Key messages to comprehend: 2023 Focused Update of the 2021 European Society of Cardiology Guidelines for the diagnosis and treatment of acute and chronic heart failure
EDITORIALS
Key messages to comprehend: 2023 Focused Update of the 2021 European Society of Cardiology Guidelines for the diagnosis and treatment of acute and chronic heart failure
Article Summary
- DOI: 10.24969/hvt.2023.443
- Page(s): 273-277
- CARDIOVASCULAR DISEASES
- Published: 09/12/2023
- Received: 21/11/2023
- Accepted: 22/11/2023
- Views: 22241
- Downloads: 3846
- Keywords: editorial
Address for Correspondence*: Zehra Ilke Akyildiz, İzmir University of Economics, Faculty of Medicine, İzmir, Turkey
E-mail: ziakyildiz@hotmail.com
Key messages to comprehend: 2023 Focused Update of the 2021 European Society of Cardiology Guidelines for the diagnosis and treatment of acute and chronic heart failure
Various randomized controlled trials have emerged following the release of the 2021 European Society of Cardiology (ESC) Guidelines on the diagnosis and management of acute and chronic heart failure (HF) (1). These studies prompted the need for the 2023 Focused Update of HF ESC guideline (2). The editorial comment on the 2023 Focused Update, presented herein, specifically tackles revisions in HF treatment recommendations based on the latest findings. It is worth to list these numerous new trials and meta-analyses, which are considered and gave inspiration to this 2023 update. The list is as follows:
ADVOR (Acetazolamide in Decompensated Heart Failure with Volume Overload) (3), CLOROTIC (Combination of Loop Diuretics with Hydrochlorothiazide in Acute Heart Failure) (4), COACH (Comparison of Outcomes and Access to Care for Heart Failure) (5), DAPA-CKD (Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease) (6), DELIVER (Dapagliflozin Evaluation to Improve the LIVEs of Patients with PReserved Ejection Fraction Heart Failure) (7), EMPA-KIDNEY (EMPAgliflozin once daily to assess cardio-renal outcomes in patients with chronic KIDNEY disease) (8), EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction) (9), EMPULSE (Empagliflozin in Patients Hospitalized with Acute Heart Failure Who Have Been Stabilized) (10),
FIDELIO-DKD (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease) (11), FIGARO-DKD (Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease) (12), IRONMAN (Effectiveness of Intravenous Iron Treatment versus Standard Care in Patients with Heart Failure and Iron Deficiency) (13), PIVOTAL (Proactive IV Iron Therapy in Haemodialysis Patients) (14,15), REVIVED-BCIS2 (Revascularization for Ischemic Ventricular Dysfunction) (16), STRONG-HF (Safety, Tolerability and Efficacy of Rapid Optimization, Helped by NT-proBNP Testing, of Heart Failure Therapies) (17), TRANSFORM-HF (Torsemide Comparison with Furosemide for Management of Heart Failure) (18), and TRILUMINATE Pivotal (Clinical Trial to Evaluate Cardiovascular Outcomes in Patients Treated With the Tricuspid Valve Repair System Pivotal) (19).
The Task Force has opted to revise the recommendations pertaining to specific sections outlined in the 2021 ESC HF Guidelines. The targeted areas for update include chronic HF, addressing conditions such as HF with mildly reduced ejection fraction (HFmrEF) and with preserved EF (HFpEF), acute HF, and the section focused on comorbidities and the prevention of HF.
Chronic heart failure
New recommendation on the use of sodium–glucose co-transporter 2 inhibitors (SGLT2 inhibitor) is based on the reduction of the primary composite endpoint based on the EMPEROR-Preserved and DELIVER trials (7, 9). EMPEROR-Preserved is the first trial in patients with HF and LVEF >40% who were randomized to empagliflozin (10 mg once daily) or placebo (9). The primary outcome, composite of cardiovascular (CV) death or hospitalization for HF, was significantly reduced however; this effect was mainly driven by the HF hospitalizations.
One year later the same results were observed in DELIVER trial (7). DELIVER randomized the HF patients with left ventricular ejection fraction (LVEF) >40% to dapagliflozin (10 mg once daily) or placebo. In both studies, reduction in HF hospitalization was noted in both type 2 diabetes mellitus (T2DM) and nondiabetics; but no reduction in CV mortality was observed.
Since then, two trials have become available with the SGLT2 inhibitors empagliflozin and dapagliflozin, in patients with HF and LVEF>40%, that justify an update in the recommendations for both HFmrEF and HFpEF as they achieved the significant reduction in primary outcome. In updated 2023 ESC HF guideline, dapagliflozin or empagliflozin are recommended in symptomatic patients with HFmrEF and HFpEF to reduce the risk of HF hospitalization or CV death as Class I-A (2). Because of these recommendations, following treatment charts are added as an update in patients with HFmrEF and HFpEF (Fig. 1).
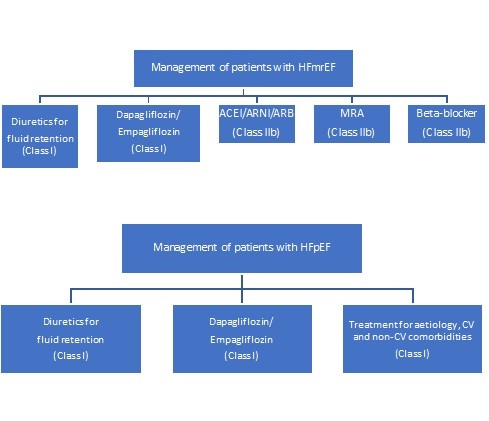
Figure 1. Treatment of HFmEF and HFpEF by SGLT2 inhibitors (modified from reference 1 under CC BY license)
ACE – angiotensin converting enzyme inhibitor, ARB – angiotensin receptor blocker, ARNI – angiotensin receptor-neprilysin inhibitor, CV- cardiovascular, HFmrEF – heart failure with mildly reduced ejection fraction, HFpEF – heart failure with preserved ejection fraction, MRA – mineralcorticoid receptor antagonist
Acute Heart Failure
Novelties in therapy and new recommendations on management strategies of acute HF are mentioned in the 2023 Focused Update.
Medical approach to acute heart failure
The trials assessing therapies and their outcomes are mentioned in this updated 2023 guideline, however no recommendation is made. These trials are ADVOR (3), CLOROTIC TRIAL (4), and EMPULSE (10). ADVOR focused on intravenous (i.v) acetazolamide in acute decompensated HF (3). Results may suggest the potential benefits of adding acetazolamide to a standard diuretic regimen for decongestion but further data on outcomes and safety are required. Oral hydrochlorothiazide in patients with acute HF was evaluated in CLOROTIC TRIAL (4). The absence of a discernible effect on clinical outcomes in this trail prevents the inclusion of any recommendation in the current guideline update. Additional information on outcomes and safety is imperative. EMPULSE tested the efficacy of the early initiation of empagliflozin in patients hospitalized for acute HF (10). The findings align with the outcomes demonstrated in patients with chronic HF, irrespective of left ventricular EF, as well as in individuals recently discharged from HF-related hospitalizations, once they achieve clinical stability.
Nevertheless, caution should be exercised in patients with T2DM who are at risk of diabetic ketoacidosis, especially those undergoing insulin treatment with altered carbohydrate intake or insulin dosage. SGLT2 inhibitors are not recommended for patients with type 1 diabetes. In updated 2023 ESC HF guideline there is no any new recommendation considering medical approach to acute HF.
Management strategies on acute heart failure
The COACH trial was about admission phase and results were consistent with a favorable effect of post-discharge care on outcomes (5). However, multinational further confirmation was needed for a guideline recommendation. 2023 updated acute HF management strategies for pre-discharge and early post-discharge were also outlined in 2021 ESC HF Guideline; however, there was no data to support these claims at that time (1). The STRONG-HF trial was about optimal medical therapy up-titration on pre-discharge and early post-discharge phases (17). In updated 2023 ESC HF guideline, an intensive strategy of initiation and rapid up-titration of evidence-based treatment before discharge and during frequent and careful follow-up visits in the first 6 weeks following a HF hospitalization is recommended to reduce the risk of HF rehospitalization or death based on the STRONG-HF trial. During the follow‐up visits, symptoms and signs of congestion, blood pressure, heart rate, NT‐proBNP values, potassium concentrations, and estimated glomerular filtration rate should be checked. Even though this is recommended, some points should be emphasized about this trial. One of them was that the patients were a carefully selected specific population according to the enrolment criteria of the STRONG-HF trial. In addition, this trial was commenced before the availability of current evidence and recommendations for SGLT2 inhibitors, which were not obligatory in the protocol (17). Furthermore, the significant reduction was only in HF hospitalization and no reduction in CV death or all-cause death alone was detected. Depending on this data, the recommendation level is given as Class I-B for pre-discharge and early post-discharge follow-up of patients hospitalized for acute HF in 2023 updated guideline (2).
Comorbidities and prevention of heart failure
Contribution of comorbidities to the progression of HF in all stages was focused in the 2021 ESC HF guidelines (1). The 2023 Focused Update of HF ESC guideline introduces new recommendations for the prevention and the comorbidities as chronic kidney disease (CKD), T2DM, and iron deficiency (2).
The data supporting the recommendations for preventing HF in individuals with both CKD and T2DM come from recent trials and meta-analysis. The DAPA-CKD included both diabetic and non-diabetic patients with CKD (6). Random assignment to dapagliflozin 10 mg once daily or placebo was made. A significant reduction by dapagliflozin compared with placebo was achieved in primary and secondary outcomes. EMPA-KIDNEY included a broader group of patients with CKD when compared to DAPA-CKD (8). The patients were randomized to empagliflozin 10 mg once daily or placebo. A significant reduction in the primary composite endpoint of progression of kidney disease or CV death was observed with empagliflozin. However, the reduction in secondary end-points as hospitalizations for HF or death due to CV causes was not significant. The result of a recent meta-analysis including DAPA-CKD, EMPA-KIDNEY, CREDENCE39a (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation), and SCORED39b (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk) was also mentioned in the updated guideline in comorbidities section (20) . This meta-analysis includes the evaluation of SGLT2 inhibitors in different clinical settings as T2DM at high risk of CV diseases, HF, CKD. Similar reduction in HF hospitalizations and CV death was observed irrespective of a history of diabetes however, results were not significant in patients without diabetes when only CKD trials were focused in the analysis. Therefore, in updated 2023 ESC HF guideline SGLT2 inhibitors (dapagliflozin or empagliflozin) are recommended in patients with CKD and T2DM to reduce the risk of HF hospitalization or CV death in this updated HF guideline as Class I-A.
The new recommendation on prevention of HF by finerenone is supported by two randomized controlled trials and a meta-analysis. The FIDELIO-DKD and FIGARO-DKD trials tested selective, non-steroidal, mineralocorticoid receptor antagonist finerenone in patients with diabetic kidney disease (11, 12).
In FIDELIO-DKD primary endpoints, composite of renal outcomes, and key secondary endpoints, CV composite outcomes, were significantly reduced by finerenone compared with placebo. In FIGARO-DKD primary endpoints, CV composite outcomes, which is mainly driven by HF hospitalizations, were significantly reduced by finerenone compared with placebo.
FIDELTY study of pooled data analysis of these trials also showed a significant a reduction in the composite CV outcome, including CV death, non-fatal stroke, non-fatal myocardial infarction, and HF hospitalizations with finerenone vs. placebo (21). This was mainly driven by the HF hospitalization reduction. In the light of these results, in updated 2023 ESC HF guideline finerenone is recommended for the prevention of HF hospitalization in patients with CKD and T2DM as Class I-A.
Iron deficiency is another common comorbidity in HF patients and it is associated with a poor prognosis (22). In the 2021 ESC HF guidelines, recommendations for the diagnosis and treatment of iron deficiency were focused (1). The 2023 Focused Update of HF ESC guideline also introduces new recommendations for iron deficiency. AFFIRM-AHF provided the role of i.v. iron in preventing HF hospitalizations and CV deaths with a borderline p value (23). However, when this analysis was reevaluated in the pre-COVID 19 period, p value gained a significance. FAIR-HF (24), CONFIRM-HF (25) trials also provided an improvement in exercise capacity, quality of life (QoL) and a reduction in HF hospitalizations. A new trial IRONMAN included patients with left ventricle EF ≤45%, and transferrin saturation <20% or serum ferritin <100 μg/L to compare i.v. ferric derisomaltose or usual care (13). Once again, in this study, HF hospitalizations and CV deaths were reduced with a borderline significance. COVID-19 sensitivity analysis of IRONMAN also showed a reduction in primary end- points with a significant p value. Furthermore, the results of FAIR-HF, CONFIRM-HF, AFFIRM-AHF, and IRONMAN have been included in meta-analyses comparing the effects of i.v. iron therapy with standard of care or placebo in patients with HF and iron deficiency (22). Individual patient data meta-analyses confirmed the impact of i.v. iron in reduction of HF hospitalizations. In the light of these results, in updated 2023 ESC HF guideline i.v. iron supplementation is recommended in symptomatic patients with HFrEF and HFmrEF, and iron deficiency, to alleviate HF symptoms and improve QoL as Class I-A and i.v. iron supplementation with ferric carboxymaltose or ferric derisomaltose should be considered in symptomatic patients with HFrEF and HFmrEF, and iron deficiency, to reduce the risk of HF hospitalization as Class IIa-A.
Conclusion
Given the steadily rising frequency and occurrence, HF has evolved into an epidemic issue. This led to medical, social and economic consequences. In this context, the QoL for HF patients has become increasingly important. This guideline emphasizes the key aspects of new modalities in HF management, which are meant to increase the QoL of these patients. These new recommendations on prevention, comorbidity and drug management of HF will lead our patients a better life expectancy. By prudently implementing these recommendations in clinical settings, we have the potential to significantly reduce the global impact of HF.
Zehra Ilke Akyildiz1*, Oben Baysan2
1Izmir University of Economics, Faculty of Medicine, İzmir, Turkey
2Guven Hospital, Ankara, Turkey
Peer-review: Internal
Conflict of interest: None to declare
Authorship: Z.I.A. and O.B. equally contributed to manuscript preparation and fulfilled authorship criteria
Acknowledgment and Funding: None to declare
References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

