Impact of inspiratory muscle training on respiratory muscle function and functional capacity in patients with heart failure: a systematic review
SYSTEMATIC REVIEW
Impact of inspiratory muscle training on respiratory muscle function and functional capacity in patients with heart failure: a systematic review
Article Summary
- DOI: 10.24969/hvt.2024.451
- CARDIOVASCULAR DISEASES
- Published: 03/01/2024
- Received: 28/02/2023
- Revised: 24/11/2023
- Accepted: 25/11/2023
- Views: 7820
- Downloads: 3822
- Keywords: heart failure, inspiratory muscle training, respiratory metaborreflex, functional capacity
Address for Correspondence: Ursula Pinelo Cavegn, Postgraduate course in Cardiovascular Physiotherapy, National Institute of Cardiology, Rio de Janeiro, RJ, Brazil; Email: ursulapinelo28@gmail.com
Úrsula Pinelo Cavegn, Luiz Fernando Rodrigues Junior
1Postgraduate course in Cardiovascular Physiotherapy, National Institute of Cardiology, Rio de Janeiro, RJ, Brazil
2Department of Physiological Sciences, Federal University of the State of Rio de Janeiro, Rio de Janeiro, RJ, Brazil
Abstract: Heart failure (HF) is one of the most prevalent diseases in the world and is characterized as a complex clinical syndrome. It is a disease that affects both the peripheral muscles and the respiratory muscles, causing damage such as reduced strength and endurance. One of the main symptoms is fatigue and exercise intolerance.
The aim of this review article is to assess whether the use of inspiratory muscle training (IMT) associated with conventional training enhances inspiratory muscle strength (PImax) and inspiratory muscle endurance (SPImax) and functional capacity in patients with HF.
Methods: A literature review was carried out on the use of IMT in patients, including 6 randomized clinical trials, searched in the PubMed, PEDro and Lilacs databases.
Results: There is evidence showing an increase in PImax and SPImax with the addition of IMT to training; in addition, an improvement in VO2 peak, circulatory power and ventilatory efficiency.
Conclusion: The use of IMT associated with conventional training is an interesting strategy for boosting gains in strength and inspiratory muscle endurance, increasing exercise tolerance and consequently improving patients' quality of life. However, more studies are needed in order to more clearly define the selection criteria and methodology for applying IMT.
Key words: heart failure, inspiratory muscle training, respiratory metaborreflex, functional capacity
Introduction
Heart failure (HF) is characterized as a complex clinical syndrome in which the heart is unable to pump blood to meet the metabolic demands of the tissues or does so under high filling pressures. It is one of the most prevalent diseases, with around 23 million cases worldwide and a 5-year survival rate of only 35% after diagnosis (1). This condition can occur as a result of biochemical, structural and congenital alterations, as well as being determined by extra-cardiac conditions such as systemic arterial hypertension (2). In this sense, at the beginning of the development of HF, the body recruits compensatory mechanisms to minimize the overload imposed on the myocardium, leading to cardiac remodeling, which will feed back into this condition (3, 4).
Cardio-sarcopenia is a condition that arises from the combination of two degenerative processes: sarcopenia, which is the gradual loss of skeletal muscle mass related to ageing, and cardiac dysfunction, characteristic of HF. Changes in cardiac function and structure trigger a series of negative effects on skeletal muscle, resulting in decreased strength, endurance and functional capacity.
This skeletal muscle dysfunction can, in turn, contribute to a vicious cycle, where muscle weakness resulting from HF leads to a sedentary lifestyle, further aggravating sarcopenia (5). Among the most common symptoms of HF are dyspnea, a feeling of fatigue and exercise intolerance (6). This can occur due to impaired muscle performance in both the peripheral and respiratory muscles, affecting exercise capacity and consequently reducing quality of life (4, 7).
Factors such as hypoxia, oxidative stress, systemic inflammation and disuse are strongly related to muscle alterations, contributing to the appearance of these symptoms (3, 8).
In turn, inspiratory muscle weakness is present in 50% of patients with HF, who will show changes in variables such as maximum inspiratory pressure (MIP), which corresponds to inspiratory muscle strength, and maximum sustained inspiratory pressure (MPSI), which corresponds to inspiratory muscle endurance.
A decrease in the predicted values of these measurements is related to an increase in the sensation of dyspnea and a worsening of functional class, implying a worse prognosis (9). In this context, several studies have used inspiratory muscle training (IMT) as a form of intervention to specifically increase the strength and endurance of the inspiratory muscles and thereby improve functional capacity. This is due to the attenuation of the respiratory metaborreflex, which is a mechanism triggered by intense respiratory effort and which leads to fatigue of the peripheral muscles. In this way, there is a redistribution of flow from the peripheral muscles to the diaphragm through sympathetic vasoconstriction, exacerbating the individual's perception of effort and limiting performance in high-intensity exercise. With improved respiratory performance, this effect is minimized, leading to greater exercise tolerance and a better quality of life (10, 11).
The general aim of this article is to review the findings related to the effects of IMT in the cardiac rehabilitation of patients with HF. The specific objectives of this article include evaluating the effect of IMT on: inspiratory muscle strength and endurance, cardiopulmonary exercise test (CPET) variables, 6-minute walk test (6MWT), quality of life (QoL) and change in NYHA functional class.
Methods
A literature review was carried out to investigate whether combining IMT with conventional training in patients with HF had a greater impact on respiratory muscle performance and the functional capacity of these individuals. This review included articles that addressed the proposed topic between 1996 and 2022. The searches were carried out in the Pubmed, PEDro and Lilacs databases, using the descriptors: inspiratory muscle training, exercise; and heart, failure. The initial search was carried out using articles that contained these terms in the title or abstract and were considered eligible for the review. The exclusion criteria were duplicate articles, those that were not in line with the research topic and studies that did not use IMT as a form of intervention. The remaining studies were included in the review.
Results
There were identified 187 possible relevant articles, with 15 being excluded as duplicates and 154 by absence of association with the study topic, remaining six studies included in the review (Fig. 1).
Considering the interventions presented by the studies (Table 1), only one study did not perform aerobic training (AT) as an intervention associated with IMT. One study performed the aerobic interval training (AIT) modality in aerobic training and the others used moderate continuous training. 3 of the 6 studies used resistance training associated with IMT.
Effect of IMT on inspiratory muscle strength and endurance
In all the studies, PImax measurements were taken pre- and post-intervention, and two studies did not assess SPImax. In the study by Winkelmann et al. (12), PImax increased significantly (110% compared to 72%) when compared to the control group, which only performed AT. Similarly, in all the other studies there was an improvement in PImax, both in the group that used IMT and in the groups that used AT and/or resistance training (RT). However, the most significant improvement occurred in the groups that used combined training. In addition, SPImax, which corresponds to inspiratory muscle endurance, was assessed in 4 studies (13-15, 17), and only showed a significant increase in the groups that underwent combined training. In the study by Kawauchi et al. (16), the low-intensity training group and the moderate-intensity training group both increased PImax, but the greatest increase was in the moderate-intensity group and occurred more quickly than in the low- intensity group. Another point worth highlighting in this study is that the patients in the control group who did not undergo any type of intervention had an 8% reduction in PImax.
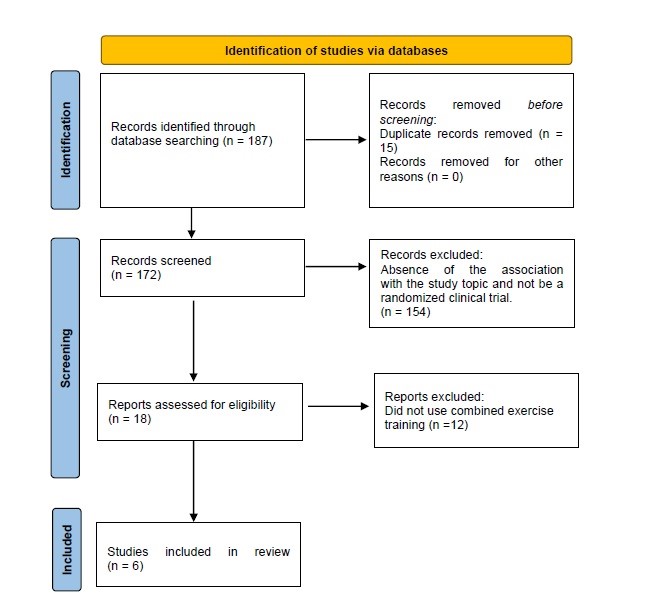
Figure 1. Identification of studies via databases
Cardiopulmonary Exercise Test Variables
In the study by Winkelmann et al. (12), interesting responses were seen in terms of the variables derived from the CPET. With the inclusion of CMT associated with aerobic training in the intervention group, significant improvements were seen in VO2 peak, circulatory power, O2 recovery kinetics and ventilatory efficiency (VE/VCO2 slope). The 2013 study by Lautaris et al. (13) corroborates the findings of the study by Winkelmann et al. (12). There was a significant improvement in VO2 peak; exercise time; ventilatory threshold and reduction in VE/VCO2 slope in the ARIS group (Aerobic Training (AT) + Resistance Training (RT) + IMT). The aerobic training group had a significant increase in VO2 peak, exercise time and ventilatory threshold. Circulatory power improved significantly in both groups.
|
Table 1. Characteristics of clinical trials and their training protocols |
|||
|
Study |
Subjects characteristics |
Training protocol |
Main outcomes |
|
Winkelmann et al. (11) 2009 |
Total sample n = 38 NYHA: N/A (1) AT: n= 19, final n = 12 M/W: 7/5 Age: 59( 9) yrs FEV1: 34(11)% PImax: 56 (13) cmH2O (2) AT + IMT: n= 19, final n= 12 M/W: 4/8 Age: 54(12) yrs FEV1: 39(12)% PImax: 57 (12) cmH2O |
Duration: 12 weeks - 3x a week. AT: 1st week 20 minutes, adding 5 minutes each week. Totaling 45 min at the end.
AT + IMT: IMT 7 times a week for 30 minutes. 30% of PImax, readjusted weekly. |
Significant improvement in PImax, peak VO2 and circulatory power in the AT + IMT group. There was also a significant decrease in the VE/VCO2 slope and ventilatory oscillations in the combined group. There were similarmprovements in QoL in both groups. |
|
Lautaris et al. (12) 2013 |
Sample: n= 27 (1) ARIS: n = 13 M/W: 10/3 Age: 57.1(11) yrs FEV1: 27.8 (8)% NYHA (II/III): 6/7 PImax: 75.3( 11) cmH2O
(2) AT: n = 14 M/W: 12/2 Age: 58.6 (8) yrs FEVI (%): 30.6 (5.4) NYHA (II/III): 8/6 PImax: 79(9.1) cmH2O
|
Duration: 12 weeks - 3x a week. (1) ARIS: AT: 70%-80% of HRmax. Initial 20 minutes and 1 min increase each session until reaching 30 minutes.
(2) AT: 70-80% HR max. Initial 20 minutes and increased by at least 1 min each session. Totaling up to 45 minutes at the end. RT: 15 minutes Quadriceps – 3x12 repetitions at 50% of the 1RM, recalculated every 2 weeks. MMSS exercises – 1 and 2 kg dumbbell (4x12 repetitions) IMT: 60% SMIP - 15 minutes 60% of SPImax. |
Significant increase in SPImax, peak VO2, exercise time, ventilatory threshold and reduction in VE/VCO2 slope in the ARIS group.
The AT group significantly improved peak VO2, exercise time and ventilatory threshold.
Circulatory power improved in both groups.
QoL and NYHA functional class improved only in the ARIS group. |
|
Adamapoulos et al. (13) 2014
|
Sample: n= 43 (1) AT/IMT: n = 21 M/W: 19/2 Age: 57.8 (11.7) yrs FEV1: 27.7 (6.7)% NYHA (II/III): 9/12 PImax: 81.9 (21.5) cmH2O
(2) AT/SHAM: n= 22 M/W: 17/5 Age: 58.3(13.2)yrs FEV1: 30.1(5)% NYHA (II/III): 12/10 PImax: 79.1(9.4) cmH2O
|
Duration: 12 weeks – 3x a week. AT: 45 min at 70-80% HR max for both groups. IMT protocol for both groups. Level 1: 60s of rest between sets. Level 2: 45s of rest. Level 3: 30s of rest. Level 4: 15s of rest. Level 5: 10s of rest. Level 6: 5s rest.
AT/IMT: 60% of SPImax in the group, readjusted every week. AT/SHAM: 10% of SPImax in the group, readjusted every week. 30 minutes - 3x a week. |
Significant improvement in SPImax, exercise time, respiratory exchange ratio , ventilation and QoL in the AT/IMT group.
No significant difference in circulatory power, VE/VCO2 slope and ventilatory threshold. VO2 peak improved in both groups. NYHA functional class improved in both groups.
|
Heart, Vessels and Transplantation 2023; 7: doi: 10.24969/hvt.2023.451
Inspiratory muscle training in HF Pinelo Cavegn, Rodrigues Jr
![]()
|
Table 1. Characteristics of clinical trials and their training protocols (continued) |
|||
|
Study |
Subjects characteristics |
Training protocol |
Main outcomes |
|
Kawauchi et al. (14) 2017
|
Sample: n= 35 (1) Control group: n= 9 M/W: 5/4 Age: 56(7) yrs FEV1: 29(7)% NYHA (II/III): 5/4 PImax: 74 (24) cmH2O (2) Low intensity training: n= 13 M/W: 6/7 Age: 54(10) yrs FEV1: 30(6)%; NYHA (II/III): 6/7 PImax: 72 (20) H2O (3) Moderate intensity training: n= 13 M/W: 8/5 Age: 56(7) yrs FEV1: 28 (5)% ; NYHA (II/III): 5/8 PImax: 70(14) cmH2O |
Duration: 8 weeks – 7x a week, supervised every 15 days by a physiotherapist. Loads were readjusted every 15 days for RT and IMT. IMT lasted 30 minutes for both groups. (1) Control group: no ntervention (2) Low intensity group: IMT at 15% of PImax, reassessed every 15 days. RT with 0.5 kg. (3) Moderate intensity group: 30% of PImax. RT with 50% of the 1RM. |
PImax increased significantly and faster in the moderate-intensity group. In the control group there was an 8% reduction in PImax. Both groups improved the 6MWT covered distance and QoL. Significant improvement in NYHA functional class in the moderate intensity group. |
|
Sadek et al. (15) 2020
|
Sample: n= 40 patients (1) Control group: n= 10 M/W: 5/5 Age: 52.6 (11.2) yrs PImax: 35.8(10.5) mmHg (2) AIT: n= 10 M/W: 5/5 Age: 51.6(13.8) PImax : 34.4(11.4) mmHg (3) IMT: n= 10 M/W: 5/5 Age: 52.5(13.7) yrs PImax: 36.4 (14.3) mmHg (4) AIT/IMT: n= 10 M/W: 5/5 Age: 51.8 (8.3) yrs PImax: 33.5 (13.1) mmHg |
Duration: 12 weeks of training – 3x a week. AIT: AT 4 min at 60% to 90% HRmax | 2 minutes at 50% HR max for 30 minutes. IMT: 20 minutes 3x a week. 60% PImax, adjusted every 2 weeks. |
Significant increase in SPImax, distance covered in the 6MWT and QoL in the AIT/IMT group. |
|
Lautaris et al. (16) 2021
|
Sample: n= 74 patients (1) AT: n= 18 Age: 64.8 (60.4–69.1) yrs M/W: 16/2 NYHA (II/III): 11/7 LVEF: 26.8 (24.7–28.8)% PImax: 83.1 (75.3–90.8) cmH2O (2) AT/RT: n= 17 Age: 67.5 (64–70.9) yrs M/W: 16/1 NYHA (II/III): 10/7 LVEF: 29.6 (27.2-32.1)% PImax: 79.8 (70.3–89.4) cmH2O (3) AT/IMT: n= 20 Age: 68.1 (65.2–71)yrs M/W: 20/0 NYHA (II/III): 11/9 LVEF: 28.8 (25.1–32.5)% PImax: 85.6 (75.6–95.5) cmH2O (4) ARIS: n= 19 Age: 63.9 (59.8–68) yrs M/W: 17/2 NYHA (II/III): 9/10 LVEF: 28.3 (25.9–30.7)% PImax: 82.6 (73.9–91.3) cmH2O |
Duration: 12 weeks of training – 3x a week. AT group: 30 min treadmill or cycle ergometer at 60-80% of HR max. 30 min of calisthenics exercises. AT/IMT group: 30 min of aerobic activity + IMT at 60% of SPImax / PImax for 30 min. AT/RT group: 30 minutes of aerobic activity + 30 minutes of resistance training at 50% of the 1 RM (3x12-15 repetitions). ARIS group: 30 min aerobic + 10 min RT with 50% 1RM (3x12-15 repetitions) + 20 min IMT with 60% SPImax / PImax. |
Significant increase in PImax in ARIS and SPImax in ARIS and AT/IMT groups. There was a trend towards an increase in peak VO2 in ARIS group. Circulatory power and CPET performance time were higher in the ARIS group. Significant improvement in QoL in the ARIS group. NYHA functional class tended to increase when compared to AT. AT/IMT obtained a significant improvement in QoL and NYHA functional class when compared to AT. |
|
Data are presented nas mean (SD), median (range) and numbers 6MWT - 6-minute walk test, AIT - aerobic interval training, ARIS - combined AT/RT/IMT, AT - aerobic training, IMT - inspiratory muscle training, LVEF – left ventricular ejection fraction, M- man, PImax - maximum inspiratory pressure, QoL - quality of life, RM - maximum repetition, RT - resistance training, SPImax - maximum sustained inspiratory pressure, W-woman, years- yrs |
|||
In another study, Lautaris et al. conducted an intervention program with 4 groups: ARIS, who performed IMT associated with aerobic and resistance training; AT + IMT, performed inspiratory muscle training associated with aerobic training; AT + RT, performed aerobic and resistance training; AT who performed only aerobic training. As a result, VO2 peak showed no significant difference between the groups, but the ARIS group tended to increase. In addition, circulatory power and CPET time were higher in the ARIS group when compared to the other groups. Parameters such as ventilatory efficiency, VE/VCO2 slope and O2 recovery kinetics did not have significant differences between the groups (17).
The study by Adamapoulos et al. (14) found no significant differences in circulatory power, ventilatory threshold and VE/VCO2 slope, like the other studies. VO2 peak improved in both groups and the intervention group achieved a significant improvement in exercise time, respiratory exchange ratio and ventilation.
The studies by Kawauchi et al. (15) and Sadek et al. (16) did not perform CPET.
6 minute walk test
In the study by Kawauchi et al. (15), the distance in the 6MWT improved significantly in the low- and moderate-intensity intervention group when compared to the control group. The ARIS group in the most recent study by Lautaris et al. (17) showed significant results for the distance covered in the 6MWT.
Sadek et al. (16) also observed a significant improvement in the combined group (HIIT + TMI) when compared to the control group. And in their study, Winkelmann et al. (12), unlike the other studies, found that both groups showed improvements in the distance covered in the 6MWT.
NYHA rating and quality of life
The study by Winkelmann et al. (12) saw similar improvements in both groups. The study by Lautaris et al. (13) showed that QoL and NYHA functional class improved only in the intervention group, which underwent combined training.
Two studies found that QoL improved more in the combined training group, but NYHA improved in both training groups (14,16). Kawauchi et al (15), in turn, obtained improved QoL in all their groups, but the moderate intensity training group significantly improved functional class and NYHA symptoms.
Finally, the most recent study by Lautaris et al. (17) showed a more significant increase in QoL in the ARIS group. NYHA class tended to improve in the ARIS group when compared to AT alone. The AT + IMT group also achieved significant improvements in QoL and NYHA when compared to AT alone.
Discussion
Inspiratory muscle training has been widely studied as a form of intervention in patients with HF, with the aim of improving their functional capacity and QoL by increasing the strength and endurance of the inspiratory muscles. Most of the studies included in this review showed that patients who underwent IMT associated with conventional training showed a significant increase in inspiratory muscle performance. These results suggest that the use of IMT is beneficial for patients with HF, attenuating the respiratory metaborreflex, improving peripheral blood flow and increasing exercise tolerance (17, 18).
The study by Meyer et al. (7) highlights that inspiratory muscle strength is an important predictor of prognosis in HF and that a reduction in PImax is related to increased mortality in these patients. When analyzing the study by Kawauchi et al. (15), we observed that patients in the control group, who did not use IMT as an intervention, reduced their PImax by 8%. These results raise the hypothesis of whether only patients with reduced PImax should be eligible for IMT or whether all HF patients should use this resource to prevent respiratory function decline and thus reduce mortality. On the other hand, studies such as those by Winkelmann et al.(12) and Sadek et al. (16), recruited patients with PImax lower than predicted for their age, categorizing inspiratory muscle weakness and showed that there was a significant improvement in PImax in the groups that used IMT as an intervention force. This raises the hypothesis that the patients who benefit most from IMT in terms of gaining inspiratory muscle strength are those with inspiratory muscle weakness.
Heart, Vessels and Transplantation 2023; 7: doi: 10.24969/hvt.2023.451
Inspiratory muscle training in HF Pinelo Cavegn, Rodrigues Jr
![]()
However, other studies have also shown that even patients without inspiratory muscle weakness can benefit from IMT. More in-depth research into this issue is therefore needed in order to define more effectively which patients meet the selection criteria for IMT.
Another relevant study for reducing mortality in HF patients is HF-ACTION (18). This study showed that a 6% increase in maximum oxygen consumption (VO2 peak) was associated with a 5% reduction in all-cause mortality and hospitalization rates. Most of the studies selected for this review showed a significant increase in VO2 peak in the combined and conventional training groups. Several studies suggest (19-21) that the most significant improvement in VO2 peak occurred in patients with reduced PImax, which raises the hypothesis that the most effective indication for IMT would be for these patients.
Although there is promising evidence of the benefits of IMT in HF patients, more studies are needed in order to clarify the best way to prescribe it, the ideal duration of treatment and whether all HF patients would benefit from using IMT as a form of intervention.
Study limitations
The heterogeneity of the included studies in terms of methodology, intervention and outcomes made it difficult to directly compare the results. In addition, most of the studies had a small sample size and limited duration, making it difficult to assess significant effects. In addition, the lack of standardization in IMT protocols and assessment measures may also have introduced bias into the results.
In terms of future prospects, it is important to carry out well-designed studies, with larger samples and longer follow-up periods, in order to provide more robust evidence on the benefits of IMT in HF patients. These issues are important for
directing the clinical application of IMT and optimizing outcomes for HF patients.
Conclusion
According to the studies analyzed, we can conclude that the use of IMT associated with other conventional training modalities in HF patients is beneficial for increasing not only inspiratory muscle strength, but also inspiratory muscle endurance. As well as exercise tolerance time, distance covered in the 6MWT, ventilatory efficiency and circulatory power. As for quality of life and NYHA classification, the results are still controversial, but exercise alone showed a
significant improvement in these parameters.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: U.P.C. and L.F.R.Jr equally contributed to the preparation of manuscript
Acknowledgment and funding: None to declare
References
| 1. Comitê Coordenador da Diretriz de Insuficiência Cardíaca. Diretriz Brasileira de Insuficiência Cardíaca Crônica e Aguda. Arq Bras Cardiol 2018; 111: 436-539. | ||||
| 2. Roger V.L. Epidemiology of HF: A Contemporary Perspective. Circ Res 2021; 128: 1421-34. doi: 10.1161/CIRCRESAHA.121.318172 https://doi.org/10.1161/CIRCRESAHA.121.318172 |
||||
| 3. Gosker HR, Wouters EF, van der Vusse GJ, et al. Skeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic HF: underlying mechanisms and therapy perspectives. Am J Clin Nutr 2000;71: 1033-47. https://doi.org/10.1093/ajcn/71.5.1033 PMid:10799364 |
||||
| 4. Drexler H, Riede U, Münzel T, König H, Funke E, Just H. Alterations of skeletal muscle in chronic HF. Circulation 1992; 85: 1751-9. doi: 10.1161/01.CIR.85.5.1751 https://doi.org/10.1161/01.CIR.85.5.1751 PMid:1315220 |
||||
| 5. Loh DR, Tan R-S, Lim WS, Koh AS. Cardio-sarcopenia: A syndrome of concern in aging. Front Med 2022; 9:1027466. doi: 10.3389/fmed.2022.1027466 https://doi.org/10.3389/fmed.2022.1027466 PMid:36388892 PMCid:PMC9640679 |
||||
| 6. Perez-Moreno AC, Jhund PS, Macdonald MR, et al. Fatigue as a predictor of outcome in patients with HF: analysis of CORONA (Controlled Rosuvastatin Multinational Trial in HF). JACC Heart Fail 2014; 2: 187-97. doi: 10.1016/j.jchf.2014.01.001. https://doi.org/10.1016/j.jchf.2014.01.001 PMid:24720928 |
||||
| 7. Meyer FJ, Borst MM, Zugck C, Kirschke A, Schellberg D, Kübler W, et al. Respiratory muscle dysfunction in congestive HF: clinical correlation and prognostic significance. Circulation 2001; 103: 2153-8. https://doi.org/10.1161/01.CIR.103.17.2153 |
||||
| 10.1161/01.CIR.103.17.2153 https://doi.org/10.1161/01.CIR.103.17.2153 PMid:11331255 |
||||
| 8. Mangner N, Weikert B, Bowen TS, Sandri M, Höllriegel R, Erbs S, et al. Skeletal muscle alterations in chronic HF: differential effects on quadriceps and diaphragm. J Cachexia Sarcopenia Muscle 2015; 6: 381-90. doi: 10.1002/jcsm.12034 https://doi.org/10.1002/jcsm.12034 PMid:26674018 |
||||
| 9. Dall'Ago P, Chiappa GR, Guths H, Stein R, Ribeiro JP. Inspiratory muscle training in patients with HF and inspiratory muscle weakness: a randomized trial. J Am Coll Cardiol 2006; 47: 757-63. 10.1016/j.jacc.2005.09.052. https://doi.org/10.1016/j.jacc.2005.09.052 PMid:16487841 |
||||
| 10. Romer LM, Polkey MI. Exercise-induced respiratory muscle fatigue: Implications for performance. J Appl Physiol 2008; 104: 879-88. doi: 10.1152/japplphysiol.01157.2007 https://doi.org/10.1152/japplphysiol.01157.2007 PMid:18096752 |
||||
| 11. Moreno AM, Toledo-Arruda AC, Lima JS, Duarte CS, Villacorta H, Nóbrega ACL. Inspiratory muscle training improves intercostal and forearm muscle oxygenation in patients with chronic hf: evidence of the origin of the respiratory metaboreflex. J Card Fail 2017; 23: 672-9. https://doi.org/10.1016/j.cardfail.2017.05.003 PMid:28499979 |
||||
| 12. Winkelmann ER, Chiappa GR, Lima COC, Viecili PRN, Stein R, Ribeiro JP. Addition of inspiratory muscle training to aerobic training improves cardiorespiratory responses to exercise in patients with HF and inspiratory muscle weakness. | ||||
| Am Heart J 2009; 158: 768.e1-7. 10.1016/j.ahj.2009.09.005. https://doi.org/10.1016/j.ahj.2009.09.005 PMid:19853695 |
||||
| 13. Laoutaris ID, Adamopoulos S, Manginas A, Panagiotakos DB, Kallistratos MS, Doulaptsis C, et al. Benefits of combined aerobic/resistance/inspiratory training in patients with chronic HF. A complete exercise model? A prospective | ||||
| randomised study. Int J Cardiol 2013; 167: 1967-72. https://doi.org/10.1016/j.ijcard.2012.05.019 |
||||
| 14. Adamopoulos S, Schmid JP, Dendale P, Poerschke D, Hansen D, Dritsas,A, et al. Combined aerobic/inspiratory muscle training vs. aerobic training in patients with chronic heart failure: The Vent-HeFT trial: A European prospective multicentre randomized trial. Eur J Heart Fail 2014; 16: 574-82. https://doi.org/10.1002/ejhf.70 PMid:24634346 |
||||
| 15. Kawauchi TS. Umeda IIK, Braga LM, Mansur AP, Rossi-Neto JM, Guerra de Moraes Rego Sousa A, et al. Is there any benefit using low-intensity inspiratory and peripheral muscle training in HF? A randomized clinical trial. Clin Res Cardiol 2017; 106: 676-85. https://doi.org/10.1007/s00392-017-1089-y PMid:28255812 |
||||
| 16. Sadek Z, Salami A, Youness M, Awada C, Hamade M, Joumaa WH, et al. A randomized controlled trial of high-intensity interval training and inspiratory muscle training for chronic HF patients with inspiratory muscle weakness. Chronic Illn 2020: 1742395320920700. https://doi.org/10.1177/1742395320920700 PMid:32370544 |
||||
| 17. Laoutaris ID, Piotrowicz E, Kallistratos MS, et al. Combined aerobic/resistance/inspiratory muscle training as the 'optimum' exercise programme for patients with chronic HF: ARISTOS-HF randomized clinical trial. Eur J Prev Cardiol 2021; 28: 1626-35. https://doi.org/10.1093/eurjpc/zwaa091 PMid:33624071 |
||||
| 18. Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ et al. HF-ACTION Investigators. Effects of exercise training on health status in patients with chronic HF: HF-ACTION randomized controlled trial. JAMA 2009; 301: 1451-9. https://doi.org/10.1001/jama.2009.457 PMCid:PMC2690699 |
||||
| 19. Cohen-Solal A, Tabet JY, Logeart D, Bourgoin P, Tokmakova M, Dahan M. A non-invasively determined surrogate of cardiac power ('circulatory power') at peak exercise is a powerful prognostic factor in chronic HF. Eur Heart J 2002; 23: 806-14. https://doi.org/10.1053/euhj.2001.2966 PMid:12009721 |
||||
| 20. Robbins M, Francis G, Pashkow F et al. Ventilatory and heart rate responses to exercise. Better predictors of HF mortality than peak oxygen consumption. Circulation 1999; 100: 2411-7. https://doi.org/10.1161/01.CIR.100.24.2411 PMid:10595953 |
||||
| 21. Chua TP, Ponikowski P, Harrington D et al. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic HF. J Am Coll Cardiol 1997; 29: 1585-90. https://doi.org/10.1016/S0735-1097(97)00078-8 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

