Evaluation of myocardial contraction fraction in transcatheter aortic valve replacement
BRIEF REPORT
Evaluation of myocardial contraction fraction in transcatheter aortic valve replacement
Article Summary
- DOI: 10.24969/hvt.2023.449
- CARDIOVASCULAR DISEASES
- Published: 23/12/2023
- Received: 24/10/2023
- Revised: 28/11/2023
- Accepted: 29/11/2023
- Views: 4186
- Downloads: 3627
- Keywords: transcatheter aortic valve replacement, aortic stenosis, myocardial contraction fraction, ventricular function
Address for Correspondence*: Andrea De Lorenzo, Instituto Nacional de Cardiologia, Rua das Laranjeiras 374, Rio de Janeiro, RJ, Brazil
Email: andlorenzo@hotmail.com Mobile: +55 21 30372288
Luana da Graça Machado, Angelo Salgado, Andrea De Lorenzo
Instituto Nacional de Cardiologia, Rio de Janeiro, RJ, Brazil
Abstract
Objective: Myocardial contraction fraction (MCF), a costless, easy-to-perform echocardiographic measure, which estimates cardiac function through the volumetric measurement of myocardial shortening, may be a useful prognostic indicator in patients undergoing transcatheter aortic valve replacement (TAVR). This study aimed to evaluate MCF in patients who underwent TAVR at a public hospital in Rio de Janeiro, Brazil.
Methods: This was a retrospective cohort study. Clinical and echocardiographic data were obtained from medical records. MCF was calculated as FDV in mL/FSV in mL x100, where FDV= final diastolic volume and FSV= final systolic volume. These were derived from the dimensions of the left ventricle. The FDV was estimated as 4.5 x (final diastolic diameter of the left ventricle) and the FSV was estimated as 3.72 x (final systolic diameter of the left ventricle). Patients were followed for 66 months, and all-cause mortality was registered.
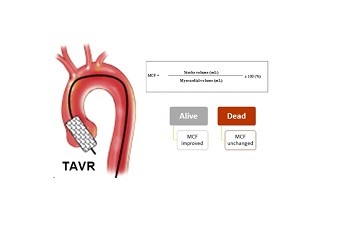
Results: Overall, 78 patients were studied. Median age was 78 years. Mortality was 56.4% over 9 years, with 29% of deaths in the first year. Pre-TAVR median MCF (45.9%) was low, while mean left ventricular ejection fraction (LVEF) was normal (57.0%). In patients who survived after the procedure, MCF increased post-TAVR, but in those who died, it decreased (49.3% vs 45.1%).
Conclusions: MCF may demonstrate left ventricular dysfunction unrecognized by LVEF measurement in patients undergoing TAVR, and may be a prognostic marker in this patient population.
Graphical abstract
Key words: transcatheter aortic valve replacement, aortic stenosis, myocardial contraction fraction, ventricular function
Left ventricular ejection fraction (LVEF), assessed by echocardiography, is a key parameter for planning transcatheter aortic valve replacement (TAVR) (1). However, myocardial dysfunction may be unrecognized by LVEF assessment, especially without the aid of myocardial strain assessment; however, the latter requires specific software, as well as operator expertise (2). A different measure of myocardial shortening, myocardial contraction fraction (MCF)- the ratio between systolic volume and myocardial volume- is considered analogous to myocardial strain (3).
MCF has been recently shown to be a prognostic marker in patients with aortic stenosis, including those undergoing TAVR (4-6); however, studies are still limited. Therefore, this study sought to evaluate MCF and its association with mortality in patients with severe aortic stenosis undergoing TAVR.
This was a retrospective study of consecutive patients aged ≥18 years who underwent TAVR at a public hospital in Rio de Janeiro from October 2011 to June 2020. The study was approved by the institutional Ethics Committee (# 4.580.337). Clinical and echocardiographic variables were obtained from medical records. All-cause death was identified from medical records or death certificates provided by the state´s Health Secretariat.
Data from echocardiograms performed immediately before TAVR, 1 week after TAVR, and at late follow-up were registered. LVEF was calculated by the Teicholz method. For the calculation of MCF, the following formula was employed (3): MCF= (FDV in mL/FSV in mL) x100, where FDV= final diastolic volume and FSV= final systolic volume. The FDV was estimated as 4.5 x (final diastolic diameter of the left ventricle [LV]) and the FSV was estimated as 3.72 x (final systolic diameter of the LV) (Fig. 1).
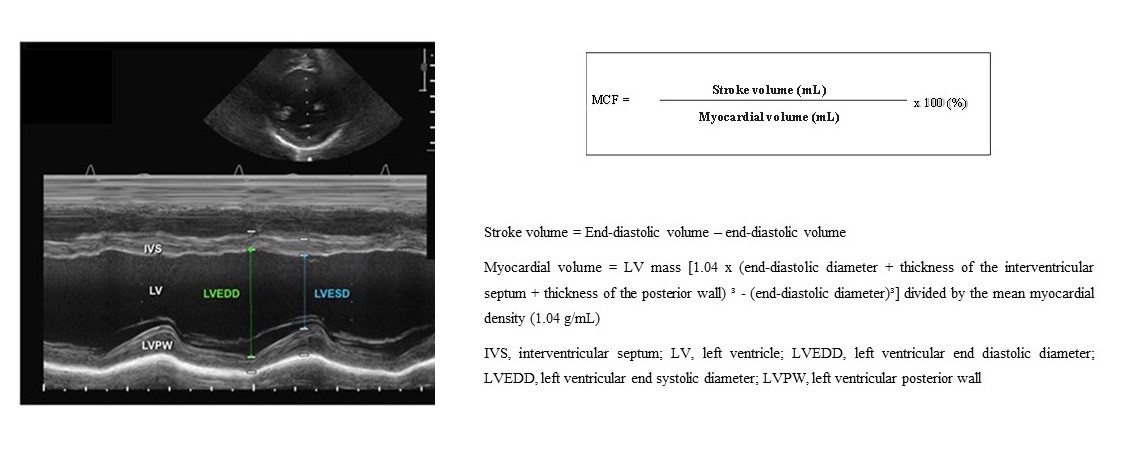
Figure 1. Myocardial contraction fraction (MCF) determination
Continuous variables are expressed as mean (SD) or median (IQR) and were compared using Mann-Whitney´s test. Categorical variables are expressed as frequencies. A p<0.05 was considered statistically significant. Survival was evaluated using Kaplan-Meier survival analysis.
Seventy-eight patients were studied. Baseline characteristics are shown in Table 1. Mean aortic valve area was 0.62 (0.42 – 0.8) cm2, LVEF was 57.3(16.3)% and MCF, 45.9 (34.8 – 54.1)%. In the 1-week evaluation, mean LVEF was 57.7 (16.6)% and median MCF was 47 (37.7 – 45.9)%. Moderate to severe aortic regurgitation was present in 26.9%, severe aortic regurgitation in 7.7%, and severe mitral regurgitation in 2.6% of patients. Regarding diastolic function, 30.7% of the patients had grade I diastolic dysfunction, and 14.1%, grade 2 diastolic dysfunction. In 14.1% of the patients, the evaluation of diastolic function was compromised due to arrhythmias or other issues, and in 7%, diastolic function was not reported. The median pre-TAVR pulmonary artery systolic pressure was 41.1 (31.5-48.5) mmHg. Right ventricular function was not evaluated.
Patients were followed for a mean of 66 months. All-cause death occurred in 56.4%. A Kaplan-Meier curve is shown in Figure 2, depicting patient survival during follow-up. Of the 78 patients, 57 completed 30 months of follow-up and 33 completed five years of follow-up.
In surviving patients, the median MCF was 43% before TAVR, raised to 49% after 1 week, and persisted at 49% at late follow-up (p<0.01). In those who died, median MCF was 48% before TAVR, 45% after 1 week, and 47% in the last echocardiogram (p>0.05) (Fig. 3).
|
Table 1. Baseline characteristics |
|
|
Variables |
Study population (n=78) |
|
Age, years |
78.6 (75.2 – 83.0) |
|
Female, n(%) |
41 (52.5) |
|
Functional class I/III (NYHA) , n(%) |
16 (20.5) |
|
Functional class III/IV (NYHA) , n(%) |
62 (79.5) |
|
Syncope, n(%) |
24 (30.8) |
|
Angina, n(%) |
32 (41.0) |
|
Known coronary artery disease, n(%) |
43 (55.1) |
|
Systemic hypertension, n(%) |
71 (91.0) |
|
Diabetes, n(%) |
21 (26.9) |
|
Dyslipidemia, n(%) |
52 (66.7) |
|
Cerebrovascular disease, n(%) |
7 (9.0) |
|
Carotid artery disease, n(%) |
17 (21.8) |
|
Peripheral artery disease, n(%) |
15 (19.2) |
|
Chronic obstructive pulmonar disease, n(%) |
14 (17.9) |
|
Chronic renal failure, n(%) |
52 (66.7) |
|
Prior coronary artery bypass, n(%) |
15 (19.2) |
|
Prior valve replacement, n(%) |
8 (10.3) |
|
Variables are expressed as number and percentage or median and interquartile range. NYHA - New York Heart Association |
|
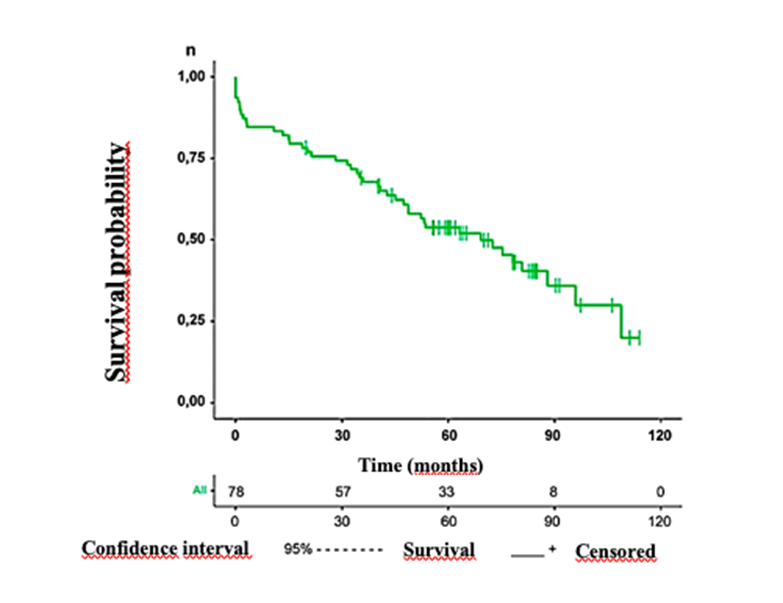
Figure 2. Kaplan-Meier curve depicting survival of the transcatheter aortic valve replacement (TAVR) patient population
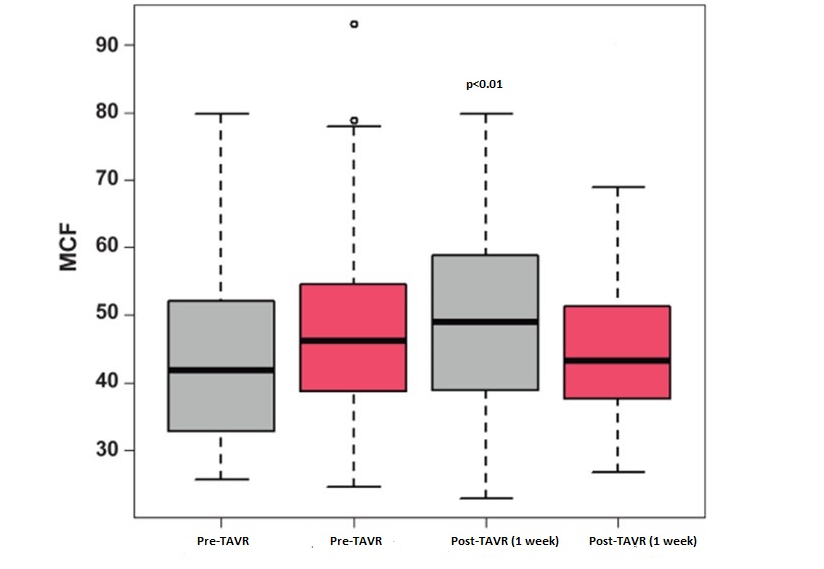
Figure 3. Myocardial contraction fraction (MCF) in patients who survived (gray boxes) or died (pink boxes) after transcatheter aortic valve replacement (TAVR). Surviving patients had increased MCF post-TAVR, while deceased patients did not show MCF improvement.
Study Limitations
This is a retrospective study from a single hospital, and therefore its results may not be extrapolated to other patient populations. Additionally, echocardiographic data were obtained from previously recorded exams, and further analyses (eg, of strain or right ventricular function) were not possible. It was also not possible to evaluate intra- or inter-observer variability of echocardiographic measures.
Conclusions
In this population of patients with severe aortic stenosis undergoing TAVR, MCF was reduced while LVEF was preserved, what may indicate unrecognized myocardial dysfunction. After TAVR, there was an increase in MCF in patients who survived and absence of increase in those who died. Therefore, MCF may be an easy, no-cost measure with prognostic value, which may merit incorporation into routine echocardiographic evaluation of patients undergoing TAVR.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship:
L.D.M., A.S., A.L. equally contributed to manuscript preparation and fulfilled authorship criteria
Acknowledgment and Funding: None to declare
References
| 1. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/ American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021; 143: e72-e227 https://doi.org/10.1161/CIR.0000000000000923 |
||||
| 2. Reisner S.A., Lysyansky P., Agmon Y., Mutlak D., Lessick J., Friedman Z. Global longitudinal strain: A novel index of left ventricular systolic function. J Am Soc Echocardiogr 2004; 17: 630-3. https://doi.org/10.1016/j.echo.2004.02.011 PMid:15163933 |
||||
| 3. King DL, El-Khoury Coffin L, Maurer MS. Myocardial contraction fraction: a volumetric index of myocardial shortening by freehand three-dimensional echocardiography. J Am Coll Cardiol 2002; 40: 325-9. https://doi.org/10.1016/S0735-1097(02)01944-7 PMid:12106939 |
||||
| 4. Rusinaru D, Bohbot Y, Kubala M, Diouf M, Altes A, Pasquet A et al. Myocardial contraction fraction for risk stratification in low-gradient aortic stenosis with preserved ejection fraction. Circ Cardiovasc Imaging 2021; 14: e012257. https://doi.org/10.1161/CIRCIMAGING.120.012257 PMid:34403263 |
||||
| 5. Al-Rashid F, Totzeck M, Saur N, Jánosi RA, Lind A, Mahabadi AA, et al. Global longitudinal strain is associated with better outcomes in transcatheter aortic valve replacement. BMC Cardiovasc Disord 2020; 20: 267. https://doi.org/10.1186/s12872-020-01556-4 PMid:32493384 PMCid:PMC7268397 |
||||
| 6. Romeo FJ, Seropian IM, Arora S, Vavalle JP, Falconi M, Oberti P, et al. Prognostic impact of myocardial contraction fraction in patients undergoing transcatheter aortic valve replacement for aortic stenosis. Cardiovasc Diag Ther 2020; 10: 12-23. https://doi.org/10.21037/cdt.2019.05.02 PMid:32175223 PMCid:PMC7044096 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

