Coronary artery disease and transcatheter aortic valve implantation: diagnostic evaluation, management and indication for percutaneous revascularization
EDITORIALS
Coronary artery disease and transcatheter aortic valve implantation: diagnostic evaluation, management and indication for percutaneous revascularization
Article Summary
- DOI: 10.24969/hvt.2023.442
- Page(s): 260-272
- CARDIOVASCULAR DISEASES
- Published: 09/12/2023
- Received: 09/12/2023
- Accepted: 09/12/2023
- Views: 5091
- Downloads: 3685
- Keywords: coronary artery disease, percutaneous coronary intervention, transcatheter aortic valve implantation
Address for Correspondence*: Marco Bernardi, Department of Clinical, Internal Medicine, Anesthesiology and Cardiovascular Sciences, Sapienza University of Rome, Piazzale Aldo Moro 5, Rome, Italy.
Email: marco.bernardi23@gmail.com; Twitter: MarcoBernardiMD; ORCID: 0000-0001-9269-8829
Dario Mafrica1, Matteo Betti2,3, Gaetano Tanzilli1, Lorenzo Lo Sasso4, Luigi Spadafora1, Giuseppe Biondi-Zoccai5,6, Marco Bernardi 1*
1Department of Clinical, Internal Medicine, Anesthesiology and Cardiovascular Sciences, Sapienza University of Rome, Italy
2Department of Clinical Sciences and Community Health, Cardiovascular Section, University of Milan, 20122 Milan, Italy
3Centro Cardiologico Monzino, IRCCS, 20138 Milan, Italy
4Catholic University of the Sacred Heart, Rome, Italy
5Department of Medical-Surgical Sciences and Biotechnologies, Sapienza University of Rome, Latina, Italy
6Mediterranea Cardiocentro, Napoli, Italy
Abstract
Aortic stenosis (AS) is the most frequent form of valvular heart disease in developed countries, and severe AS is a major cause of morbidity and mortality in the elderly. Since many of these patients often have a high or prohibitive risk for surgical aortic valve replacement (SAVR), transcatheter aortic valve implantation (TAVI) has emerged as an established therapy and is now becoming a common practice, even in low-risk patients. As severe AS and coronary artery disease (CAD) are often concomitant, a matter of debate is whether, how, and when to treat coexisting CAD. The aim of this commentary is to analyze the rationale for the diagnostic evaluation and management of CAD in TAVI candidates, as proposed by the recent EAPCI/ESC consensus statement.
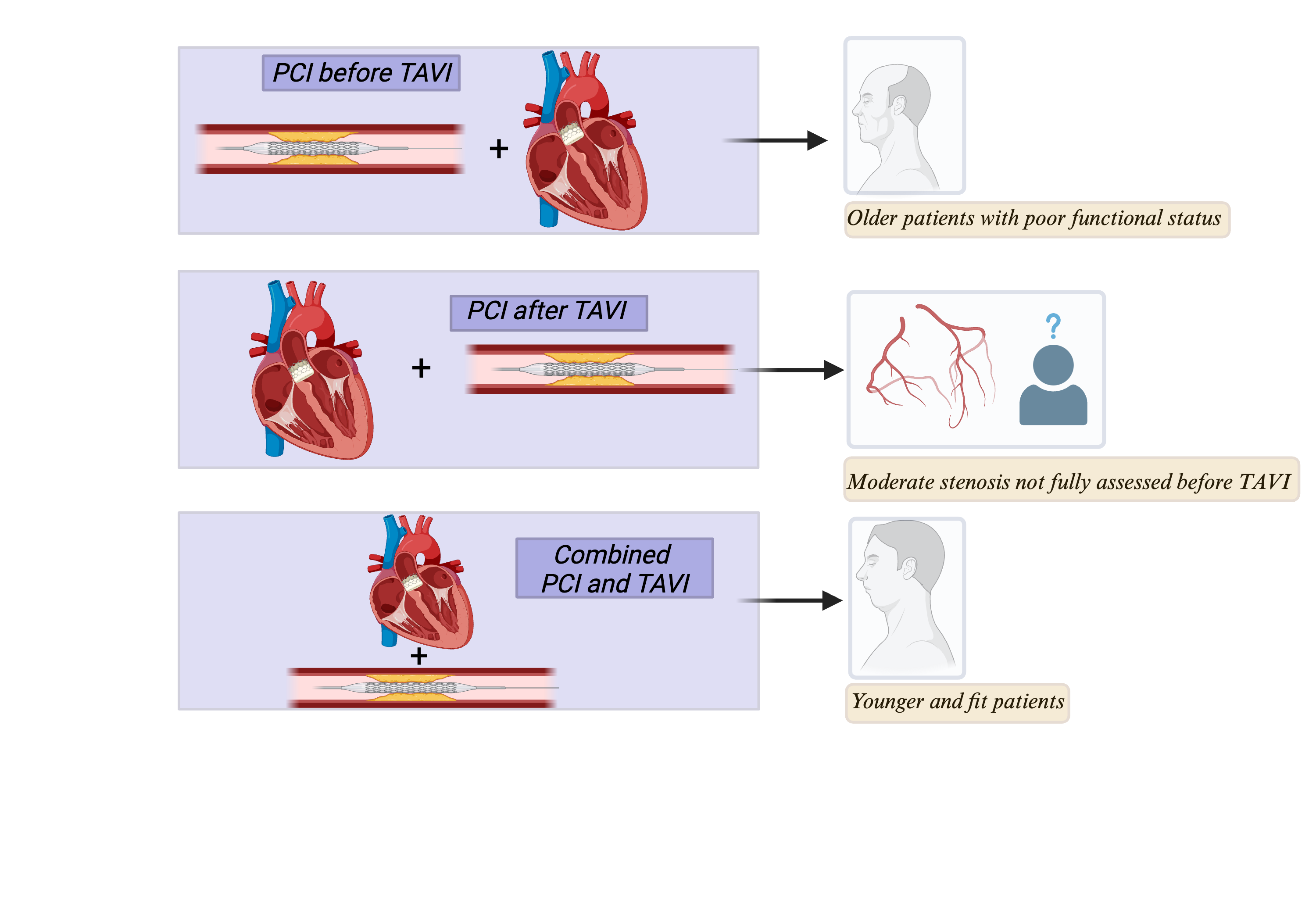
Graphical abstract: Management of CAD in TAVI candidates
CAD - coronary artery disease, PCI -percutaneous coronary intervention, TAVI -transcatheter aortic valve implantation;
Key words: coronary artery disease, percutaneous coronary intervention, transcatheter aortic valve implantation
Judge a man by his questions rather than his answers.
Voltaire
Transcatheter aortic valve implantation (TAVI) has been a revolution for the treatment of severe aortic stenosis (AS) in patients who are either at high risk for traditional surgical aortic valve replacement (SAVR) or inoperable, with proven survival benefits (1, 2). To date, TAVI is recommended in older patients (>75 years), or in those who are high risk (STS-PROM/EuroSCORE II >8%) (3).
Several studies have demonstrated the association between AS and coronary artery disease (CAD), which increases with the age, as the risk factors for aortic stenosis are similar to those for atherosclerotic disease (4). Specifically, CAD has been reported in ≥50% of AS patients undergoing both SAVR and TAVI (5). Given this strong association, assessment of CAD in patients undergoing TAVI is often necessary.
In a recent consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) in collaboration with the European Society of Cardiology (ESC) Working Group on Cardiovascular Surgery, Tarantini G. et al. (6), provided a comprehensive overview of the management of CAD in patients with AS undergoing TAVI analyzing unresolved questions about coronary revascularization in this setting.
The current standard of care in the assessment of CAD in TAVI candidates remains invasive coronary angiography (ICA), which is recommended with a Class I recommendation before considering valve intervention in cases of history of CAD, suspected myocardial ischemia, left ventricular systolic dysfunction, in men >40 years of age and postmenopausal women, and in case of one or more cardiovascular risk factors (3).
The role of coronary CT angiography (CCTA) is limited to patients with a low probability of CAD as an alternative to ICA (Class IIa). It is essential to consider that coronary calcification, which is frequent and increases with age, may compromise image quality, preventing CCTA from providing a proper characterization of the plaque (3, 7, 8). Currently, it is possible to evaluate coronary stenosis using intracoronary physiology tools, enabling a deeper understanding of the hemodynamic mechanisms implicated in ischemia (9). However, it should be noted that AS may introduce potential pitfalls. Indeed, severe AS determines a reduced coronary flow firstly because of the stenosed aortic valve and secondly because of the simultaneous compression of the microcirculation from the hypertrophic contracting myocardium which is enhanced by the elevated intraventricular pressure (10). These opposing forces to the forward flow in the coronary artery could counteract the effect of intracoronary or intravenous adenosine and may, therefore attenuate the response of the coronary microcirculation to adenosine (10). Thus, FFR could potentially lead to an underestimation of the severity of the lesion, especially considering that AS itself acts as an effective tandem lesion proximal to the epicardial coronary stenosis (6). These aspects suggest that iFR may be a better option for evaluating coronary stenoses in cases of AS, as it does not require hyperemia, but observational data are inconsistent. Hence, the role of coronary invasive physiology and conventional thresholds of functional flow reserve (FFR) and instantaneous wave-free ratio (iFR) needs to be further clarified in patients with AS.
Another issue with the management of CAD in patients undergoing TAVI is the timing of PCI. While it is clear that an acute coronary syndrome needs urgent revascularization of the culprit vessel, doubts still remains about the best timing for performing PCI in TAVI candidates with chronic coronary syndrome. As a fact, it is challenging to ascribe symptoms such as angina, syncope and dyspnea to the valvular heart disease or to the ischemic condition, especially in older adults; consequently, current ESC guidelines recommend PCI only in case of a severe (>70%) coronary artery stenosis in proximal segments (Class IIa) but do not advice about timing (3, 11). Tarantini et al. (6), analyzed pros and cons of performing PCI before, after or combined with TAVI (6).
Performing PCI before TAVI allows an easier coronary access, especially if a self-expanding transcatheter heart valve (THV) with a supra-annular leaflet position such as the Evolut R (Medtronic) is selected for implantation. Also, this strategy reduces the risk of ischemia-induced hemodynamic instability, especially when doing rapid ventricular pacing during valve release. Furthermore, the total amount of contrast used to perform PCI and TAVI is divided in two procedures, lowering the risk of contrast-induced nephropathy. On the other hand, the procedure would carry a higher risk of hemodynamic instability due to AS and, as previously mentioned, untreated AS may alter intracoronary physiology.
Another possibility is performing PCI after TAVI. In this case, a non-stenotic aortic valve would allow a better assessment of intracoronary physiology, lower risk of hemodynamic instability during complex PCI, and reduced contrast use compared to concomitant procedures.
An important disadvantage would be the more challenging coronary access, which is also one of the main concerns with the expansion of TAVI to younger patients, since they have a high probability of CAD progression requiring ICA years after the TAVI procedure.
As explained by Tarantini and colleagues (6), to preserve coronary access in this setting, it is crucial to consider an accurate THV selection based on stent frame and leaflet height with respect to the recommended annular positioning, as this is a key factor influencing coronary access after TAVI (6). In fact, a balloon-expandable intra-annular THV such as a SAPIEN 3 or SAPIEN 3 Ultra (Edwards Lifesciences) would facilitate this purpose (12). Other determining factors are sinus sizes, height and width of the sinotubular junction (STJ), and coronary take-off height and position. Furthermore, commissural alignment should always be pursued to optimize coronary access, especially when implanting a tall-frame THV (6).
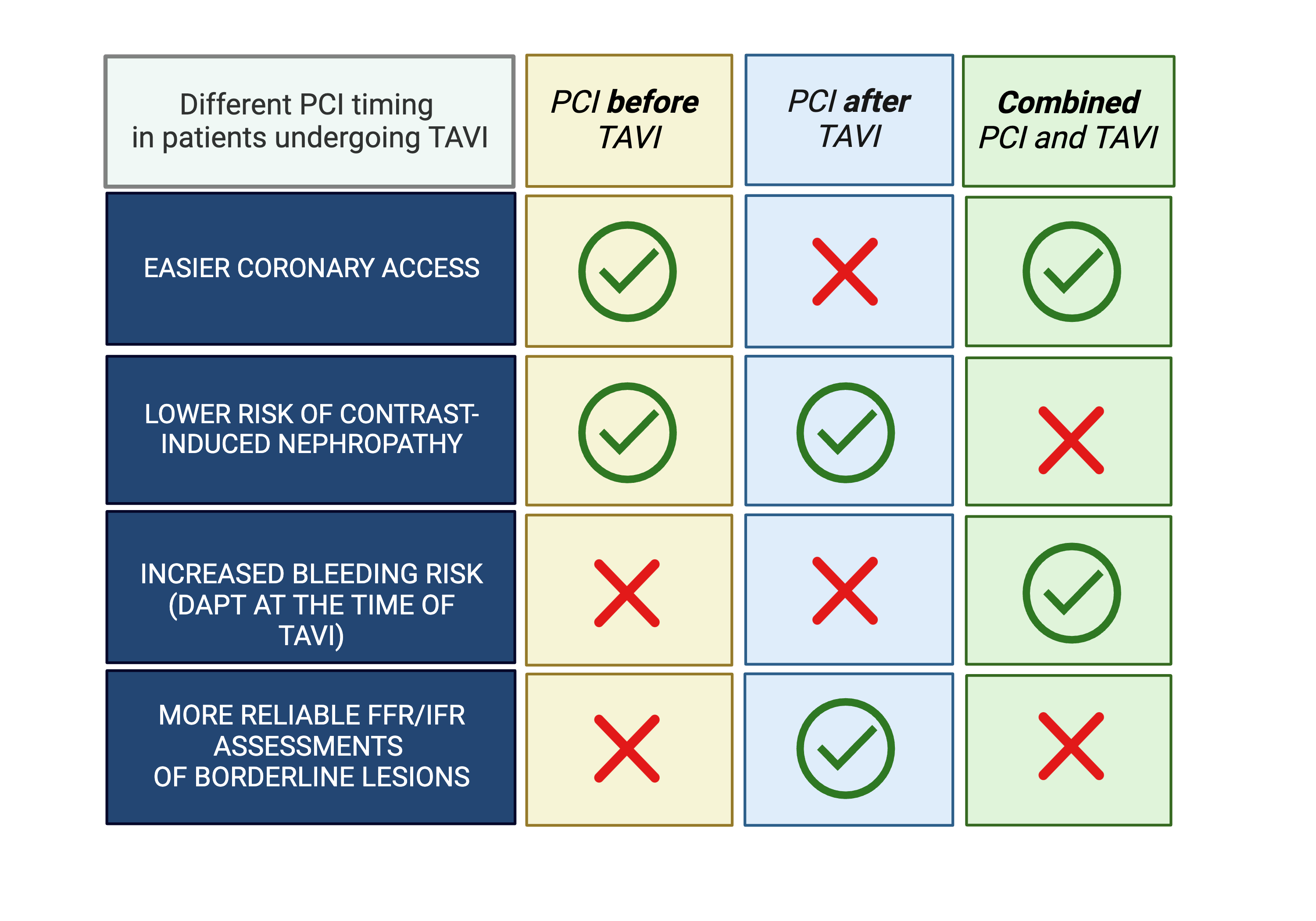
Figure 1. Different PCI timing in patients undergoing TAVI
PCI - percutaneous coronary intervention, TAVI - transcatheter aortic valve implantation
The last option is represented by combined PCI and TAVI. This choice would increase the risk of contrast-induced acute kidney injury and would require dual antiplatelet therapy at the time of TAVI, increasing the bleeding risk.
Considering these three possibilities, the decision for the timing of PCI and TAVI must be adapted and discussed case-by-case (Fig. 1). For instance, for older patients with poor functional status, it may be reasonable to perform PCI before TAVI due to the lower probability of performing ICA again and the lower ischemic risk during the TAVI procedure, in particular if a THV with supra-annular leaflets is selected. Conversely, for younger and “fit” patients it may be more indicated to perform PCI and TAVI in the same procedure. Finally, performing ICA and subsequent PCI after TAVI allows to obtain better accuracy from intracoronary physiology, which could be useful in case of a moderate stenosis documented in CCTA but not fully assessed before TAVI.
In conclusion, the management of CAD in TAVI candidates is still a matter of debate. The progressively younger age of these patients has raised more doubts about the timing in which the two procedures must be performed. The decision must be based on the complexity of the coronary anatomy, selection of valve prosthesis, symptoms, and patient comorbidities. Further evidence is awaited.
Peer-review: Internal
Conflict of interest: Giuseppe Biondi-Zoccai has consulted for Amarin, Balmed, Cardionovum, Crannmedical, Endocore Lab, Eukon, Guidotti, Innovheart, Meditrial, Microport, Opsens Medical, Terumo, and Translumina, outside the present work.
All other authors report no conflict of interest.
Authorship: D.M., M.B., G.T., L. L. S., L.S., G.B.-Z., M.B. equally contributed to manuscript preparation and fulfilled authorship criteria, D.M. and M.B. equal authorship criteria contribution
Acknowledgment and Funding: None to declare
References
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

