Candidemia in critically ill patients admitted to a cardiothoracic intensive care unit: a retrospective, observational study
ORIGINAL RESEARCH ARTICLE
Candidemia in critically ill patients admitted to a cardiothoracic intensive care unit: a retrospective, observational study
Article Summary
- DOI: 10.24969/hvt.2017.44
- Page(s): 46-55
- CARDIOVASCULAR DISEASES
- Published: 05/04/2018
- Received: 18/02/2018
- Revised: 04/04/2018
- Accepted: 04/04/2018
- Views: 10567
- Downloads: 8828
- Keywords: intensive care, cardiac surgery, candidemia, mortality, hemodialysis, heart dysfunction
Address for Correspondence: Cristiane da Cruz Lamas, Instituto Nacional de Cardiologia, Rua das Laranjeiras 374, 5th floor- Laranjeiras, 22240-006 Rio de
Janeiro, Brasil, Email: cristianelamas@gmail.com, Phone: +55(21) 991628048.
1Instituto Nacional de Infectologia Evandro Chagas, Fundacao Oswaldo Cruz (FIOCRUZ), Rio de Janeiro, Brasil
2 Cardiovascular Research Unit, Instituto Nacional de Cardiologia, Rio de Janeiro, Brasil
3Universidade do Grande Rio (Unigranrio), Rio de Janeiro, Brasil
4Microbiology Laboratory, Instituto Nacional de Cardiologia, Rio de Janeiro, Brasil
5Cardiothoracic Intensive Care Unit, Instituto Nacional de Cardiologia, Rio de Janeiro, Brasil
6Biostatistics Department, Instituto Nacional de Cardiologia, Rio de Janeiro, Brasil
7Infection Control Unit, Instituto Nacional de Cardiologia, Rio de Janeiro, Brasil
Abstract
Objective: Candidemia is a severe condition, with high mortality. Objectives: Our objectives were to estimate the incidence, predictors, and the outcomes of candidemia in adult patients admitted to a cardiothoracic intensive care unit.
Methods: A retrospective study using a cardiothoracic intensive care unit database prospectively implemented in a tertiary referral center for cardiac surgery. Patients with candidemia were identified by microbiology laboratory reports. Survival was compared between groups with and without candidemia. Univariate and multivariate analysis were performed to determine factors associated with candidemia outcomes.
Results: There were 21 adult patients with candidemia in the 2997 adult patients admitted during the 6-year period (2009-2015), with an incidence of 1.1 cases per 1000 patients-day. Candidemia was an independent predictor of mortality (HR: 2.67, CI 1.54-4.62). Cases had higher pulmonary artery systolic pressure, PASP (28.57% vs. 11.50%, p=0.03) and Euroscore (4.76 vs. 2.85, p<.0001); more infective endocarditis (14.29% vs. 2.76%, p<0.02) and mediastinitis (23.81% vs. 2.22%, p<0.0001). Cardiopulmonary bypass times differed between groups (124 vs. 100 minutes, p=0.02). Postoperatively, the candidemia group had more cardiogenic shock (38.10% vs. 9.55%, p<0.0001); hemodialysis (42.86% vs. 3.31%; p<0.0001), and higher sequential organ failure assessment (SOFA) scores (4 [3-5] vs. 3 [1-4], p<0.02). Mortality at 30 and 60-days was 28.5% and 47.6%, in the candidemia group compared to 10.3% and 11.1% in the control group, respectively (p<.0001). C. parapsilosis (50%) and C. albicans (18%) were the most prevalent species.
Conclusions: Candidemia was an uncommon infectious complication, but associated with a significant mortality. Our data support the identification of high-risk cardiac surgery patients who may benefit from empirical antifungal therapy.
Keywords: intensive care; cardiac surgery; candidemia; mortality; hemodialysis; heart dysfunction
Introduction
Candidemia is the most significant fungal infection in critically ill patients and requires a prolonged hospital stay (1, 2). Its attributable mortality in the intensive care unit (ICU) setting ranges from 30-40% (3, 4). Candida albicans is still the predominant pathogen isolated in patients with invasive candidiasis, but in the last years, there has been a global shift favoring non-albicans species (5, 6).
The identification of risk factors associated with candidemia in critically ill patients is crucial for providing the opportunity for early intervention with antifungal therapy and to curb the enormous burden of the infection. In patients admitted to general medical or surgical ICUs the risk of candidemia is related to increasing age, severity of illness, exposure to broad-spectrum antibiotics, recent abdominal surgery, hemodialysis (HD), receipt of parenteral nutrition, corticosteroids, and indwelling central venous catheters (CVC) (6, 7). However, in a subset of patients undergoing cardiac surgery, the characteristics related to cardiac intervention (i.e.; duration of cardiopulmonary bypass (CPB), aortic cross-clamp time, length of surgery) may render those patients more susceptible to candidemia compared to other high-risk patients.
As there are very few studies addressing candidemia in the setting of cardiothoracic ICU (CICU) (8, 9), we sought to estimate the incidence, predictors, and the outcomes of candidemia in critically ill patients admitted to a Brazilian CICU over a 6-year period.
Methods
Study design
This was a retrospective, observational, single-center study conducted in the CICU unit of Instituto Nacional de Cardiologia (INC), Rio de Janeiro, Brazil. INC is a 165-bed tertiary care referral center for cardiology and cardiac surgery, funded by the Brazilian Unified Health System (SUS). Approximately 4000 patients are admitted annually and an average of 550 adults undergo open cardiac surgery (coronary artery bypass grafts, valve replacement, aortic surgery, combined surgery, and heart transplantation). The CICU is responsible for handling all adult critically ill patients submitted to cardiac surgery. All blood culture (BC) positive samples for Candida from adult patients admitted to CICU were identified through the Microbiology laboratory’s database. These patients’ medical charts were fully reviewed. For all patients (candidaemic and non-candidaemic), data were obtained from the CICU prospectively collected database which included clinical, echocardiographic, surgical and outcome variables, in the years 2009-2015. Ethical approval was obtained from INC’s review board via Plataforma Brasil in 2016. The IRB did not deem consent forms necessary as the study was retrospective and patients’ confidentiality maintained. The study was conducted according to the principles expressed in the Declaration of Helsinki.
Laboratory investigations
Candida isolates were identified and tested for antifungal susceptibility at the Microbiology laboratory. BC were incubated in the Bact Alert® (Biomerieux, France) system and isolates were identified by the Vitek 2® (Biomerieux, France). Antifungal susceptibility was performed using the Vitek 2® (10). Minimum inhibitory concentrations breakpoints were interpreted from the Clinical & Laboratory Standards Institute’s supplement for yeasts.
Data collection
Variables were recorded in a standardized case report form. Pre-operative variables included age, gender, body mass index (BMI), presence of valve lesions, dysfunction of aortic or mitral valve prosthesis, left ventricular ejection fraction (LVEF), pulmonary artery systolic pressure (PASP), presence of active infective endocarditis (IE), coronary artery disease, diabetes mellitus (DM), smoking, systemic hypertension, serum creatinine, and the European system for cardiac operative risk evaluation estimates (Euroscore). Variables related to the cardiac surgery procedure included CPB time and total duration of surgery. After the cardiac intervention, the sequential organ failure assessment score (SOFA) on admission to CICU, the highest serum glycaemia in the first 24 hours after surgery, and the presence of hemodialysis were extracted.
Definitions
Candidemia was defined as the recovery of Candida species from one or more peripheral BC or one or more BC taken from a recently (i.e.; less than 30 minutes) inserted central-line. A case was defined as a patient with ICU-acquired candidemia if it occurred more than 48 h after CICU admission or less than 48 h after discharge from CICU. Date of onset of candidemia was taken as the date when the first positive BC was drawn.
Early treatment was defined as appropriate initiation of antifungals within 48 hours after BC culture collection, or when the patient was on preemptive antifungal therapy when BCs were collected.
Assessment of outcomes
Patients were followed after cardiac surgery until hospital discharge or until death occurred. The pre-specified outcomes were all-cause in-CICU mortality, and 30 and 60-day mortality rates stratified to the presence of candidemia during CICU admission.
Statistical analysis
Categorical variables were expressed as frequencies (%) and compared with χ² or the Fisher exact tests. Continuous variables were expressed as the mean and standard deviation (SD), if normally distributed, or median and interquartile ranges (IQR), assuming a non-normal distribution. Student t-test and Mann-Whitney U test were used for comparing continuous variables, according to data normality. A p value<0.05 was considered statistically significant for all analysis. A univariate analysis was done to assess the risk factors for candidemia during CICU admission, followed by a multivariate logistic regression analysis. Similarly, a univariate analysis was done to determine predictors associated with survival after CICU admission. We then applied a Cox proportional hazard model to test the association between candidemia and mortality after adjusting for potential confounders. Comparisons of survival at 30 and 60-days after CICU admission were performed with Kaplan-Meier curves. The log-rank test was used to compare the curves. All analyses were performed with the use of R software (version 3.1.0) and SPSS (version 20, Chicago, IL, USA).
Results
Between January 2009 and January 2015, 21 episodes of candidemia were identified in adults in the CICU. There were 2997 adult patients admitted to the CICU in the same study period. This corresponded to a cumulative incidence of 1.1 per 1000 patients-day. Table 1 shows the main characteristics of patients according to the presence of candidemia.
Patients with candidemia had significantly higher preoperative PASP values above 60 mmHg (28.57% vs. 11.50%, p = 0.03); higher preoperative Euroscore (4.76 vs. 2.85%, p<.0001); and more active IE as well as mediastinitis at the time of heart surgery. The CPB time was the only intraoperative variable that differed statistically between the groups (124 vs. 100 min, p = 0.02). In the post-operative period, the candidemia group had more cardiogenic shock (38.10% vs. 9.55%, p < 0.0001), were more often submitted to hemodialysis (42.86% vs. 3.31%; p<0.0001), and had a higher SOFA score (4 [3-5] vs. 3 [1-4], p < 0.02). There were also differences between the groups as to length of hospital stay (46 [30.5-62.5] vs. 14 [10-22] days, p<0.0001) and CICU stay (27 vs. 3 days, p<0.0001). On multivariate logistic regression analysis, variables that were independent predictors of candidemia were the presence of hemodialysis (OR: 20.4; CI 6.76- 61.4), mediastinitis (OR: 9.5; CI 2.89- 31.4) and length of hospital stay (OR: 1.01; CI 1.00- 1.02).
Mortality outcomes
During CICU stay, the mortality rate was 66.7% in the candidemia group compared to 11.9% in the non-candidemia group (p< 0.0001). The mortality rate at 30 days and 60 days was 28.5% and 47.6%, in the candidemia group, compared to 10.3% and 11.1% in the non-candidemia group (p=0.01; p<0.0001, respectively). The median survival time after CICU admission was significantly lower in the candidemia group compared to controls (65 [25.9-104] vs. 115 [79.5-150.4] days). Figure 1 shows the Kaplan-Meier curves stratified for the presence of candidemia.
Non-survivors were older and had a higher proportion of candidemia. Moreover, those patients had a more lengthy CPB time compared to those who survived (135 [99-185] vs. 100 [80-130] minutes, p<0.0001] (Table 2). On investigation of the risk factors for mortality in our cohort, the presence of candidemia during CICU stay remained a significant predictor after adjusting for other variables (Table 3).
|
Table 1. Baseline characteristics of patients admitted to CICU stratified to the presence of candidemia |
|||
|
Characteristics |
Candidemia (N = 21) |
Non-candidemia (N = 2976) |
p |
|
Age, years |
60 [45-77] |
59[50-67] |
0.56 |
|
Male sex, n(%) |
14 (66.67) |
1794 (60.28) |
0.66 |
|
BMI (kg/m2) |
25.06±4.26 |
26.47±4.78 |
0.17 |
|
ICU-LOS (days) |
27 (13-36) |
3 (2-5) |
<0.0001 |
|
Total hospital-LOS (days) |
46 [30.5-62.5] |
14 [10-22] |
<0.0001 |
|
Comorbidities |
|
|
|
|
DM, n(%) |
4 (19.05) |
620 (21.15) |
1 |
|
Smoking, n (%) |
7 (33.33) |
1038 (35.41) |
1 |
|
Hypertension, n (%) |
10 (47.62) |
2143 (73.11) |
0.01 |
|
Preoperative variables |
|
|
|
|
Euroscore |
4.76 ± 2.7 |
2.85 ± 2.27 |
<0.0001 |
|
LVEF, % |
60.52 ± 16.35 |
59.08 ± 13.89 |
0.63 |
|
Creatinine, mg/dl |
0.50 [0.50-0.90] |
0.70[0.50-1] |
0.82 |
|
PASP>60 mmHg, n(%) |
6 (28.57) |
337 (11.50) |
0.03 |
|
CAD, n(%) |
9 (42.86) |
1701 (58) |
0.23 |
|
Aortic stenosis, n(%) |
0 (0) |
9 (0.34) |
1 |
|
Mitral stenosis, n(%) |
0 (0) |
8 (0.29) |
1 |
|
Aortic regurgitation, n(%) |
2 (11.11) |
354 (13.72) |
<0.0001 |
|
Mitral regurgitation, n(%) |
6 (33.33) |
705 (29.41) |
0.91 |
|
Tricuspid regurgitation, n(%) |
2 (11.76) |
439 (16.61) |
1 |
|
Acute active infective endocarditis, n(%) |
3 (14.29) |
81 (2.76) |
0.02 |
|
Acute active mediastinitis, n(%) |
5 (23.81) |
65 (2.22) |
0.0001 |
|
Intraoperative variables |
|
|
|
|
CBP time, min |
124 [110-140] |
100 [80-135] |
0.02 |
|
Total surgery duration, hours |
5.05 [4.17-5.50] |
4.25 [3.40-5.23 |
0.06 |
|
Postoperative variables |
|
|
|
|
Hemodialysis, n(%) |
9 (42.86) |
97 (3.31) |
<0.0001 |
|
Shock, n(%) |
8 (38.10) |
280 (9.55) |
0.0001 |
|
SOFA score on 1st day after surgery |
4 [3-5] |
3[1-4] |
0.02 |
|
Peak glycaemia, mg/dl |
195 [145-209] |
181 [151-210] |
0.91 |
|
Data are presented as number (percentage), median [IQR] and mean ± SD p values in bold means statistically significant results. CAD - coronary artery disease; CPB- cardiopulmonary bypass; DM -diabetes mellitus; EUROSCORE-European system for cardiac operative risk evaluation; IQR -interquartile range; LOS -length of stay; LVEF -left ventricular ejection fraction; SD- standard deviation; PASP -pulmonary artery systolic pressure; SOFA -sequential organ failure assessment score. |
|||
|
Table 2. Factors significantly associated with survival after cardiothoracic ICU admission by univariate analysis |
||||||
|
Variable |
Alive (N=2587) |
Dead (N=365) |
OR |
95%CI |
p |
|
|
Lower estimate |
Higher estimate |
|||||
|
Candidemia, n (%) |
7 (0.3) |
14 (3.8) |
14.8 |
5.9 |
37.1 |
<0.0001 |
|
Age, years |
58 [49-66] |
65 [55-72] |
1.03 |
1.02 |
1.04 |
<0.0001 |
|
Perioperative creatinine, mg/dl |
0.69 [0.50-1.00] |
0.70 [0.50-1.45] |
1.41 |
1.2 |
1.65 |
0.03 |
|
BMI, kg/m2 |
26.56 ± 4.71 |
25.78 ± 5.14 |
0.965 |
0.942 |
0.988 |
0.004 |
|
Perioperative LVEF , % |
59.31±13.73 |
57.54±15.01 |
0.991 |
0.983 |
0.999 |
0.023 |
|
Perioperative PASP , mmHg |
15 [15-33] |
24 [15-45] |
1.02 |
1.01 |
1.02 |
<0.0001 |
|
Total surgery duration, hours |
4.15 [3.37-5.12] |
5.30 [4.25-6.50] |
1.62 |
1.5 |
1.73 |
<0.0001 |
|
CBP time, min |
100 [80-130] |
135 [99-185] |
1.01 |
1.01 |
1.02 |
<0.0001 |
|
Peak glycaemia, mg/dl |
180 [152-209] |
184 [147-232] |
1 |
1 |
1 |
0.004 |
|
SOFA score on 1st day after surgery |
3 [1-4] |
5 [3-6] |
1.67 |
1.56 |
1.78 |
<0.0001 |
|
ICU length of stay, days |
3 [2-5] |
4 [2-11] |
1.01 |
1.00 |
1.02 |
<0.0001 |
|
Data are presented as number (percentage), median [IQR] and mean ± SD BMI - body mass index; CI - confidence interval; CPB - cardiopulmonary bypass; IQR - interquartile range; LVEF- left ventricular ejection fraction; SD - standard deviation; OR -odds ratio; PASP - pulmonary artery systolic pressure; SOFA -sequential organ failure assessment score. |
||||||
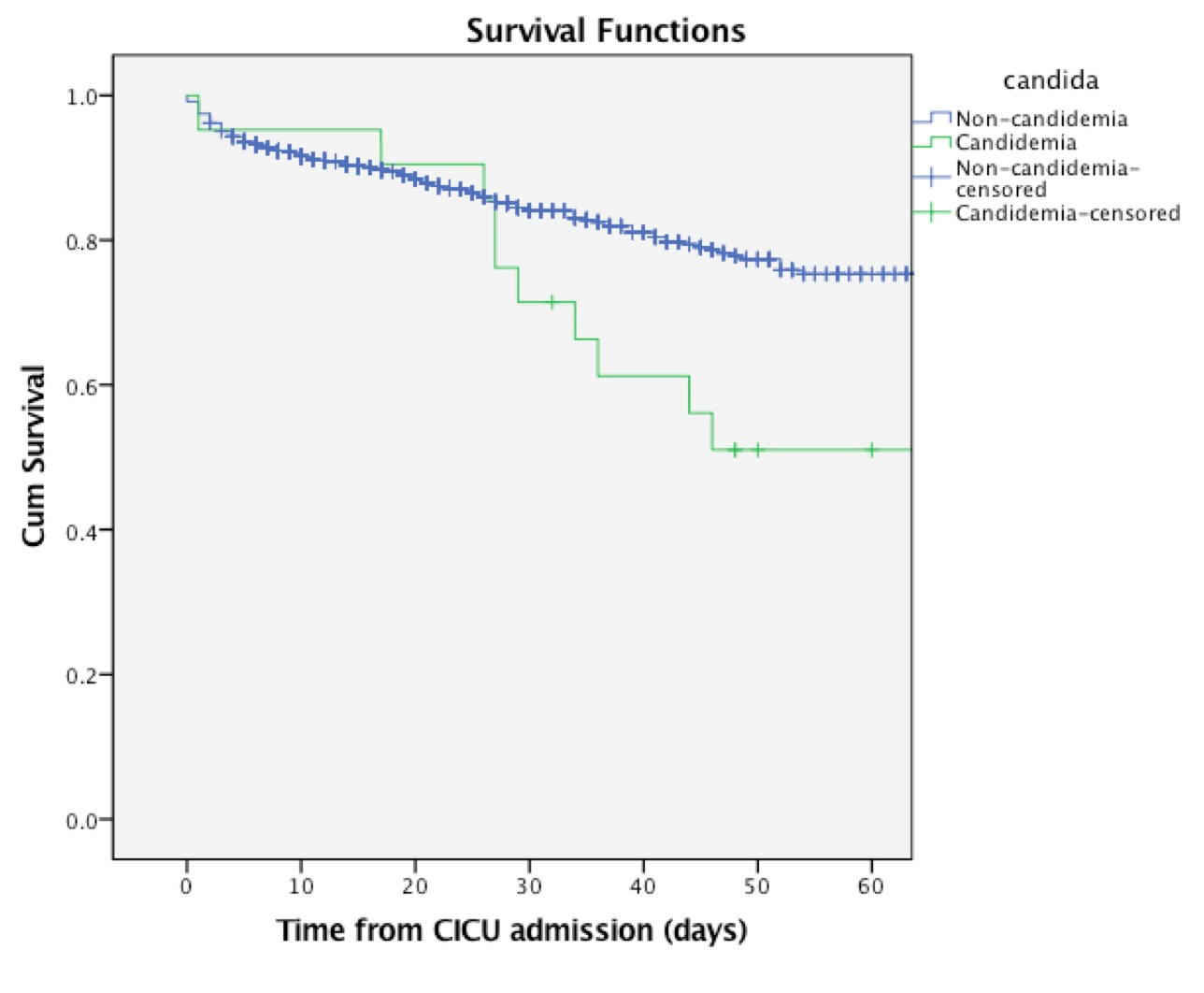
Figure 1. Kaplan Meier survival curve stratified by the presence of candidemia: patients with ICU-acquired candidemia (n=21) versus control (n=29769). Log Rank test - 12.757; p<0.0001.
CICU- cardiothoracic intensive care unit, Cum – cumulative, ICU intensive care unit
|
Table 3. Multivariate analysis of hospital mortality after cardiothoracic ICU admission by a Cox regression model |
|||
|
Variable |
Adjusted HR |
95% CI |
p |
|
Candidemia |
2.67 |
1.54-4.62 |
0.0001 |
|
Age |
1.02 |
1.01-1.03 |
0.0001 |
|
Creatinine |
1.22 |
1.09-1.36 |
0.0001 |
|
BMI |
0.96 |
0.94-0.98 |
0.003 |
|
LVEF |
0.99 |
0.98-1.002 |
0.143 |
|
PASP |
1.00 |
1.001-1.012 |
0.013 |
|
CPB time, min |
1.009 |
1.007-1.011 |
0.0001 |
|
Total surgery duration, hours |
1.000 |
0.995-1.006 |
0.892 |
|
Peak glycemia |
1.00 |
0.99-1.00 |
0.98 |
|
SOFA score |
1.34 |
1.26-1.42 |
0.0001 |
|
ICU-LOS |
0.97 |
0.969-0.986 |
0.0001 |
|
BMI - body mass index; CPB- cardiopulmonary bypass; ICU-LOS- intensive care unit length of stay; LVEF- left ventricular ejection fraction; SOFA - sequential organ failure assessment score. |
|||
Candidemia outcomes
Characteristics of patients with candidemia are shown in Table 4.
The median time from CICU admission to the diagnosis of candidemia was 36 days [IQR 18.5-93.5]. The rate of non-albicans: albicans Candida was 4.5:1. Multispecies candidemia occurred in 1 patient (C. parapsilosis and C. tropicalis in the same BC bottle). Before the onset of candidemia, three (14.2%) patients underwent abdominal surgery; eight were given corticosteroids (38%) and three parenteral nutrition (14.2%). The majority (76%) had been on mechanical ventilation for more than seven days before the candidemia onset. Underlying renal disease was present in 18 patients, in whom acute failure occurred in 12 (57%) and chronic renal failure was already present in six (28.5%). The definite infective focus for Candida infection was determined for only 5 (23.8%) patients (mediastinitis in 4, IE in 1). Preemptive antifungal therapy was recorded in 3 (14%) cases. Prior antifungal exposure did not increase the chances for subsequent non-Candida albicans infections (p=0.55). The median duration of antifungal therapy was 13 days [IQR 4.5-29.5]. The CVC access was removed after the diagnosis of candidemia in all cases, as per CICU protocol. Combined antifungal therapy was given to over half the patients. Early appropriate therapy was given in 11 (52%), but it was not associated with survival compared to those whose treatment initiation was delayed (p=0.28). All Candida isolates recovered were sensitive to the antifungals tested, apart from one C. krusei isolate that was resistant to fluconazole, and one C. glabrata isolate that was resistant to fluconazole and voriconazol (Supplementary Table).
Fourteen (66%) patients died during CICU admission from infective complications. The mortality rate according to Candida species was not significantly different (p = 0.07).
Discussion
To our knowledge, this is the third study to date investigating candidemia in adults in a CICU (8, 9). We have reported the epidemiology, characteristics, and outcomes of adult critically ill patients with candidemia after cardiac surgery. We found that the overall incidence of candidemia during the 6-year period was 1.1 cases per 1000 admissions. Factors associated with candidemia were related to the general ICU population (i.e.; ICU-LOS, HD, shock, Euroscore, SOFA) as well as to the cardiac intervention (i.e.; CPB time). Moreover, our study for the first time points out that candidemia is a strong predictor of mortality in cardiac surgery patients.
![]()
|
Table 4. Demographic, clinical, microbiological, and outcomes features of 21 patients with ICU-acquired candidemia. |
||||||||||
|
Year
|
Gender |
Age, years |
Type of surgery |
CBP time, min |
RRT |
Preop. broad-spectrum ATB sage |
Preop. Corticoste-roids usage |
Candida spp |
Antifungal therapy |
Outcome |
|
2014 |
M |
29 |
Heart transplant |
117 |
+ |
+ |
- |
C. dubliniensis |
None |
Death |
|
2014 |
M |
82 |
CABG |
65 |
- |
+ |
+ |
C. arapsilosis C. tropicalis |
Echin |
Death |
|
2014 |
F |
34 |
VR |
213 |
- |
+ |
- |
C. albicans |
L-AmB |
Death, after CICU discharge |
|
2014 |
M |
36 |
Aortic surgery |
110 |
+ |
+ |
_ |
C. parapsilosis |
dAmB+echino |
Death, after CICU discharge |
|
2013 |
F |
34 |
VR |
130 |
- |
+ |
- |
C. parapsilosis |
Azole+dAmB+echino |
Alive |
|
2013 |
F |
81 |
VR |
95 |
- |
+ |
- |
C. parapsilosis |
Echino |
Death |
|
2013 |
M |
78 |
VR |
163 |
- |
+ |
- |
C. parapsilosis |
dAmB |
Death |
|
2013 |
M |
65 |
VR |
135 |
- |
+ |
- |
C. parapsilosis |
Azole+L-AmB |
Death |
|
2013 |
M |
79 |
CABG |
135 |
+ |
+ |
+ |
C. parapsilosis |
Echino |
Death |
|
2013 |
M |
79 |
VR |
140 |
+ |
+ |
+ |
C. parapsilosis |
Echino |
Death |
|
2013 |
M |
67 |
CABG |
122 |
+ |
+ |
+ |
C. parapsilosis |
dAmB + echino |
Death |
|
2012 |
M |
60 |
VR |
125 |
- |
+ |
- |
C. glabrata |
L-AmB + echino |
Alive |
|
2012 |
M |
77 |
CABG |
50 |
+ |
+ |
- |
C. krusei |
echino |
death |
|
2012 |
M |
45 |
Heart transplant |
124 |
- |
+ |
+ |
C. parapsilosis |
Azole+L-AmB |
Alive |
|
2012 |
M |
46 |
Aortic surgery |
180 |
+ |
+ |
+ |
C. tropicalis |
dAmB+echino |
Death |
|
2012 |
M |
52 |
Heart transplant |
90 |
+ |
+ |
+ |
C. albicans |
Azole+L-AmB+echino |
Alive |
|
2011 |
F |
58 |
VR |
169 |
+ |
+ |
- |
C. tropicalis |
Echino |
Death |
|
2011 |
F |
69 |
VR |
115 |
+ |
+ |
- |
C. glabrata |
Azole+dAmB |
Death |
|
2011 |
F |
69 |
CABG |
120 |
- |
+ |
- |
C. albicans |
none |
Alive |
|
2010 |
M |
51 |
CABG |
90 |
- |
+ |
- |
C. parapsilosis |
Azole |
Death |
|
2009 |
F |
43 |
CABG |
189 |
+ |
+ |
+ |
C. albicans |
Echino |
Death |
|
ATB - antibiotics; CABG- coronary artery bypass graft; CBP -cardiopulmonary bypass; CICU- cardiothoracic intensive care unit; dAmB -deoxycolate amphotericin B; Echino-echinocandin; M- male; F- female; L-AmB- liposomal amphotericin b; RRT -renal replacement therapy; preop. –preoperative, VR- valvar replacement surgery |
||||||||||
Our epidemiological data showed that the incidence of candidemia is substantially lower than reported elsewhere. Michalopoulos found an incidence of 7.7/1000 admissions (9), similar to what was observed in the study by Pasero et al., of 13/1000 admissions (8), but in contrast to our incidence of 1.1/1000. Furthermore, we found that C. parapsilosis was the predominant species recovered in candidemia patients, which is in agreement with data from South America (11). In a similar fashion, in two recent population-based surveys conducted in Latin America and Europe, the most common non-albicans Candida was C. parapsilosis (26.5% and 22.7%, respectively) (12, 13). In fact, there has been an increasing trend for non-albicans infections, possibly due to more exposure to CVC, parental nutrition, and antifungal selection pressure (especially echinocandins) (11).
Several factors have been identified to be associated with candidemia after cardiac surgery. Mechanical ventilation ≥ 10 days, bacteremia, CPB>120 minutes, and diabetes mellitus were independent predictors of candidemia (9), while in another study the variables that were independently associated with the development of candidemia were parenteral nutrition, sepsis, SAPS score, ICU-LOS ≥ 20 days (8). We confirmed the already known “general” risk factors for candidemia, but our findings strongly suggest that a lengthy CPB time is also associated with it. A prolonged CPB time is associated with postoperative splanchnic hypoperfusion, leading to a subsequent increased risk of microbiota translocation into the systemic circulation (14). This lengthy ischemia period, combined with a selection of gut microbiota by broad-spectrum antibiotics, points the gut as the probable focus for the candidemia. From the infection control perspective, the focus of infection was presumed to be catheter-related for most patients, but this was difficult to prove, as virtually all patients after cardiac surgery have CVCs. On the other hand, the use of femoral HD does present a high risk of infection. Some authors advocate the retention of CVCs following the diagnosis of candidemia (15), but in a recent review, CVC removal reduced mortality (16). It is interesting to note that candidemic patients had significantly higher PASP values before surgery, reflecting the severity of their heart dysfunction. This may also have contributed to further gut hypoperfusion, even after surgery.
The mortality rate related to candidemia found in our study was 66.6%, similar to what was found in other studies conducted in the region (17, 18). Moreover, the mortality rates of the candidemia group remained significantly higher after 30 and 60 days of CICU admission compared to controls. This might be partially explained by the severity of the underlying preoperative morbidities (i.e.; pulmonary hypertension, active IE, higher Euroscore), higher severity of illness at ICU admission (i.e.; SOFA score) and late diagnosis of candidemia. The mortality rate observed could be partially related to the delay in recognizing candidemia after CICU admission (median time was 36 days in our CICU). These figures are in contrast to the median of 17 days observed in other similar studies (8, 18, 19). It may be that the delay in recognizing candidemia was due to use of “regular” aerobic/anaerobic BC bottles, and not mycosis bottles, as physicians in the CICU often do not routinely request specific bottles for fungi. Evidence shows that the sensitivity to invasive candidiasis is markedly increased by including a mycosis bottle (20, 21). Further, our patients with candidemia had more pulmonary hypertension and active IE at the time of surgery, which have been shown to be independent risk factors for death after cardiac surgery (22). Lastly, another explanation for the observed higher mortality in our study was the fact that we did not exclude subjects with invasive bacterial infections preceding surgery, those with immunodeficiency, or those that required exposure to an invasive device before surgery as was the case in previous works, which refer to the cardiac surgery scenario (8, 9).
Study Implications
Our study has several implications. First, we identified that ICU-acquired candidemia is a risk factor associated with increased mortality in cardiac surgery patients. Consequently, empirical antifungal treatment initiation with high-dose fluconazole or an echinocandin in high-risk cardiac surgery patients (i.e.; those with kidney injury, severe pulmonary hypertension, and prolonged CPB time) may be advisable in the scenario of unexplained fever or hypothermia or altered inflammatory markers. Meta-analyses of clinical trials have consistently shown that fluconazole prophylaxis was associated with a reduction in invasive candidiasis rate, but not in all-cause mortality (23).
Although enormous challenges persist concerning the identification of the patients that will benefit most from antifungal prophylaxis, we feel that the benefits associated with this strategy justify the theoretical risks of the emergence of fluconazole-resistant strains, drug toxicity, and costs. Also we are aware that conventional BC for Candida exhibit reduced sensitivity (approximately 50%) and slow turnaround times (24) and therefore the implementation of a nonculture diagnostic framework such as measuring serum mannan and anti-mannan antibodies, β-D-glucan assays, and polymerase chain reaction assays into clinical practice would be desirable to shorten the time to diagnosis and initiation of antifungal therapy (25).
Secondly, despite the low numbers of isolates, our study provides for the first time the epidemiology and the resistance patterns of the circulating Candida species in a referral CICU in Brazil. Based on our findings, C. parapsilosis is responsible for half of the candidemia cases. Collaborating with the current treatment guidelines, and taking into account our antimicrobial susceptibility pattern, we support the empirical use of fluconazole due to its excellent efficacy, favorable gastrointestinal absorption, and low cost compared to echinocandins and amphotericin B (26). However, in those who have had previous exposure to azoles or are hemodynamically unstable, echinocandins should be considered first-line therapy (26). We recognize that in-vitro studies showed decreased activity of echinocandin against isolates of C. parapsilosis, despite clinical data demonstrating that the initial use of an echinocandin-based regimen does not increase the chances of clinical failure in candidemia due to C. parapsilosis (27).
Study Limitations
There are several limitations to the current study, the most important of which is the low number of candidemia cases over a 6-year period. Also, our findings refer to the epidemiology of candidemia in a CICU referral center in Brazil. Extrapolations outside this scenario may be inappropriate. Furthermore, we were unable to describe the impact of different Candida species isolates, multispecies candidemia, and the effects of early antifungal initiation, on candidemia-related mortality. Previous data have shown that mortality is species-specific (i.e.; low mortality among patients with C. parapsilosis and C. krusei) (28); mortality increases in multispecies candidemia (29); and mortality decreases with early initiation of appropriate treatment (30).
Conclusions
Candidemia in critically ill patients who have cardiac surgery is an uncommon and late infectious complication but associated with a very high mortality. Candidemia is an independent risk factor for mortality after cardiac surgery, and it was associated with general ICU (HD, Euroscore and SOFA scores) and cardiac surgery (CPB time) variables. In an attempt to decrease candidemia-related mortality, and the subsequent CICU crude mortality, we suggest that empirical systemic antifungal therapy should be initiated promptly in high-risk cardiac surgery patients presenting with an infectious syndrome. Further investigation in a larger, prospective study, of the impact of candidemia in cardiac surgery patients, is necessary to confirm our findings.
Peer-review: External and internal
Conflict of interest: The authors declare that they have no conflict of interest.
Ethical approval: Ethical approval has been granted by the local IRB.
Informed consent: informed consent has been waived by the IRB due to the retrospective nature of the study.
Authorship: J.A.S.M., T.M.P., J.A.A., S.O., M.G.C., L.P.F., R.Q.G., M.B.F., A.R.F., C.C.L. contributed equally to the study and preparation of manuscript.
Acknowledgements
We wish to acknowledge the support of the medical and nursing staff of the cardiothoracic intensive care unit for their precious clinical care.
Funding: Jose Moreira is supported by a scholarship from Programa de Estudantes-Convenio de Pos-graduacao (PEC-PG, CAPES/CNPQ). Thiago Peixoto had a student personal grant from FUNADESP/Unigranrio. Cristiane Lamas had a personal research grant from FUNADESP/Unigranrio from 2013-2015, and has a current research grant from FAPERJ.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
|
Supplementary Table. In vitro antifungal susceptibility of the clinical isolates in post cardiac surgery patients (n=22) |
||||
|
Species (no of isolates) |
Drug |
Resistance strains (%) |
Intermediate strains (%) |
Susceptible strains (%) |
|
C. parapsilosis (n=11) |
Amphotericin B |
|
|
100 |
|
|
Fluconazol |
|
|
100 |
|
|
Voriconazol |
|
|
100 |
|
|
Flucytosine |
|
|
100 |
|
|
Caspofungin‡ |
|
|
9 |
|
|
Anidulafungin‡ |
|
|
9 |
|
|
Micafungin |
|
|
NT |
|
C. albicans (n=4) |
Amphotericin B |
|
|
100 |
|
|
Fluconazol |
|
|
100 |
|
|
Voriconazol |
|
|
100 |
|
|
Flucytosine |
|
|
100 |
|
|
Caspofungin§ |
|
|
25 |
|
|
Anidulafungin |
|
|
NT |
|
|
Micafungin§ |
|
|
25 |
|
C. tropicalis (n=3) |
Amphotericin B |
|
|
100 |
|
|
Fluconazol |
|
|
100 |
|
|
Voriconazol |
|
|
100 |
|
|
Flucytosine |
|
|
100 |
|
|
Caspofungin |
|
|
NT |
|
|
Anidulafungin |
|
|
NT |
|
|
Micafungin |
|
|
NT |
|
C. glabrata (n=2) |
Amphotericin B |
|
|
100 |
|
|
Fluconazol |
50 |
|
50 |
|
|
Voriconazol |
50 |
|
50 |
|
|
Flucytosine |
|
|
100 |
|
|
Caspofungin |
|
|
NT |
|
|
Anidulafungin |
|
|
NT |
|
|
Micafungin |
|
|
NT |
|
C. krusei (n=1) |
Amphotercin B |
|
|
100 |
|
|
Fluconazol |
100 |
|
|
|
|
Voriconazol |
|
|
100 |
|
|
Flucytosine |
|
100 |
|
|
|
Caspofungin |
|
|
NT |
|
|
Anidulafungin |
|
|
NT |
|
|
Micafungin |
|
|
NT |
|
C. dubliniensis (n=1) |
Amphotecin B |
|
|
100 |
|
|
Fluconazol |
|
|
100 |
|
|
Voriconazol |
|
|
100 |
|
|
Flucytosine |
|
|
100 |
|
|
Caspofungin |
|
|
NT |
|
|
Anidulafungin |
|
|
NT |
|
|
Micafungin |
|
|
NT |
References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

