The role of revascularization in chronic coronary disease: a reappraisal stemming from the most recent AHA/ACC/ACCP/ASPC/NLA/PCNA guidelines
EDITORIALS
The role of revascularization in chronic coronary disease: a reappraisal stemming from the most recent AHA/ACC/ACCP/ASPC/NLA/PCNA guidelines
Article Summary
- DOI: 10.24969/hvt.2024.453
- CARDIOVASCULAR DISEASES
- Published: 06/01/2024
- Received: 15/12/2023
- Accepted: 16/12/2023
- Views: 5910
- Downloads: 3900
- Keywords: chronic coronary heart disease, percutaneous coronary intervention, coronary bypass surgery, coronary revascularization, guidelines
Address for Correspondence: Marco Borghi, Department of Clinical and Experimental Medicine, Policlinic "G. Martino," University of Messina, Messina, Italy
E-mail: marco.borgi9@gmail.com
Marco Borgi1, Giuseppe Biondi Zoccai2,3 Francesco Versaci4
1 Department of Clinical and Experimental Medicine, Policlinic "G. Martino," University of Messina, Messina, Italy
2 Department of Medical-Surgical Sciences and Biotechnologies, Sapienza University of Rome, Latina
3 Mediterranea Cardiocentro, Napoli, Italy
4 UOC UTIC Emodinamica e Cardiologia, Ospedale Santa Maria Goretti, Latina, Italy
Abstract
In the recently published clinical practice guidelines by the American Heart Association/American College of Cardiology, a central part has been dedicated to the revascularization, as new evidence came up increasing our knowledge on this relevant topic. Providing symptom relief, preventing major adverse cardiovascular events and improving long-term survival is ultimately the aim of coronary revascularization. Such ambitious goals should be actively pursued, as suggested thoroughly in the text, sharing the clinical decision making with the patient.
Aim of this commentary is to highlight critical messages from the recent guideline, especially in complex clinical scenarios such as angiographically intermediate coronary artery stenoses, significant left main disease and multivessel disease with severe left ventricular dysfunction.
Key words: chronic coronary heart disease, percutaneous coronary intervention, coronary bypass surgery, coronary revascularization, guidelines
The 2023 AHA/ACC multi-society Guidelines (1) provide a valuable state-of-the-art tool for the clinician, helpful in everyday clinical practice. The first relevant change is evident at first glance, as American guidelines now embrace the term “Chronic coronary disease” (CCD) instead of the previous “stable ischemic heart disease”, in this sense approaching the European guidelines (2) which already define it as “chronic coronary syndrome”. Under this term, a very large and heterogeneous population is encompassed, including symptomatic and asymptomatic patients either with obstructive or non-obstructive coronary artery disease, who eventually suffered from myocardial infarction (MI), or with heart failure (HF). Such a heterogeneous, difficult-to-treat population deserves personalized approach, tailored upon peculiar presentation and comorbidities, to deal with multifaceted complexity of individual patients.
Enormous progresses in clinical research brought medical therapy to the forefront in patients with CCD, representing an essential tool in the patient management, with excellent clinical results in recent trials. In patients with CCD, treatment is aimed at symptom relief, prevention of nonfatal events and improving long-term survival.
In a substantial proportion of patients, such aims can be safely achieved with optimal medical therapy that perhaps often represents the first therapeutical step (3, 4). Revascularization, on the other side, still remains the cornerstone of treatment in a substantial proportion of patients, and its indication represents a challenge for the clinician, who should decide what approach to favor in each patient.
Since previous guidelines published in 2012 (5), important trials evaluated revascularization in different settings, adding to pre-existing evidence, shaping current approach in CCD (Fig. 1). It is relevant to evaluate on a case-by-case basis, establishing for each case the aim of revascularization, and deciding consequently the approach. As for symptoms management, revascularization is warranted (COR 1, LOE A) in patients with limiting angina despite optimal medical therapy, as it is proved to be superior to medical therapy when significant stenosis amenable to revascularization are present (6).
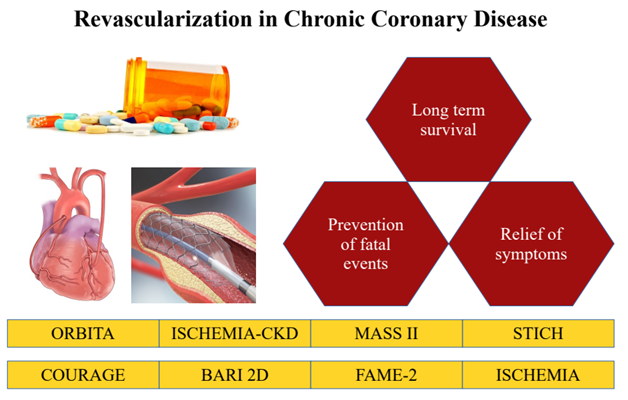
Figure 1. Role of revascularization in chronic coronary disease (in red), as informed by several landmark clinical trials (in yellow)
In this setting, in patients with angiographically intermediate stenosis, use of physiologic guidance is strongly suggested (COR 1 LOE A) either with fractional flow reserve (FFR) or with non-hyperemic pressure ratios (e.g. iFR) when no previous evaluation for ischemia is present, on the basis of the results, among others, of the FAME-2 (Fractional Flow Reserve Guided Percutaneous Coronary Intervention Plus Optimal Medical Treatment [OMT] Versus OMT) trial (7).
Notably, American guidelines shed light on the economic aspects of such approach, showing that a physiology-driven revascularization represents a high economic value intervention. In addition to invasive tools such as hyperemic and non-hyperemic indices, attention should be tributed to validated non-invasive angiography-based indexes, among which quantitative flow ratio (QFR), vessel FFR (vFFR) and FFR-angio, that could help in correct planning of coronary percutaneous intervention (8-10).
A large section is dedicated to evaluating benefit of revascularization in different categories of patients, as several studies brilliantly showed that mortality benefit conferred by revascularization is not homogenously distributed, being notably higher in patients with left main disease or ventricular dysfunction. Perhaps, in patients with significant left main disease or multivessel disease and severe left ventricular dysfunction, defined as left ventricular ejection fraction (LVEF) < 35%, revascularization is indicated to improve survival (COR 1 LOE B-R); in patients with preserved LVEF with multivessel disease both percutaneous coronary intervention (PCI) and coronary bypass surgery (CABG) are appropriate; the aim of revascularization, other than improving symptoms, is to lower the risk of cardiovascular events such as spontaneous MI, unplanned urgent revascularization and cardiac death (11).
The decision between PCI and CABG certainly represents a difficult choice in specific patient population, such as left main disease, diabetic patients and three-vessel disease. Most of the current evidence comes from old studies, not taking into account the enormous progresses both in medical therapy and in PCIs, with new generation of drug-eluting stents and refined plaque modification techniques, useful in cases of high technical complexity, such as calcific stenosis. A solid indication to CABG still remains in patients with significant left main involvement and high-complexity coronary artery disease (CAD) (COR 1 LOE B-R), as well as in patients with diabetes and multivessel CAD with involvement of LAD who are appropriate candidates for CABG (COR 1 LOE A).
The reason for this indication lies in the very different nature of the two interventions, with CABG providing long-term protection against proximal disease progression and plaque rupture, providing alternative route for blood that is unhindered by upstream native CAD and hence providing increased blood flow to jeopardized myocardium distal to the stenosis, a completely different approach from PCI that results in a focal intervention with no effect on eventual upstream or downstream de-novo lesions.
Complexity according to guidelines should be evaluated using Syntax score (12-13), with 33 representing the established cut-off to estimate CAD complexity: it is reasonable to offer CABG if Syntax score > 33 in patients with CCD (COR 2a, LOE B-R), whereas for lower complexity CAD with Syntax score < 33, PCI may be considered as an alternative to CABG to reduce MACE (COR 2b LOE B-R).
Such angiographical considerations should be always balanced with consideration regarding clinical status, as in patients with CCD who are poor candidates for surgery, it is reasonable to choose PCI over CABG to improve symptoms and reduce major adverse cardiac events (MACE) (COR 2a, LOE B-NR). In this setting, risk scores are useful tools to objectively assess clinical status, providing a rough estimate of surgical risk, useful for clinicians, patients and the patient’s family, hence helping in taking informed decisions regarding treatment (13). STS score has been derived from data on patients undergoing CABG in United States and is periodically updated, predicting risk of adverse outcomes in patients undergoing CABG such as death, renal failure, stroke, prolonged ventilation, deep sternal wound infection, reoperation and prolonged length of stay. EuroScore II (14) is another valuable option, taking into account similar risk factors, with predicted high surgical risk with values higher than six points. Both risk scores do not take into account all possible risk factors, and perhaps should be integrated by considering overall patient frailty, MELD score for assessing cirrhosis, and malnutrition, best assessed with Malnutrition Universal Screening Tool (MUST). Shared decision making in this context is of utmost importance, and hence extensively discussed in the document, as it contributes to better patients’ understanding of treatment options, with realistic awareness of potential clinical benefits and harms. In the context of difficult decisions regarding best treatment strategy, a Heart Team approach including representatives from interventional cardiology, cardiac surgery and clinical cardiology is recommended to improve patient outcomes (COR 1, LOE B-NR). Enormous technical progress significantly improved outcomes, pushing forward the boundaries in patients once deemed unsuitable for revascularization; nonetheless, careful decision-making regarding planning and choice of the revascularization strategy still remains the most important step in the management of chronic coronary disease patients, and the present Guidelines certainly represent a valuable tool in helping physicians’ decisions.
Peer-review: Internal
Conflict of interest: None to declare
Authorship: M.B., G.B. Z. and F.V. equally contributed to the study and fulfilled authorship criteria
Acknowledgement and Funding: None to declare
References
| 1.Virani SS, Newby KL, Arnold SV, Bittner V, Brewer LPC, Demeter SH, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines Circulation 2023; 148: e9-e119. https://doi.org/10.1161/CIR.0000000000001183 |
||||
| 2.Knuuti J, Wijns W, Saraste A, Cappodano D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC), Eur Heart J 2020; 41: 407-77. https://doi.org/10.15829/1560-4071-2020-2-3757 |
||||
| 3.Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O'Brien SM, Boden WE, et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease N Engl J Med 2020; 382: 1395-407 | ||||
| 4.Pham V, Moroni A, Gall E, Benedetti A, Zvelonghi C, Picard F. Revascularization and medical therapy for chronic coronary syndromes: lessons learnt from recent trials, a literature review. J Clin Med 2023; 12: 2833. https://doi.org/10.3390/jcm12082833 PMid:37109169 PMCid:PMC10141707 |
||||
| 5.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012; 126: e354-e471. https://doi.org/10.1161/CIR.0b013e318277d6a0 |
||||
| 6.Rajkumar CA, Foley M, Ahmed-Jushuf F, Nowbar AN, Simader FA, Davies JR, et al. A placebo-controlled trial of percutaneous coronary intervention for stable angina. N Engl J Med 2023; 389: 2319-30. Doi: 10.1056/NEJMoa2310610 https://doi.org/10.1056/NEJMoa2310610 PMid:38015442 PMCid:PMC7615400 |
||||
| 7.De Bruyne B, Pijls NHJ, Kalesan B, Barbato E, Tonino PAL, Piroth Z, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease trial. N Engl J Med 2012; 367:991-1001. DOI: 10.1056/NEJMoa1205361 https://doi.org/10.1056/NEJMoa1205361 PMid:22924638 |
||||
| 8.Biscaglia S, Verardi FM, Tebaldi M, Guiducci V, Caglione S, Campana R, et al. QFR-Based virtual PCI or conventional angiography to guide PCI: The AQVA Trial. JACC Card Interv 2023; 16: 783-94. https://doi.org/10.1016/j.jcin.2022.10.054 PMid:36898939 |
||||
| 9.Xu B, Shengxian T, Song L, Jin Z, Yu B, Fu G, et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): a multicentre, randomised, sham-controlled trial. Lancet 2021; 398: 2149-59. Doi:10.1016/S0140-6736(21)02248-09 https://doi.org/10.1016/S0140-6736(21)02248-0 PMid:34742368 |
||||
| 10.Escaned J, Berry C, De Bruyne B, Shabbir A, Collet C, Lee JM, et al. Applied coronary physiology for planning and guidance of percutaneous coronary interventions. A clinical consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the European Society of Cardiology. EuroIntervention 2023; 19: 464-81. DOI: 10.4244/EIJ-D-23-00194 https://doi.org/10.4244/EIJ-D-23-00194 PMid:37171503 |
||||
| 11.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, et al. Coronary-Artery Bypass Surgery in Patients with Left Ventricular Dysfunction. N Engl J Med 2011; 364: 1607-16. https://doi.org/10.1056/NEJMoa1100356 PMid:21463150 PMCid:PMC3415273 |
||||
| 12.Serruys PW, Morice MC, Kappetein AP, Colombo A, Homes DR, Mack MJ, et al. Percutaneous Coronary Intervention versus Coronary-Artery Bypass Grafting for Severe Coronary Artery Disease. N Engl J Med 2009; 360: 961-72. https://doi.org/10.1056/NEJMoa0804626 PMid:19228612 |
||||
| 13.Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American Col- lege of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022; 145: e18-e114. https://doi.org/10.1161/CIR.0000000000001060 |
||||
| 14.Nashef SAM, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, et al. . EuroSCORE II. Eur J Cardiothorac Surg 2012; 41: 734-44. doi: 10.1093/ejcts/ezs043 https://doi.org/10.1093/ejcts/ezs043 PMid:22378855 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

