Single-center adult cardiac surgery practice during the COVID-19 era
ORIGINAL RESEARCH ARTICLE
Single-center adult cardiac surgery practice during the COVID-19 era
Article Summary
- DOI: 10.24969/hvt.2023.439
- Page(s): 347-354
- CARDIOVASCULAR DISEASES
- Published: 03/12/2023
- Received: 05/07/2023
- Revised: 30/10/2023
- Accepted: 01/11/2023
- Views: 4213
- Downloads: 3535
- Keywords: COVID-19, COVID-19 markers, NT-pro-BNP, congestive heart failure, cardiac surgery, early outcomes, cardiac surgery procedures
Address for Correspondence*: Damirbek Abibillaev, Faculty of Medicine, International Ala-Too University, Bishkek, Kyrgyz Republic; Department of Pharmacology, Kyrgyz State Medical Academy, Bishkek, Kyrgyz Republic
Email: kg.damir.da@gmail.com
Elmira Tukusheva 1, Taalaibek Kudaiberdiev 1, Damirbek Abibillaev 1,2*, Irina Achmedova 1, Akmaral Kurmanbekova 1,3, Aizhamal Islamova 1
1Department of Consultative-Diagnostic Unit, Research Institute of Cardiac Surgery and Organ Transplantation, Bishkek, Kyrgyz Republic
2Faculty of Medicine, International Ala-Too University, Bishkek, Kyrgyz Republic
3Department of Pharmacology, Kyrgyz State Medical Academy, Bishkek, Kyrgyz Republic
Abstract
Objective: Like other activities, cardiac surgical practice was also affected by pandemics. During the COVID-19 outbreak, it was crucial distinguishing the preoperative data of COVID-19 positive candidates for open-heart surgery from COVID-19 negative counterparts, as well as the influence of positive viral markers on earlier postoperative results.
Methods: We retrospectively analyzed 177 patients who were operated in the Research Institute of Heart Surgery and Organ Transplantation from September 2020 to December 2021. Patients were categorized according to COVID-19 positive (CP) and COVID-19 negative (CN) groups. Baseline clinical, laboratory, imaging markers and perioperative findings were compared. Some of the perioperative findings were considered outcome variables. Due to unequal distributions, nonparametric tests were used for statistical analysis. Continuous variables were represented by mean and standard deviations, whereas categorical ones were by absolute count and percentage.
Results: No substantial differences were detected in this small comparison study. Baseline clinical, laboratory and echocardiographic findings revealed similar conditions in both groups. Only hemoglobin levels were found higher in CP group (142.8 (18.2) vs 137.7 (19.9), p=0.03). The mostly performed operation was CABG-surgery in each group (22 vs 37 cases). There were no differences in intraoperative variables. Most of the patients of CN group presented by exacerbated heart failure signs in earlier postoperative period. Single mortality case was observed in CN group.
Conclusion: In our cohort of patients, clinical, laboratory, imaging and perioperative parameters were not differed significantly in CP and CN groups altogether with some exceptions. Cardiac surgery was found as the relatively safe procedure during pandemics era. Congestive heart failure was the main event in the earlier post-op period.
Graphical abstract
Keywords: COVID-19, COVID-19 markers, NT-pro-BNP, congestive heart failure, cardiac surgery, early outcomes, cardiac surgery procedures
Introduction
COVID-19 is the most life-threatening infection caused by RNA-coated coronavirus 2 (SARS-CoV-2), firstly emerged in 2019 in Wuhan province of China and declared as the pandemic by WHO due to its epidemiological background (1). As of July 15, 2022, more than 560 million cases have been reported worldwide and more than 6.3 million deaths have been confirmed, making the COVID-19 pandemic one of the deadliest in history (2).
SARS-CoV-2 primarily affects the respiratory system, manifesting a number of clinical scenarios, from asymptomatic course to severe respiratory conditions (3, 4). Acute heart failure (HF) and other cardiac involvements are the main clinical manifestations observed in infected patients in the late stages of the disease (3, 5)
Patients with cardiovascular diseases (CVD) are more prone to myocardial damage during the course of COVID-19 and presented with the higher risk of death (3, 6, 7) The prevalence of CVD in COVID-19 patients were ranged from 4% to 40% and associated with adverse outcomes, re-hospitalizations and increased mortality (8, 9).
Per se, cardiac surgery itself is considered as the traumatic factor on the myocardium, and these are the fairly complex procedures given the bypass and aortic cross-clamp, which exerts additional myocardial damage beyond the COVID-19 infection (10). N-terminal pro-brain natriuretic peptide (NT pro-BNP) was found as a reliable marker of cardiac injury in patients undergoing cardiac surgery and it was correlated with invasive ventilation time, duration of hospital stay and augmented inotropic support (11). It was also elevated in patients infected with COVID-19 (12).
Nonetheless, little is known about the outcomes of patients who underwent cardiac surgery during the COVID-19 pandemics, particularly those infected with SARS-CoV-2. The correlation of COVID-19 and cardiac markers also remains controversial.
In this article, we aimed to analyze the preoperative clinical, laboratory and imaging markers, intraoperative variables and postoperative outcomes of COVID-19 infected adult patients, who underwent cardiac surgery and compare with the cohort of patients without COVID-19.
Methods
Study design: single -center retrospective cross-sectional observational study.
Study population: The three hundred patients were hospitalized for the open-heart surgery in Research Institute of Heart Surgery and Organ Transplantation (RIHSOT hospital) from September 2020 to December 2021. All surgically operated adult patients in this timeline were included into study regardless of volume of the operation (isolated vs simultaneous), repetition (first time vs recurrent), mode of surgery (elective vs emergency). The patients were divided into 2 groups according to presence or absence of COVID-19. The retrospective data derived from case histories and discharge summaries. The data of pediatric patients, involving hybrid procedures, transcatheter intervention were excluded from the study. The inconclusive data regarding the viral test and/or perioperative findings also removed from the study.
The study was complied with 1975 Helsinki Declaration and Institutional Ethical Board of RIHSOT. Due to retrospective design, patient consents were not required.
COVID-19 testing
were categorized into two groups according to COVID-19 markers (both IgG and IgM antibodies were taken into account): COVID-19 positive (when at least one of the viral markers tested positive regardless of acuity of disease) and COVID-19 negative (when none of the tests were tested positive).
Immunoglobulin analysis was based on ELISA method (BGI Genomic analyzer, China). Cut-off values for both Ig G and IgM established for 5 AU/ml. Due to retrospective design of study we could not found sufficient data regarding the use of RT-PCR values. Because of these circumstances, we classified patients in accordance with sole immunoglobulin data.
Data collection
Baseline demographic parameters (age, gender, underlying diagnosis and type of surgery), clinical (presence of acute coronary syndrome during hospitalization, NYHA functional class of HF presence of exacerbated or newly emerged dyspnea, presence of pleural effusion and atrial fibrillation during admission, presence of symptomatic tachycardia, systolic and diastolic blood pressure values, baseline oxygen saturation) and laboratory (D-dimer, ferritin, prothrombin time, NT pro-BNP, hemoglobin levels, erythrocyte sedimentation rate, leucocytes, lymphocytes, platelets, C-reactive protein (CRP), hepatic transaminases, creatinine and uremic markers) some echocardiographic markers (left atrial antero-posterior size, estimated systolic pulmonary pressure, left and right ventricular chamber sizes, ejection fraction) were analyzed. NT pro-BNP was analyzed according to ECLIA method (Beijing Hotgen Biotech analyzer, China). Cut-off value was established as 430 pg/ml. Furthermore, some perioperative data (cardiac-pulmonary bypass time, duration of intensive care stay, duration of mechanical ventilation, prolonged inotropic support) and mortality were also included in the analysis.
Statistical analysis
Continuous or quantitative variables are depicted as mean and standard deviation, median, maximal and minimal ranges. Qualitative variables are presented as absolute count and percentage. Nonparametric Mann Whitney U test and Chi square analysis were used for comparison of continuous and categorical variables. We also calculated odds ratios. Significance was adjusted for p-value less than 0.05.
Results
Overall, three hundred patient reports were analyzed. Due to inconclusive data, 123 were excluded. One hundred seventy-seven patients were included in the study and further categorized in accordance with COVID-19 positivity. Ninety patients tested positive and this group depicted as CP (COVID-19 positive) and 87 patients were tested negative and noted as CN (COVID-19 negative). Baseline demographic, clinical, laboratory and echocardiographic data were analyzed separately. Perioperative parameters and earlier post-operative (10-15 days when patient still found in hospital wards) outcomes were also compared between groups.
Demographic characteristics
Patient groups were equally matched by age and gender distribution. The underlying structure of diagnostic conditions also did not show significant difference. In CP, rheumatic heart disease (RHD) was prevailed, whereas in CN coronary artery disease (CAD) was found prevalent. Due to lower occurrence rate, other disorders were grouped into “other” term. This heterogeneous group included aortic, pericardial diseases and other mediastinal conditions, which necessitated cardiothoracic surgery. In both groups, CABG was mostly performed procedure by 20 (22.2%) vs 27 (31%) cases. Valve surgery was the second most performed operation including both isolated mitral, aortic and combined procedures. Due to scarcity of data, we could not identify exact valve surgery types, i.e. repair or replacement. Most likely, most of the performed option was valve replacement rather than valve repair, because of abundancy of the long-standing rheumatic heart disease with severely damaged valve morphology in our cohort of patients. The results are highlighted in Table 1.
|
Table 1. Baseline demographic data of patient groups |
|||
|
Parameters |
CP (n=90) |
CN (n=87) |
p |
|
Age, years |
56.3(12) 58 (18-74) |
53.5(15.2) 57 (18-80) |
0.31 |
|
Male sex, n(%) |
45 (50) |
39 (44.8) |
0.49 |
|
Main diagnosis, n(%) CAD VHD CHD Others |
30 (33.3) 36 (40) 15 (16.7) 9 (10) |
36 (41.4)) 27 (31) 19 (21.8) 5 (5.7) |
0.33 |
|
Type of surgical operation, n(%) CABG MVR AVR CVR CHD Other |
20 (22.2) 20 (22.2) 8 (8.9) 6 (6.7) 8 (8.9) 28 (31.1) |
27 (31) 12 (13.8) 11 (9.3) 2 (2.3) 1 (1.1) 34 (39.1) |
0.04 |
|
Data are presented as n(%), mean (SD) and median (min-max) Mann Whitney U and Chi-square tests AVR – aortic valve surgery, CABG – coronary artery bypass graft surgery, CAD – coronary artery disease, CHD – congenital heart disease, CN – COVID-19 negative patients, CP – COVID-19 positive patients, CVR – combined aortic and mitral valve surgeries, MVR – mitral valve surgery, Other – other cardiac surgery operations, including tricuspid valve surgery, pericardiocenthesis, aortic reconstruction etc, VHD – valvular heart disease |
|||
Preoperative clinical findings
Interestingly, patients in the COVID-19 negative group mostly presented by acute coronary syndromes upon hospitalization (p=0.04). Almost in 70-80% patients, progressive dyspnea was manifested during admission, but no significant difference was found between groups. According to NYHA gradation of HF in both groups, patients presented mostly by class III, i.e. symptoms occurred in moderate physical activity. A reliable marker of heart failure, NT pro-BNP was found only in selected subjects without significant difference among groups. Other clinical markers such as blood pressure, and oxygen saturation also did not show differences among COVID-19 positive and negative patients. All preoperative findings are depicted in Table 2.
|
Table 2. Baseline preoperative clinical findings |
|||
|
Variables |
CP (n=90) |
CN (n=87) |
p |
|
Presence of ACS during hospitalization, n(%) |
6 (6.7)
|
14 (16.1) |
0.04 |
|
CHF according to functional class (NYHA), n(%) II III IV |
53 (58.9) 34 (37.8) 3 (3.3) |
59 (67.8) 28 (32.2) 0 |
0.14 |
|
Presence of dyspnea on admission1, n(%) |
74 (82.2) |
64 (73.6) |
0.13 |
|
Presence of cough on admission, n(%) |
2 (2.2) |
2 (2.3) |
0.20 |
|
Presence of respiratory auscultative abnormalities2, n(%) |
8 (8.9) |
11 (12.6) |
0.42 |
|
Presence of pleural effusion on admission3, n(%) |
6 (6.7) |
5 (5.7) |
0.80 |
|
Presence of atrial fibrillation on admission4, n(%) |
14 (15.6) |
11 (12.6) |
0.57 |
|
Tachycardia on admission5, n(%) |
14 (15.6) |
10 (11.5) |
0.43 |
|
NT pro-BNP elevated cases6, n(%) |
5 (5.6) |
5 (5.7) |
0.95 |
|
Systolic blood pressure, in mm of Hg |
119.9(18) 120 (80-220) |
115.2(13.5) 120 (80-150) |
0.22 |
|
Diastolic blood pressure, in mm of Hg |
72.9(11.2) 70 (53-90) |
71.9(8.5) 70 (40-100) |
0.79 |
|
O2 saturation |
96.3(1.8) 96 (90-100) |
95.9(2.2) 96 (85-100) |
0.41 |
|
Data are presented as n(%), mean (SD) and median (min-max) Mann Whitney U and Chi-square tests ACS – acute coronary syndrome, CHF – chronic heart failure, CN – COVID-19 negative patients, CP – COVID-19 positive patients, NT pro-BNP - N-terminal pro-brain natriuretic peptide 1 Emergence of new-onset or progressing of previously detected dyspnea 2 Presence of any kind of respiratory abnormalities, such as rales, rhonchi, crepitations etc. 3 Pleural effusion confirmed by chest X-ray 4 Atrial fibrillation documented by 12-lead ECG 5 Symptomatic tachycardia (fatigue, breathlessness, pre-syncope, etc) 6 The values above 430 pg/ml accepted as elevated |
|||
Preoperative laboratory and echocardiographic findings
Contrarily, COVID-19 negative patients showed higher D-dimer and NT-pro-BNP values in contrast to main group patients (p<0.05 and p=0.03, respectively). From laboratory markers only hemoglobin values were higher in COVID-19 positive cases (p=0.02). Platelets also were detected merely higher in CP, but significance was not noted. Remaining laboratory and echocardiographic parameters did not show reliable differences, although heart chambers tend to be dilated in main group of patients. All preoperative laboratory and echocardiographic data are presented in Table 3.
|
Table 3. Baseline preoperative laboratory and echocardiographic findings |
|||
|
Variables |
CP (n=90) |
CN (n=87) |
p |
|
D-dimer, mg/L FEU |
0.6(0.8) 0.4 (0.1-4.4) |
1.5(1.9) 0.7 (0.1-6.3) |
0.05 |
|
Ferritin, ng/ml |
166(109.4) 162 (11-432) |
317.4(208.5) 373 (81-600) |
0.24 |
|
Prothrombin time, ms |
74.4(19.9) 81 (14-101) |
77.4(18.7) 81 (13-103) |
0.41 |
|
Fibrinogen, mg/dl |
3676.6(908.7) 3552 (1776-7548) |
3711.3(693.5) 3996 (2222-5328) |
0.69 |
|
NT pro-BNP, pg/ml |
8653.5(17623.6) 1501.5 (1116-44619) |
21611.1(22022.9) 5303 (1527-44803) |
0.03 |
|
Hemoglobin, g/L |
142.8(18.2) 144 (86-189) |
137.7(19.9) 138 (67-196) |
0.02 |
|
ESR, mm/h |
7.8(10.6) 4 (1-50) |
8.3(8.5) 5 (1-35) |
0.13 |
|
Leucocytes, 109/L |
6.7(2.5) 6 (4-12) |
8.5(2.1) 8.5 (7-10) |
0.32 |
|
Lymphocytes, %L |
32.4(10.9) 32 (9-62) |
29.9(12) 28 (8-67) |
0.10 |
|
Platelets, 109/L |
234.3(48.3) 230 (14-414) |
223.6(32.7) 222.5 (162-313) |
0.06 |
|
CRP, mg/L |
20.5(7.5) 18 (12-36) |
25.6(10.7 24 (12-46) |
0.19 |
|
ALT, U/L |
31.9(27.2) 23.5 (10-129) |
37.6(22) 34 (12-106) |
0.11 |
|
AST, U/L |
29.2(15) 25 (12-87) |
33.7(16.5 29 (12-97) |
0.06 |
|
Creatinine, µmol/L |
95.9(25) 92 (65-195) |
80.7(30.3) 78.5 (70-195) |
0.07 |
|
Urea, mmol/l |
4.9(0.8) 5 (3-6) |
9.5(5.4) 8 (5-17) |
0.05 |
|
BUN, mg/dl |
17.5(1.3) 17 (16-20) |
19.1(3.5) 17.5 (16-28) |
0.31 |
|
Glucose, mmol/L |
6.1(0.8) 6 (5-7) |
8(5.8) 6 (5-20) |
0.66 |
|
LA antero-posterior, mm |
43.1(13) 41 (14-90) |
40.6(22.1) 39 (14-77) |
0.21 |
|
SPAP, mm of Hg |
43.9(16) 40 (22-110) |
41.2(12.1) 39 (17-75) |
0.46 |
|
EF (Simpson), % |
59.7(10.5) 62 (29-82) |
59.6(11.8) 60.5 (32-86) |
0.76 |
|
EDD, mm |
52.3(10.1) 51 (20-89) |
49.2(13.9) 49.5 (4-84) |
0.22 |
|
ESD, mm |
39.1(18.6) 34 (10-128) |
37.5(15.2) 34 (10-88) |
0.98 |
|
RV, mm |
25.6(4.5) 25 (15-41) |
24.8(4.1) 25 (12-38) |
0.41 |
|
Data are presented as mean (SD) and median (min-max), Mann Whitney U test BUN – blood urea nitrogen, ALT – alanine aminotransferase, AST – aspartate aminotransferase, CN – COVID-19 negative patients, CP – COVID-19 positive patients, CRP – C-reactive protein, EDD – end-diastolic volume, EF – ejection fraction, ESD – end-systolic volume, ESR – erythrocyte sedimentation rate, LA – left atrium, NT pro-BNP - N-terminal pro-brain natriuretic peptide, RV – right ventricle, SPAP – systolic pulmonary blood pressure |
|||
Heart, Vessels and Transplantation 2023; 7: doi: 10.24969/hvt.2023.439
Cardiac surgery outcomes in COVID patients Tukusheva et al.
![]()
Perioperative findings and hospitalization period outcomes
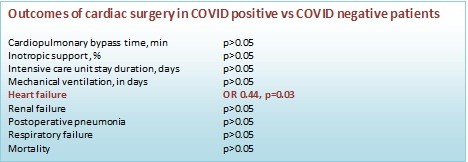
Similarly, perioperative findings also did not reveal significant changes in terms of exact operative indices, such as cardiac-pulmonary bypass time, inotropic support, duration of invasive ventilation and ICU stay. Nonetheless, duration of CPB time was little prolonged in CP group. Among complications, HF exacerbation occurred twice more in COVID-19 negative patients and by 56% less in COVID positive patients (OR – 0.44, p=0.03). Prolonged cases of ICU duration were similar in both groups. Other complications did not show significant differences. Single case of death was noted in CN group during early post-op. All these values have been shown in Table 4.
|
Table 4. Perioperative findings and early postop outcomes
|
|||||
|
Variables |
CP (n=90) |
CN (n=87) |
OR |
p |
|
|
CPB time, in minutes |
97.8(35) 93 (30-190) |
92.9(31.4) 90 (30-170) |
NA |
0.55 |
|
|
INO support1 |
8 (8.9) |
12 (13.8) |
0.6 |
0.30 |
|
|
ICU duration, in days |
20 (22.2) |
21 (24.1) |
1.1 |
0.76 |
|
|
Mechanical ventilation, in days |
3 (3.3) |
5 (5.7) |
0.56 |
0.44 |
|
|
Heart failure2 |
13 (16.1) |
24 (25.6) |
0.44 |
0.03 |
|
|
Renal failure3 |
2 (1.7) |
2 (1.7) |
0.96 |
0.97 |
|
|
Post-op pneumonia4 |
1 (1.1) |
0 |
NA |
0.32 |
|
|
Respiratory failure5 |
4 (4.4) |
3 (3.4) |
1.3 |
0.73 |
|
|
Early post-op mortality |
0 |
1 (1.1) |
0 |
0.30 |
|
|
|
Data are presented as n(%), mean (SD) and median (min-max) Mann Whitney U and Chi-square tests CN – COVID-19 negative patients, CPB – cardiopulmonary bypass time, CP – COVID-19 positive patients, INO support – inotropic support, ICU duration – duration of intensive care unit stay, OR –odds ratio 1 Accentuated inotropic support – either more than two inotropes used and/or support exceeded 48 h in early postop; 2 Exacerbation of previously diagnosed congestive heart failure, i.e. progression of heart failure symptoms (dyspnea, orthopnea etc.) or emergence of de novo heart failure, evidenced by echocardiography, regardless of classification by EF; 3 Emergence of acute kidney injury or progression of chronic kidney disease according to current KDIGO guidelines; 4 Lung consolidation evidenced by chest X-ray in post-op period 5 Respiratory failure evidenced by either pulse oxymetry or fluctuation of arterial blood gas |
||||
Discussion
According to literature, immunoglobulin positivity based on either Ig G or Ig M considered as a weak test. Most of the studies convinced on reliability of nucleid acid testing (23). However, some studies revealed the combination of both immunoglobulins, i.e. IgG-IgM assays accurately predicted the clinical and laboratory features of the disease (24). Consequently, according to above-mentioned findings, our Ig-based classification approach somehow advocated taking into account of lack of RT-PCR data. Per se, we intended for sharing of overall conditions in cardiac surgical practice during COVID-19 era.
Generally, our findings did not demonstrate overall differences between COVID-19 positive and negative groups. Roughly, cardiac surgical practice during COVID-19 era did not suffer from tremendous negative outcomes. As evidenced by several authors by literature volume of operations substantially decreased due to lockdown (13). Furthermore, these patient cohorts were presented by poor post-operative outcomes, i.e. prolonged ICU and ward stay, increased need for inotropic and ECMO support. Some studies clearly identified the occurrence of adverse outcomes especially during the 0 and 4th weeks of infection, a “peri-COVID-19” period (14).
Initially patients were equally presented by respiratory-driven symptoms in both groups, however HF exacerbation was dominated in negative group which was also supported by higher NT-pro BNP levels. Despite the evidenced role of natriuretic peptides in therapeutic patients, limited knowledge regarding the perioperative efficacy of these predictors is available in a current literature (11, 15). Anyway, to show the exact role of natriuretic peptides the large- scale studies are necessary.
Nonetheless, we found signs of hemocoagulation in CP group, i.e. increased hemoglobin and thrombocyte levels. As published in previous papers, platelet count was elevated along with other thrombosis markers, such as fibrinogen and D-dimer levels in COVID-19 patients (16, 17). Strikingly in our study, D-dimers were elevated not in CP patients but CN ones. Some researchers reported that, contrary to conventional coagulation markers, thromboelastometric and thrombodynamic markers predicted future outcomes reliably in selected populations (18, 19).
Various changes of cardiac geometry were observed in COVID-19 patients in accordance with direct or indirect COVID-19 damage to cardiomyocytes. These complex pathophysiologic mechanisms presented by versatile clinical manifestations, including exacerbation of existing HF, emergence of de novo cardiac symptoms, rhythm and conduction disturbances, increased severity of coronary artery disease, fluctuation of blood pressure levels (6, 8, 20). In our study, cardiac chambers of infected patients were mildly enlarged compared to non-infected individuals. Per se, chamber dilatation had to be observed in a CN group patients owed to higher NT- proBNP levels and frequent HF cases. Tendency of chamber dilatation could be explained by a certain degree of myocardial damage in infected patients during perioperative period.
Furthermore, post-operative pneumonia and respiratory failure are frequently seen in CP patients in spite of lack of significance. This finding one more emphasizes the impact of COVID-19 infection on earlier outcomes. If we rely on baseline clinical data, such as progressive dyspnea, abnormal respiratory auscultative findings, no significant differences were noted. Classically, acute respiratory distress syndrome is one of the result of cytokine storm and the main cause of severe respiratory failure and increased mortality (21, 22). Nevertheless, respiratory events were not differed in both groups.
Study limitations
Due to lack of adequate study design and patient data, small sample size and short follow-up, we could not gain answers for several questions. However, it was very difficult for developing countries obtaining reliable diagnostic and prognostic viral markers, such as RT-PCR at that disaster of outbreak. For this reason, we largely depended on immunoglobulin analysis for categorization of patients. In upcoming manuscripts, needed data will be complemented and analyzed widely. On the other hand, clinical heterogeneity can lead to inaccurate conclusions, though we could not reliably distinguish the acuity of viral infection in this cohort of patients. Exact surgical outcomes had to be assessed during interim and long-term follow-up periods.
Conclusion
To sum up, COVID-19 pandemics affected every field of medicine including elective and urgent cardiac surgery. In our cohort of patients, clinical, laboratory, imaging and perioperative parameters were not differed significantly in CP and CN groups. Clinical heterogeneity in preoperative baseline data raised further assumptions regarding the superiority of laboratory and imaging markers of the disease. Early post-operative findings were not found significantly different among groups. Paradoxically, congestive HF exacerbation or its de novo emergence prevailed in COVID-19 negative individuals during early post-operative period. Cardiac surgery found as the relatively safe and doable procedure during pandemics era.
Ethics: Institution Ethics committee approval was obtained. Informed consent of patients was not required as to retrospective design analysis of data.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: E.T., T.K., D.A., I.A., A. K., and A.I. equally contributed to data collection, statistical analysis, writing and primary review of manuscript, discussion and review of complete final version of manuscript
Acknowledgment: We greatly appreciate all our collegues-heroes of medicine who sacrificed their health and life during COVID-19 pandemics period
Funding: None to declare
References
- 1.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020; 579: 265–9. doi: 10.1038/s41586-020-2008-3.
- 2.Our world in data. Available from: https://ourworldindata.org/covid-vaccinations?country=OWID_WRL
- 3.Farshidfar F, Koleini N, Ardehali H. Cardiovascular complications of COVID-19. JCI Insight 2021; 8; 6: e148980.
- doi: 10.1172/jci.insight.148980
- 4.Sattar Y, Ullah W, Rauf H, Virk HUH, Yadav S, Chowdhury M, Connerney M, et al. COVID-19 cardiovascular epidemiology, cellular pathogenesis, clinical manifestations and management. Int J Cardiol Heart Vasc ;29: 100589. doi: 10.1016/j.ijcha.2020.100589
- 5.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020; doi: 10.1001/jama.2020.6775
- 6.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020; 5: 811-8. doi: 10.1001/jamacardio.2020.1017.
- 7.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; doi: 10.1001/jama.2020.2648
- 8.Aggarwal G, Cheruiyot I, Aggarwal S, Wong J, Lippi G, Lavie CJ, et al. Association of cardiovascular disease with coronavirus disease 2019 (COVID-19) Severity: A Meta-Analysis. Curr Probl Cardiol 2020; 45: 100617. doi: 10.1016/j.cpcardiol.2020.100617
- 9.Dalia T, Lahan S, Ranka S, Acharya P, Gautam A, Goyal A, et al. Impact of congestive heart failure and role of cardiac biomarkers in COVID-19 patients: A systematic review and meta-analysis. Indian Heart J 2021; 73: 91-8. doi: 10.1016/j.ihj.2020.12.002
- 10.Karfis EA, Kontogiorgi P, Papadopoulos G, Tzimas P, Seferiadis C, Athanasulas C, et al. Cardiac valve surgery and myocardial damage: the role of cardiac troponin I. J Cardiovasc Surg (Torino) 2010; 51: 409-15.
- 11.Jogia, PM, Kalkoff M, Sleigh JW, Bertinelli A, La Pine M, Richards AM, et al. (2007). NT-Pro BNP Secretion and Clinical Endpoints in Cardiac Surgery Intensive Care Patients. Anaesth Inten Care 2007; 35: 363–9. doi: 10.1177/0310057X0703500307
- 12.Aspromonte N, Cappannoli L, Scicchitano P, Massari F, Pantano I, Massetti M, et al. Stay Home! Stay Safe! First post-discharge cardiologic evaluation of low-risk-low-BNP heart failure patients in COVID-19 era. J Clin Med 2021; 10: 2126. doi: 10.3390/jcm10102126
- 13.Rubino AS, DE Santo LS, Pisano A, di Mauro M, Benussi S, Borghetti V, et al. . Cardiac surgery practice during the COVID-19 outbreak: a multicentre national survey. Eur J Cardio-Thorac Surg 2021; 59: 901–7. doi: 10.1093/ejcts/ezaa436
- 14.Deng JZ, Chan JS, Potter AL, Chen YW, Sandhu HS, Panda N, et al. The risk of postoperative complications after major elective surgery in active or resolved COVID-19 in the United States. Ann Surg 2022; 275: 242-6. doi:10.1097/SLA.0000000000005308
- 15. Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994; 90: 195–203. doi.org/10.1161/01.CIR.90.1. 195
- 16.Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020; 136: 489-500. doi: 10.1182/blood.2020006520
- 17.Suh YJ, Hong H, Ohana M, Bompard F, Revel MP, Valle C, et al. Pulmonary embolism and deep vein thrombosis in COVID-19: A systematic review and meta-analysis. Radiol 2021; 298: E70-E80. doi: 10.1148/radiol.2020203557.
- 18.Vuimo TS, Tsarenko SV, Filimonova EV, Seregina EA, Karamzin SS. Correction of anticoagulant therapy in patients with severe COVID-19 virus infection using a thrombodynamics coagulation assay. Clin Appl Thromb Hemost 2022; 28: 10760296221142862. doi: 10.1177/10760296221142862
- 19.Aires RB, Soares AASM, Gomides APM, Nicola AM, Teixeira-Carvalho A, da Silva DLM, et al. Thromboelastometry demonstrates endogenous coagulation activation in nonsevere and severe COVID-19 patients and has applicability as a decision algorithm for intervention. PLoS One 2022; 17: e0262600. doi: 10.1371/journal.pone.0262600.
- 20.Nicol M, Cacoub L, Baudet M, Nahmani Y, Cacoub P, Cohen-Solal A, et al. Delayed acute myocarditis and COVID-19-related multisystem inflammatory syndrome. ESC Heart Fail 2020; 7: 4371–6. doi: 10.1002/ehf2.13047.
- 21.Gibson PG, Qin L, Puah SH. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust 2020; 213: 54-56.e1
- 22.Ashimov ZI, Kudaiberdiev TZ, Abibillaev DA, Gaybyldaev ZJ, Zaripov DE et al. The effects of tocilizumab on clinical and laboratory features of patients with severe COVID-19: a single-center experience. Heart Vessels Transplant 2020; 4: 132-8.
- 23.Nikam C, Suraweera W, Fu SHH, Brown PE, Nagelkerke N, Jha P. PCR test positivity and viral loads during three SARS-CoV-2 viral waves in Mumbai, India. Biomedicines 2023; 11: 1939. doi:10.3390/biomedicines11071939
- 24.Xie J, Ding C, Li J, Wang Y, Guo H, Lu Zh, et al. Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test. J Med Virol 2020; 92: 2004-10. doi:10.1002/jmv.25930
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

