Hospital predictors of deep vein thrombosis after ischemic stroke: A systematic review
SYSTEMATIC REVIEW
Hospital predictors of deep vein thrombosis after ischemic stroke: A systematic review
Article Summary
- DOI: 10.24969/hvt.2024.459
- Page(s): 284-292
- CARDIOVASCULAR DISEASES
- Published: 06/02/2024
- Received: 23/09/2023
- Revised: 22/01/2024
- Accepted: 24/01/2024
- Views: 8183
- Downloads: 3512
- Keywords: Acute stroke. ischemic stroke. deep venous thrombosis. predictors. systematic review
Address for Correspondence: Elmira Mamytova, Department of Neurology and Clinical Genetics named after A.M. Murzaliev of the Kyrgyz State Medical Academy named after I.K. Akhunbayev. Bishkek. Kyrgyzstan
Email: elmira.mamytova@yahoo.com
ORCID: ORCID: Torokulova K.T.- 0009-0002-7126-3476 Bijalieva G.S -0009-0005-3407-4305 Akulich E.N. - 0009-0007-4268-7591 Mamytova E.M.- 0000-0002-4322-5555Mamytova A.J.- 0009-0007-1637-6984 Akhmadeeva L.R.-0000-0002-1177-6424
Karlygach T. Torokulova1, Gulmira S. Biyalieva2, Elena N. Akulich 2, Elmira A. Mamytova1, Aina J. Mamytova3, Leila R. Akhmadeeva4
1Department of Neurology and Clinical Genetics named after A.M. Murzaliev of the Kyrgyz State Medical Academy named after I.K. Akhunbayev. Bishkek. Kyrgyzstan.
2International Higher School of Medicine "Vedanta". Bishkek. Kyrgyzstan
3Department of Internal Diseases of the Kyrgyz State Medical Institute of Retraining and Advanced Training named after S.B. Daniyarov. Bishkek. Kyrgyzstan
4Department of Neurology of the Bashkir State Medical University of the Ministry of Health of the Russian Federation. Ufa. Russian Federation
Abstract
Objective: In the acute stage of ischemic stroke, deep vein thrombosis (DVT) is more common than in the recovery stage. The stratification of the risk of developing DVT is crucial when making a decision in terms of correct diagnostic and treatment options in stroke patients.
The aim of the study was to review systematically up-to-date published studies on predictors for DVT in patients with acute ischemic stroke.
Methods: Study design - systematic review of observational studies. Russian scientific citation database of RSCI, English scientific citation database of PubMed, Web of Science, Embase for the period of time 2018-August 2023 were used for studies search. Data from selected studies were extracted, including study design, data source, outcome definition, sample size, and predictors. The most common demographic, clinical and/or laboratory markers for predicting stroke-related DVT were presented.
Results: Totally 234 studies were reviewed, and after the selection process, nine studies were included in this article. The incidence of DVT in patients with acute ischemic stroke ranged from 0.53 % to 19.8 %. The most frequently pointed predictors were D-dimer level, age, sex, lower limb plegia, and high NIHHS scores.
Conclusion: Identification of individuals with high risk of venous limb thrombosis after ischemic stroke is crucial for targeted thromboprophylaxis. Advanced knowledge of predictors and biomarkers is needed to guide clinical decision-making and develop risk prediction models.
Key words: Acute stroke. ischemic stroke. deep venous thrombosis. predictors. systematic review
Introduction
Cerebrovascular diseases, the most common of which is stroke, primarily affect middle-aged and older people and cause significant harm to both their physical and mental state (1).
These diseases carry a significant financial burden and cause significant suffering both for the patients themselves and for their families, as well as have significant socio-economic consequences (2).
Stroke is the third leading cause of death worldwide. The risk of death and disability due to cerebrovascular diseases is constantly increasing due to the fact that approximately two-thirds of survivors have varying degrees of motor deficit and other conditions that lead to delayed recovery (3).
Stroke survivors often need early rehabilitation measures after their condition is stabilized, since rehabilitation measures are the main mechanism by which functional recovery and independence in everyday life are achieved. But deep vein thrombosis (DVT) is one of the most common and a fatal complication in patients in the post-stroke period and one of the leading causes hindering the process of early rehabilitation (4).
Since the presence of DVT can lead to a delay in rehabilitation measures, the risk of developing DVT in stroke patients should be assessed at an early stage in order to actively intervene to prevent its development.
Therefore, the stratification of the risk of developing DVT is crucial when making a decision in terms of correct diagnostic and treatment options in stroke patients.
The purpose of this systematic review article was to analyze and present up-to-date data on the most common predictors of DVT and the mechanisms of their development in stroke patients in the early post-stroke period.
Methods
Study design – systematic review.
Search strategy
Russian scientific citation database of RSCI, PubMed, Web of Science, EMBASE for the period of time 2018-August 2023 were used for search of evidence. We used the following keywords: “Deep vein thrombosis”, “Stroke”, “Cerebral stroke”, “Acute stroke”, “Ischemic stroke”, “prognosis”, “Risk factor”, “Predictor”, “outcomes” and “Risk Score.” Data from selected studies were extracted. including study design, data source, outcome definition, sample size, predictors. The most common demographic, clinical and/or laboratory markers for predicting stroke-related DVT were presented.
For the systematic review, we utilized the PRIZMA system (Preferred Reporting Items for Systematic review and Meta-Analysis Protocols). This system helps frame the review's objective, search strategy, and study inclusion and exclusion criteria. The key items of our systematic review are described below:
• P (Population): Patients with acute ischemic stroke in early stage.
• I (Intervention model): Predictors for DVT in acute ischemic stroke patients that were developed and published (predictors ≥ 2).
• C (Comparator): No competing group.
• O (Outcome): The outcome is connected with DVT rather than its subgroups.
• S (Setting): To characterize the prediction of DVT in patients with acute ischemic stroke, facilitating the implementation of preventive measures to prevent adverse events.
Inclusion and exclusion criteria
The inclusion criteria were: (1) studies involving patients with acute ischemic stroke; (2) prospective single- and multicenter cohort studies; (3) retrospective cross-over studies; (4) reported predictors of DVT; (5) the outcome of interest was DVT; (6) full text articles; (7) studies published within period of 2019-2023.
The exclusion criteria were: (1) studies published out of period from 2019-2023; (2) not written in English or Russian; (3) not full -text articles.
Study selection
Preliminary, duplicate studies were excluded, and then the rest studies were reviewed based on their titles and abstracts to determine their eligibility. The inclusion and exclusion criteria were assessed, articles full-texts were looked through and the reference lists of all eligible studies were examined to identify any potentially relevant studies.
Data collection
The collected information from the selected studies was categorized into two groups: (1) Basic information: included details such as the authors, years of publication, research design, participants, scientific data source, and sample size. (2) Predictors information: encompassed information about predictors were presented.
Quality assessment
To recognize the quality and potential bias risk of the included studies, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) assessment tools were employed (5). The GRADE approach provides guidance for rating quality of evidence and based on six factors: study design, risk of bias, inconsistency, indirectness, imprecision, and other considerations (i.e., publication bias). This assessment tools were designed for evaluating the quality of evidence in systematic reviews.
Data synthesis
We pre-specified that only a qualitative synthesis would be carried out in case of insufficient data on each predictor and/or substantial clinical and methodological heterogeneity across the included studies. For a qualitative synthesis, characteristics, results, and risk of bias of the included studies were presented in tabular and narrative formats.
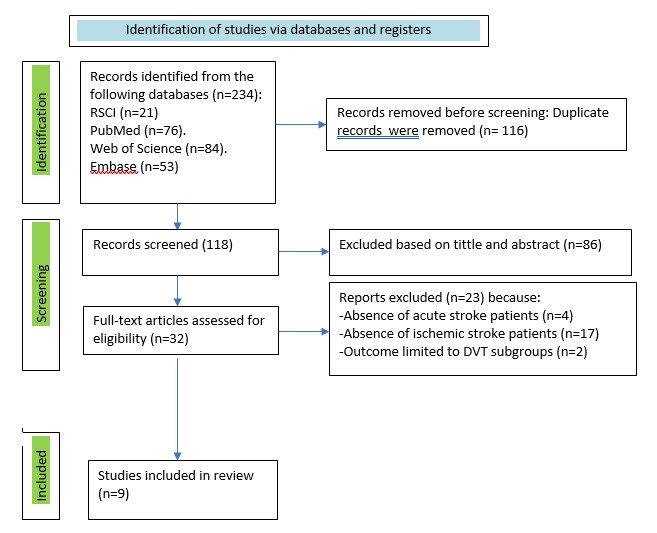
Figure 1. Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flowchart of literature search and selection in our study
Results
Study selection
Figure 1 demonstrates the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 flowchart results of search process.
In the beginning of search 234 indexed records were yielded totally. Overall, 116 duplicate records were identified and removed across all databases, 118 titles and abstracts were screened for eligibility. The next was reviewing for further evaluation of 32 articles. After the subsequent evaluation, 4 studies were excluded as they did not focus on acute stroke. Additionally, 17 studies were found to be inconsistent with ischemic stroke population of the review, 2 studies had outcomes limited to subgroups. Ultimately, 9 studies were included in this review.
Study characteristics
Tables 1 - 4 summarize characteristics of the 9 included studies such as the authors, year, type of study, predictors, and frequency of DVT. All articles were published between 2019 and 2023. Of the included studies, 2 were prospective (single-centered study) and 7 were retrospective. Regarding the study subjects, all patients were with acute ischemic stroke. The sample sizes ranged from 239 to 146,062 participants across the studies.
Totally, 23 predictors of DVT were presented by the authors of the studies, which were grouped into 4 groups:
1) Demographic indicators (Table 1);
2) Anamnesis data or concomitant diseases (Table 2);
3)Data on the severity of the condition or stroke (Table 3);
4) Laboratory markers (Table 4).
|
Table 1. General characteristics of DVT predictors based on demographic data in patients with ischemic stroke |
|||
|
Authors /Year |
Study design |
Demographic predictor |
DVT case |
|
Li et al. (2023)7 |
Retrospective |
Age
|
13.2% |
|
Cheng et al. (2021)14 |
Retrospective |
21.9% |
|
|
Xi et al. (2021)9 |
prospective. single-center cohort |
14.4% |
|
|
Mori el al. (2021)11 |
retrospective cross-over study |
17.6% |
|
|
Ha et al. (2020)8 |
prospective. stroke registry |
Sex (Women) |
13.1%
|
|
Cheng et al. (2021)14 |
Retrospective |
21.9% |
|
|
Liu et al. (2021)25 |
Retrospective |
15.8% |
|
|
DVT- deep venous thrombosis |
|||
|
Table 2. General characteristics of DVT predictors based on data from patient history and/or concomitant conditions in patients with ischemic stroke |
|||
|
Authors /Year |
Study design |
Demographic predictor |
DVT case |
|
Huang et al. (2021)12 |
Retrospective
|
Infections (pneumonia) |
19.8% |
|
Cheng et al. (2021)14 |
Retrospective |
21.9% |
|
|
Wang et al. (2023)13 |
Retrospective
|
Atrial fibrillation |
N/A |
|
Cheng et al. (2021)13 |
Retrospective |
21.9% |
|
|
Xi et al. (2021)9 |
prospective. single-center cohort |
Concomitant malignant neoplasms |
14.4% |
|
Cheng et al. (2021)14 |
Retrospective |
21.9% |
|
|
DVT- deep venous thrombosis |
|||
|
Table 3. General characteristics of DVT predictors based on data on the severity of the condition or stroke (scales) in patients with ischemic stroke |
|||
|
Authors /Year |
Study design |
Demographic predictor |
DVT case |
|
Zhu et al. (2020)10 |
retrospective |
High score on the Caprini scale |
0.53% |
|
Zhu et al. (2020)10 |
retrospective |
High score on the NEWS scale |
0.53% |
|
Liu et al. (2021)25 |
retrospective |
Long hospital stay |
15.8% |
|
Huang el al. (2021)12 |
retrospective
|
Increased muscle tone |
19.8% |
|
Li et al. (2023)7 |
retrospective |
Low score on the Berg scale |
13.2% |
|
Mori el al. (2021)11 |
retrospective cross-over study |
Low score on the Rankin (or Bartel) scale |
17.6% |
|
Xi et al. (2021)9 |
prospective. single-center cohort |
Lower limb plegia |
14.4% |
|
Liu et al. (2021)25 |
retrospective |
15.8% |
|
|
Cheng et al. (2021)14 |
retrospective |
21.9% |
|
|
Ha et al. (2020)8 |
prospective. single-center cohort |
Cardioembolic stroke
|
13.1% |
|
Ha et al. (2020)8 |
prospective. single-center cohort |
High scores NIHHS |
13.1%
|
|
Mori el al. (2021)11 |
retrospective cross-over study |
17.6% |
|
|
Zhu et al. (2020)10 |
retrospective cross-over study |
0.53% |
|
|
Mori el al. (2021)11 |
retrospective cross-over study |
Low body mass index |
17.6% |
|
DVT- deep venous thrombosis |
|||
|
Table 4. General characteristics of DVT predictors based on laboratory markers in patients with ischemic stroke |
|||
|
Authors /Year |
Study design |
Demographic predictor |
DVT case |
|
Li et al. (2023)7 |
retrospective |
Elevated D-dimer (+ elevated C-reactive protein) |
14.4% |
|
Ha et al. (2020)8 |
prospective. single-center cohort |
13.1% |
|
|
Xi et al. (2021)9 |
prospective. single-center cohort |
14.4% |
|
|
Huang el al. (2021)12 |
retrospective |
19.8% |
|
|
Mori el al. (2021)11 |
retrospective cross-over study |
17.6% |
|
|
Zhu et al. (2020)10 |
retrospective |
0.53% |
|
|
Mori el al. (2021)11 |
retrospective cross-over study |
Elevated or decreased triglyceride levels |
17.6% |
|
Liu et al. (2021)25 |
retrospective |
Deceleration of APTT |
15.8% |
|
Mori el al. (2021)11 |
retrospective cross-over study |
Low hemoglobin level |
17.6% |
|
Wang et al. (2023)13 |
retrospective |
Elevated plasma homocysteine levels |
N/A |
|
Zhu et al. (2020)10 |
retrospective |
Elevated thrombin and prothrombin time |
0.53% |
|
Xi et al. (2021)9 |
prospective. single-center cohort |
Hypoalbuminemia |
14.4% |
|
Mori el al. (2021)11 |
retrospective cross-over study |
17.6% |
|
|
Wang et al. (2023)13 |
retrospective |
Increase of indirect bilirubin in plasma |
N/A |
|
APTT - activated partial thromboplastin time, DVT- deep venous thrombosis |
|||
Predictors of the development of DVT in stroke patients differ among different authors, as well as its frequency of DVT.
The most frequently identified predictors were elevated level of D-dimer (and C-reactive protein), older age (over 65 years), female sex, the presence of plegia in the extremities and a high score on the NIHHS scale, the remaining predictors were less common (16-20).
Several other predictors of DVT development after stroke have also been identified such as atrial fibrillation, initial severity of stroke, subtype of stroke, length of hospital stay, previous venous thrombosis, increased body mass index (BMI), presence of malignant tumors, changes in the levels of some clinical and laboratory variables, such as high scores on the Berg equilibrium scale, activated partial thromboplastin time (APTT) and others (21-24).
Discussion
Stroke is one of the leading causes of disability and mortality worldwide. It accounts for 42% of disability-adjusted life years associated with neurological disorders, it causes long-term disability in almost 50% of survivors. While the incidence of stroke is decreasing in high-income countries, it is increasing significantly in low- and middle-income countries (to which Kyrgyzstan belongs) (6). In Russia, for example according data of Republic of Bashkortostan, since 2016 till 2018 the incidence of strokes has gradually decreased up to 348.2 cases per 100 thousand adults (7).
In the Kyrgyz Republic, the incidence of stroke according to the cerebral stroke registry is 2.6-2.67 cases per 1000 population, mortality – 1.17, hospital mortality – 14.3%, mortality in those who died at home – 51.3% (8).
The incidence of ischemic stroke is 1.87 per 1000 population (men - 1.64. women - 2.06); hemorrhagic stroke - 0.54 per 1000 population (8).
Stroke can be divided into ischemic stroke and hemorrhagic stroke, in which ischemic stroke accounts for a large proportion of cases. Hemorrhagic stroke accounts for 10-15% of stroke cases annually (6).
Stroke survivors often need early rehabilitation measures after their condition is stabilized, since rehabilitation measures are the main mechanism by which functional recovery and independence in everyday life are achieved. But deep vein thrombosis is one of the most common and fatal complications in patients in the post-stroke period and one of the leading causes hindering the process of early rehabilitation (9).
However, the development of DVT can occur as early as the 2nd day, reaching a peak between 2 and 7 days (11).
There is both symptomatic DVT and asymptomatic DVT after stroke, which differ in their prevalence. The incidence of DVT among patients after stroke ranges from 10 to 80%, depending on the diagnostic approach, evaluation time and pharmacological thromboprophylaxis (12).
But there is insufficient data on the prevalence and features of the course of asymptomatic DVT.
Deep vein thrombosis (DVT) is the formation of a blood clot that forms in deep veins, usually in the legs, but can occur in the veins of the hands, as well as in the mesenteric and cerebral veins.
Physical examination most often demonstrates unilateral swelling of the extremities, redness and discomfort over the vein and, possibly, a noticeable "heaviness" where the thrombosis is located. Muscle spasm is another typical manifestation of DVT in the lower limb. DVT should be suspected if the patient has any of the above-mentioned clinical symptoms for a certain period of time. However, since clinical signs and symptoms can be very diverse and unique for each person, the prognosis of DVT based solely on medical representation is unreliable. Thus, visual investigations, such as Doppler ultrasound, can confirm or exclude its presence (13).
Three fundamental factors underlie the development of DVT: damage to the venous wall, the tendency of blood to hypercoagulation and slow blood flow (14). Most of the factors that are more or less related to the development of thromboembolic events revolve around these 3 events.
Its development is caused by abnormal hypercoagulation activity with slowing of blood flow in deep veins and insufficiency of venous valves, which occur in paralyzed limbs and can lead to pulmonary embolism (PE). The prevalence of immobilized patients after stroke ranges from 10% to 75%, depending on the method of diagnosis and evaluation time. Decreased motor activity is an important risk factor for the development of DVT; and therefore, stroke patients are considered to have a high risk of developing DVT (12).
Venous congestion, endothelial damage or inflammation, as a rule, lead to an increased blood clotting state.
Activation of the blood clotting cascade, as well as aggregation of platelets and blood cells occur simultaneously, resulting in the formation of a blood clot. A blood clot can cause complete or partial occlusion of a vein, which leads to venous congestion, lymphedema, and possibly ischemia of surrounding tissues. In some cases, DVT can spread further and lead to pulmonary vein thromboembolism (15).
Let's consider the explanation of the mechanisms of development of only the most common predictors. The description of each of the predictors was not part of the purpose of our review.
For example, the laboratory indicator of blood coagulation activity as a D-dimer is a product of the breakdown of a venous thrombus and has also been identified as an indicator of the prediction of DVT in various conditions. The D-dimer is a byproduct of the degradation of cross-linked fibrin by plasmin. It is mainly produced by secondary fibrinolysis after the formation of a blood clot, and elevated levels of D-dimer reflect the ongoing or potential formation of a blood clot: therefore, the determination of the level of D-dimer is used in the clinical diagnosis of various thromboembolic diseases. The most representative diseases are deep vein thrombosis or pulmonary embolism, and the D-dimer is widely used in combination with clinical scales to exclude these diseases in patients with low clinical probability. D-dimer has also been proposed as a diagnostic tool for various cardiovascular, cerebrovascular and aortic diseases, but has not yet reached such a consensus as venous thromboembolism (25).
The D-dimer has also been used as an indicator in patients with ischemic stroke. Its level is closely related to the size or severity of stroke lesions and short- and long-term prognosis. In addition, the level of D-dimer indicates the etiological mechanism of stroke. D-dimer levels are higher in cardiogenic strokes, which form fibrin-rich clots, than in strokes caused by other mechanisms. In strokes caused by the disease of large arteries, which form a platelet-rich thrombus or occlusion of small vessels based on lipohyalinosis, the level of increase in D-dimer was relatively low. In addition, its very high values have recently been proposed as one of the main etiologies of cryptogenic stroke, especially in embolic strokes in unverified cases of the source of embolism. In these patients, the D-dimer was recognized as the most powerful diagnostic marker, playing an important role as a predictor of early neurological deterioration, stroke recurrence and mortality (26).
Studies show that the sensitivity of the D-dimer test was measured as 85-95% and the specificity was 25-50% to predict DVT at its values of more than 0.5 micrograms/ml. Thus, the level of D-dimer is an important factor in the development of DVT in patients with stroke. Given that D-dimer levels can be easily measured in any facility, D-dimer levels should be regularly checked in all stroke patients (19).
Another important predictor is old age, which was largely associated with DVT (16), in particular, age ≥65 years. Age at the level of ≥65 years should cause clinical suspicion and can be used as an independent factor for predicting the risk of a thromboembolic event. With increasing age, the blood gradually enters a state of hypercoagulation, and the mobility of patients gradually decreases. Hypercoagulation and reduction of age-related motor activity easily contributes to the formation of blood clots, which are confirmed by elderly patients. This is due to the following factors:
(1) elderly adult patients have concomitant osteoporosis, which contributes to difficulty in walking, reduces endurance during exercise and a decrease in muscle mass, which in turn leads to a decrease in the function of muscle contractility. contributing to a slow and stagnant state of blood flow, thereby ultimately the formation of blood clots;
(2) it is known that aging reduces the elasticity and integrity of the intima endothelium. In addition, due to atrophic processes in intima against the background of aging, there is a lack of complete closure of the venous valves, in turn, the coagulation system is activated by damaged endothelial cells, which lead to increased blood viscosity (16).
One more important factor that plays a role in the development of venous blood clots is gender. The risk of developing DVT seems to vary among different sexes, with women being more at risk. It is believed that a woman's life has frequent fluctuations in prothrombotic activity. In youth, pregnancy, menstrual cycles and oral contraceptives are common; after which menopause is inevitable and hormone replacement therapy can be used which can have its potential impact on the development of cardiovascular diseases including DVT.
In addition, studies conducted in the USA demonstrated that middle-aged women are beginning to experience a temporary trend towards an overall increase in the prevalence of cardiovascular diseases, while the increase in the prevalence of these diseases among men is mainly
due to an increase in bad habits such as smoking and diseases such as diabetes and hypertension (23).
Stroke severity is one of reasons for the higher prevalence of DVT. Patients admitted to hospital with higher NIHSS scores (with a median of 14) relatively often had DVT.
It is widely recognized that paralysis in stroke patients promotes the development of DVT. Compared to mild stroke, moderate stroke has 80.8% sensitivity and 61% specificity, while severe stroke has 34.62% sensitivity and 91.00% specificity (18-20).
Patients with higher NIHSS scores and DVT stay longer in hospital and have a higher risk of immobility, as was expected. In terms of stroke features, patients having hemorrhagic stroke are more likely to have a DVT (23). In patients with ischemic stroke, lacuna subtypes were less likely to lead to DVT than subtype is connected with cardioembolism (atrial fibrillation (20).
Neurologists also observed that lower extremity muscle weakness was the strongest independent predictor of DVT (16, 23, 25). The loss of venous pump function caused by muscle weakness may contribute to DVT formation. In clinical practice, those patients who actively move both their paralyzed and non-paralyzed extremities have less risk of thromboembolic events. Additionally, this risk decreases if caregivers to passively move the patient's paralyzed extremities (25).
Another equally important tool for predicting the risk of developing DVT is the Berg balance scale (SBB) that consists of 14 points, each of which has gradations from 0 to 4, which must be summed up to score from 0 to 56 points. Scores of 0-20 indicate a high risk of falls, 21-40 indicate an average risk of falls, and 41-56 indicate a good balance or a low risk of falls. The lower the SBB score, the worse the ability to maintain balance. Patients with stroke and poor balance often have reduced daily activity due to fear of falling while walking or out of bed. Therefore, the level of patient mobility or the degree of its limitation is an established risk factor; its accuracy has been confirmed as an important clinical tool for identifying patients with suspected DVT (27).
Study limitations
Despite the data presented above, there is currently no reliable assessment system for predicting DVT, which is accepted by doctors everywhere in hospitals after ischemic and even more so hemorrhagic stroke.
An effective model of risk stratification for DVT at the hospital level after a stroke would be useful for identifying high-risk patients and for implementing individual preventive strategies. Therefore further population studies are needed, including in Kyrgyzstan.
Conclusion
Identification of individuals at high risk of venous limb thrombosis after ischemic stroke is crucial for targeted thromboprophylaxis. Advanced knowledge of predictors and biomarkers is needed to guide clinical decision-making and develop risk prediction models. The most common demographic, clinical and/or laboratory markers for predicting stroke-related DVT are presented.
Peer-review: External and Internal
Conflict of interest: None to declare
Authorship: K.T.T., G.S. B., E.N. A., E. A. M, J.Z. M., and L.R.A. equally contributed to the study and manuscript preparation and fulfilled authorship criteria
Acknowledgment and funding: None to declare
References
1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation 2021; 143: e254-e743.
2.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018: 49: 158.
3.Duy KN, Nguyen DT, Le MV, Le LD, Tran HC, et al. Enoxaparin in the prevention of deep vein thrombosis in patients with acute ischemic stroke. Trends Med Sci. 2022; 2: e122415.
4.Maufus M, Elias A, Barrellier M-T, Pernod G, Medicine FSV. Diagnosis of deep vein thrombosis recurrence: Ultrasound criteria. Thromb Res 2018; 161: 78–83.
5.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations BMJ 2008; 336: 924-6.
6.Fujita Y, Nakatsuka H, Namba Y, Mitani S, Yoshitake N, Sugimoto E, et al. The incidence of pulmonary embolism and deep vein thrombosis and their predictive risk factors after lower extremity arthroplasty: A retrospective analysis based on diagnosis using multidetector CT. J Anesth 2015; 29: 235–41.
7. Li F, Wei C, Huo S, Liu X, Du J. Predictors of deep-vein thrombosis for acute stroke at admission to a rehabilitation unit: A retrospective study. Front Neurol 2023; 14:1137485.
8.Ha SH, Kim Y-J, Heo SH, Chang D-I, Kim BJ. Prediction of deep vein thrombosis by ultrasonography and D-dimer in Asian patients with ischemic stroke. BMC Neurol 2020; 20:257.
9.Xi P, Zhi W, Qingmei C, Lan X, Qi F. Development and validation of a nomogram for lower extremity deep venous thrombosis in patients after acute stroke. J Stroke Cerebrovasc Dis 2021; 30: 237.
10.Zhu X, Zhang T, Zhou L, Yin X, Dong Q. Stratification of venous thromboembolism risk in stroke patients by Carpini score. Ann Palliat Med 2020; 9: 626-631.
11.Mori T, Yoshioka K, Tanno Y. Frequency of deep vein thrombosis at admission for acute stroke and associated factors: a cross-sectional study. Thrombosis J 2021; 19: 62.
12.Yucai H, Guo C,Song K, Li Ch, Ding N. Association of clinical and laboratory variables with in-hospital incidence of deep vein thrombosis in patients after acute ischemic stroke: A retrospective study. Medicine 2021; 100: e24601.
13.Wang Y, Cao M, Liu X, Sun Y, Wang Y, Jin R. Nomogram prediction for lower extremity deep vein thrombosis in acute ischemic stroke patients receiving thrombolytic therapy. Clin Appl Thromb Hemost 2023; 29: 10760296231171603.
14.Cheng HR, Huang GQ, Wu ZQ, Lin GQ, Song JY, Liu YT, et al. Individualized predictions of early isolated distal deep vein thrombosis in patients with acute ischemic stroke: a retrospective study. BMC Geriatr 2021; 21: 140.
15.Tøndel BG, Morelli VM, Hansen JB, Braekkan SK. Risk factors and predictors for venous thromboembolism in people with ischemic stroke: A systematic review. J Thromb Haemost 2022;,20: 2173-86.
16.Henke PK, Kahn SR, Pannucci CJ, Secemsky EA, Evans NS, Koharana AA, et al. Call to action to prevent venous thrombo- embolism in hospitalized patients: a policy statement from the American Heart Association. Circulation 2020; 141:e 914- e931.
17. Klochichina OA. Analysis of the average long-term morbidity and mortality from stroke in the regions of the Russian Federation included in the federal program of reorganization of care for stroke patients. SS Korsakov Journ of Neurol and Psych 2020;120: 12: 37-41
18. Hsieh MT, Hsieh CY, Tsai TT, Wang YC, Sung SF. Performance of ICD-10-CM diagnosis codes for identifying acute ischemic stroke in a national health insurance claims database. Clin Epidemiol 2020; 12: 1007-13.
19.Samudinova TT, Kulov BB, Turgumbaev DD, Abirova AB. Epidemiology of stroke in the city of Bishkek according to the register (2017-2018). Healthcare of Kyrgyzstan 2021; 3: 90-103.
20.Geraldini F, De Cassai A, Correale C, Andreatta G, Grandis M, Navalesi P, et al. Predictors of deep-vein thrombosis in subarachnoid hemorrhage: A retrospective analysis. Acta Neurochir 2020; 162: 2295–301.
21.Khan MT, Ikram A, Saeed O, Afridi T, Sila CA, Smith MS, et al. Deep vein thrombosis in acute stroke—A systemic review of the literature. Cureus 2017; 9: e1982.
22.Abdelmalik BHA, Leslom MMA, Gameraddin M, Alshammari QT, Hussien R, Alyami MH. Assessment of lower limb deep vein thrombosis: characterization and associated risk factors using triplex Doppler imaging. Vasc Health Risk Manag 2023; 19: 279-87.
23. Wang Y, Shi Y, Dong Y, Dong Q, Ye T, Fang K. Clinical risk factors of asymptomatic deep venous thrombosis in patients with acute stroke. Clin Appl Thrombosis Hemostasis 2019; 25:1076029619868534.
24.Needleman L, Cronan JJ, Lilly MP, Merli GJ, Adhikari S, Hertzberg BS, et al. Ultrasound for lower extremity deep venous thrombosis. Circulation 2018; 137:1505–15.
25. Liu Z, Liu D, Guo ZN, Jin H, Sun T, Ni C. Incidence and risk factors of lower-extremity deep vein thrombosis after thrombolysis among patients with acute ischemic stroke. Pharmgenomics Pers Med 2021; 14:1107-14.
26.Chi G. Lee JJ. Montazerin SM. Marszalek J. Association of D-dimer with short-term risk of venous thromboembolism in acutely ill medical patients: A systematic review and meta-analysis. Vas Med 2022; 27: 478–86.
27.Ha SH, Kim Y-J, Heo SH, Chang D-I, Kim BJ. Prediction of deep vein thrombosis by ultrasonography and D-dimer in Asian patients with ischemic stroke. BMC Neurol 2020; 20: 257.
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

