once daily, T/D - twice daily |
|||||
A fundamental point is to analyze the images in the three planes (axial, coronal and sagittal), since only in this way we can see the real extension and proximal compromise, which in partial visualizations can be overlooked, this information allows defining the best approach strategy ( Fig. 1, 2).
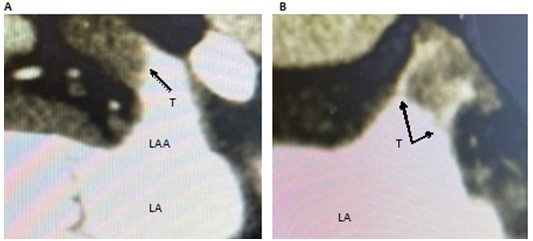
Figure 1. A Sagittal view, a distal thrombus, apparently in contact with the LAA wall and finishing smooth with the proximal segment free. B Coronal view of the same patient showing contrast between the thrombus and the LAA aall and thin prolongations / debris spreading to the ostium
LA - left atrium, LAA - left atrium appendage, T – thrombus
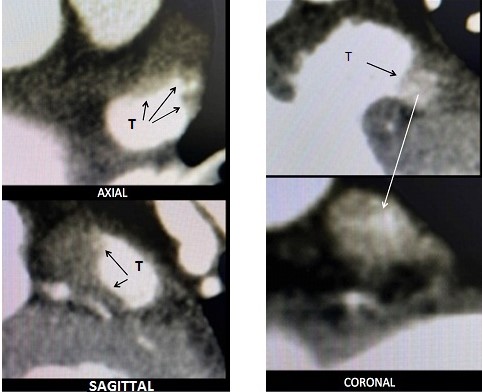
Figure 2. The coronal view suggest a distal occupation of one lobe (anterior), but in the axial view the thrombus is seen in the body of the LAA and sagittal view shows its prolongation to the ostium
LAA – left atrial thrombus
B) Management of prior anticoagulation
It is the only opportunity where we perform a bridge to parenteral anticoagulation with low molecular weight heparin adjusted for the patient's weight from 72 hours prior to the procedure.
C) Choice of device
All the devices used were double-sealed (plug/umbrella + disc), Amulet (Abott) and Lambre (Lifetech).
D) Technique
Right and left femoral artery and venous accesses were made. A decapolar catheter was placed in the His bundle zone. Bilateral carotid arteriography was performed to assess their patency. Filters were then positioned in the common carotid arteries and remain undeployed. Unfractionated heparin 80 IU/Kg was administered as an initial bolus and subsequent corrections were guided by the activated coagulation time.
Transseptal puncture was performed under radiological and transesophageal ultrasound (TEE) guidance; immediately prior to cross the septum, the carotid filters were deployed.
Sheath exchange was performed in the left superior pulmonary vein, pigtail catheter was placed close to the LAA ostium, and subselectively a very low-pressure manual injection of 2.5 cm of contrast was injected to confirm its position. Measurements (size) were made from the previous CT and TEE at the procedure (Fig. 3)
The technique used was the no touch described in other publications (see in Discussion). There was no need for recaptures or repositioning.
In all cases the device could be implanted successfully. No leaks at the time of implantation or follow-up were observed. No device-related thrombi (DRT) were detected in TEE and CT controls at 3 and 6 months, respectively.
E) Subsequent pharmacological management
Eight patients were administered apixaban 5 mg every 12 hours for 90 days, and after DRT was ruled out on TEE, double antiplatelet therapy (100 mg aspirin and 75 mg clopidogrel daily) was administered. In the remaining patients, the regimen used was dual antiplatelet therapy.
In one patient, there was a vascular complication (pseudoaneurysm) that was surgically resolved. No bleeding or embolic complications were recorded during clinical follow-up. In the control TEE at 90 days and CT at 180 days, there were no leaks or thrombi related to the device.
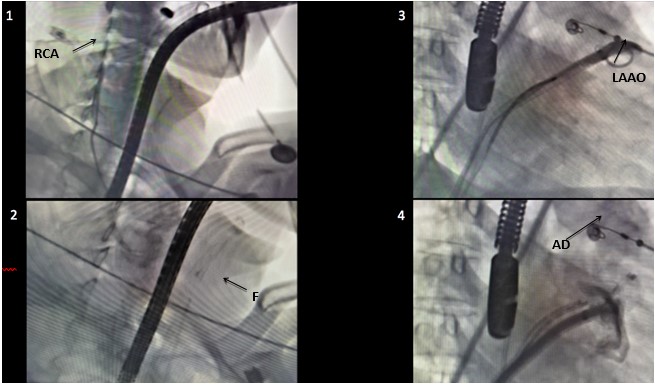
Figure 3. 1. Carotid angiography, right carotid artery (RCA). 2. Undeployed filter in left carotid artery. 3. Subselective small contrast injection close to the LAA ostium (LAAO). 4. Amulet device (AD) deployed in the LAA confirming it sealing by angiography.
LAA – left atrial appendage
Discussion
The occupying lesion of the LAA is classically considered a contraindication to performing procedures in the left atrium and especially in the LAA (18-21).
Patients under OAC who present with thromboembolic events or if LAA occupying lesion is detected in images (TEE and/or CT) constitute a very high-risk population, and pose a great therapeutic challenge and there is no a universally accepted way to deal with this problem (18, 20, 21).
The incidence of LAA thrombus or LAA sludge in patients under adequate OAC is little known. In our recent experience, it is 4%, almost all of them were under treatment with rivaroxaban 20 mg once daily. The number reported in the consulted literature varies from 4 to 24% (20, 24 - 28).
The most frequently found arrhythmia was persistent AF (18).
In our series, the average CHADS-VASC2 score (the most used in the assessment of embolic risk in this population) was 1.4, suggesting that the real risk could be underestimated using this score alone (29-31), in other series the average was 3 (18). We believe that the evaluation of left atrial size, presence of spontaneous contrast, LAA mechanic dysfunction, evaluated by low velocity in the ostium, morphology - multilobed LAA or cauliflower were associated with a higher incidence of TE events - (32 - 35) could contribute to personalize the embolic risk in addition to the CHADS-VASc2 Score.
The most frequently adopted therapies are intensification of anticoagulation, surgical removal and percutaneous closure of the LAA (18).
Modification of anticoagulation can be by changing to a parenteral regimen (intravenous with unfractionated heparin or subcutaneous with low molecular weight heparin), or in the oral route by changing or increasing the dose of the drug used, and a third modality, adding antiplatelet therapy to the OAC (20, 36, 37, 38).
Efficacy in thrombus resolution ranged from 41-78%, total or partial, depending on the series consulted, regimen used, and reassessment time (18, 20, 21, 30, 39, 40)
In a study comparing intensification of anticoagulation versus primary LAA closure, the incidence of bleeding was 9.6 vs 2.9 and cerebrovascular events 3.8 vs 0%, respectively (20). Jin et al. (31), reported the absence of embolic or hemorrhagic events both during the procedure and during follow-up. It should be noted that 4 of the 7 patients treated had a proximal location of the thrombus, defining a population with a higher risk of material detachment. In a meta-analysis prior to the previous article, which included 58 patients, one suffered a stroke, one major bleeding, and DRT was detected in two, one of whom was under dual antiplatelet therapy and the other under direct anticoagulant (18).
In order to be able to continuously assess the neurological status of patients and act promptly in case of alterations, it has been proposed to perform the procedure under local anesthesia in those who tolerate the transesophageal probe (31).
In case of opting for percutaneous closure, the no touch technique is used, paying special attention to avoiding recaptures and contrast injections inside the LAA. The ball shape is formed in the ostium and advances smoothly to the landing zone (18, 20, 41-47). The Amulet device allows, due to its structure, to adopt after the conformation of the ball another one similar to a triangle, increasing the surface of the distal end of the device and thus covering a greater area when advancing in the body of the appendage, thus increasing the protection of migration of thrombus fragments in case of its detachment (29).
The incidence of acute brain lesions detected in magnetic resonance imaging has been described in 48% after LAA occlusion (48), so using of cerebral protection systems is prudent, but it was variable, remaining at the discretion of the operator, 9 to 100% (18, 20, 25, 43, 44, 49, 50), reserving it in general for patients with proximal location of the thrombus. The devices used were: Sentinel (Boston Scientific), with right radial arterial access 6 French and it protects bilateral carotid flow; Triguard System (Keystone Heart) that also covers all three supra-aortic vessels (brachiocephalic, left common carotid and left subclavian arteries- including the protection of the vertebral arteries-) (50), and the individual carotid filter (31), with one 8F and two 6F femoral arterial approach, respectively. It should be kept in mind that cerebral protection devices are designed for cerebral but not systemic protection. The arguments used by those who do not use them are cost, fear of complications in vascular access, and incomplete protection.
The location of the thrombus in most of the publications was defined as proximal or distal taking 50% of the body of the LAA measured in most of the series in TEE at 45° in its longitudinal long axis (18). We made the evaluation based on CT images in the three views (axial, coronal and sagittal), since partial views can generate incomplete visualization of the thrombus, especially in its proximal extension.
Appendages that present a large proximal bending (chicken wing) for technical reasons of approach and embolic risk, proximal should be defined from the ostium to the beginning of the curve. The thrombus position conditioned the strategy, with a greater indication of intensification of anticoagulation in those with a proximal location and younger patients and reserving primary closure for distal thrombus, older patients or with a formal contraindication for anticoagulation (20, 29). In some centers, both procedures were combined, with initial anticoagulation intensification followed by percutaneous closure, specifically in proximal localized thrombi (20, 25, 31). In addition to the obvious hope of their dissolution or reduction in size, there is the hypothesis that they could change their structure turning less friable, and those organized and older would have less possibility of displacement and embolization (21). Those that protrude from the ostium into the atrial cavity were considered a contraindication for direct percutaneous closure (20).
Double closure devices, Plug/Umbrella + disc, were the most used, between 75-85% for the Amulet, ACP and Lambre group vs 15-25% for Watchman (specifically Watchman Flex) (18, 20, 24, 25, 31, 50). Recently, a beneficial synergistic effect of continuing OAC plus LAA occlusion in high-risk patients without contraindications for anticoagulation in reducing systemic and cerebral embolic events, as well as all-cause mortality, has been proposed (4, 24, 51, 52).
In our series, the presence of DRT was not detected during follow-up; its incidence has been reported to be 1.5 - 14% (20, 24, 53, 54). Margonatto et al. (24), reported 13% of DRT in their patients, occurred in those who had been discharged under dual antiplatelet therapy, and it resolved when switching to OAC.
Study limitations
This study has several limitations, first the small sample, second it was done by one experimented operator in a single center, and its results should not be extrapolated directly.
More studies are needed in order to validate this therapy as well as to assess the thrombogenic risk in this defined subpopulation.
Conclusions
In patients with nonvalvular AF and failure of anticoagulation with presence of LAA thrombus or sludge, primary percutaneous LAA closure is a possible therapy using specific techniques of image analysis, programming and performance of the procedure.
Left atrial appendage thrombus has been considered as a classic contraindication to performing procedures in the left atrium and even more LAA closure. In patients with failure of OAC, the intensification of anticoagulation is the most widespread course of action, but due to the increase in bleeding and the high rate of failure to resolve the thrombus, percutaneous primary LAA closure is a possible therapy using specific techniques of analysis, programming and performance of the procedure.
Ethics: All patients provided informed consent for all procedures and
study was approved by Ethics Committee
Peer-review: External and Internal
Conflict of interest: None to declare
Authorship: L.A.A. and J.J.L. equally contributed to the study and preparation of manuscript
Acknowledgement and funding: None to declare
References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

Fitz Roy Mountain, Santa Cruz Province, Patagonia, Argentina. Luis Arabia, Cordoba, Argentina
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

