Echocardiographic evaluation of heart valve prosthetic dysfunction
REVIEW
Echocardiographic evaluation of heart valve prosthetic dysfunction
Article Summary
- DOI: 10.24969/hvt.2017.46
- Page(s): 10-16
- CARDIOVASCULAR DISEASES
- Published: 13/03/2018
- Received: 26/02/2018
- Accepted: 12/03/2018
- Views: 22869
- Downloads: 10094
- Keywords: Prosthetic heart valves, transthoracic echocardiography, transesophageal echocardiography, prosthetic valves dysfunction, Doppler ultrasound
Address for Correspondence: Yuriy Ivaniv,
7/1 Karmaliuk str., Lviv, 79010, Ukraine
E-mail: yivaniv@mail.lviv.ua
Authors
Diagnostic Radiology Department, Danylo Halycky National Medical University, Lviv, Ukraine
Abstract
Patients with replaced heart valve submitted to echocardiographic examination may have symptoms related either to valvular malfunction or ventricular dysfunction from different causes. Clinical examination is not reliable in a prosthetic valve evaluation and the main information regarding its function could be obtained using different cardiac ultrasound modalities. This review provides a description of echocardiographic and Doppler techniques useful in evaluation of prosthetic heart valves. For the interpretation of echocardiography there is a need in special knowledge of prosthesis types and possible reasons of prosthetic function deterioration. Echocardiography allows to reveal valve thrombosis, pannus formation, vegetation and such complications of infective endocarditis as valve ring abscess or dehiscence. Transthoracic echocardiography requires different section plane angles and unconventional views. Transesophageal echocardiography is more often used than in native valve examination due to better visualization of prosthetic valve structure and function. Three-dimensional echocardiography could provide more detailed visual information especially in the assessment of paravalvular regurgitation or valve obstruction.
The evaluation of the cardiac prosthetic valve is a challenge for the echocardiographic examination. This is partly due to the large variety of prostheses. Since 1950 in the world a big variety of artificial heart valve models have been introduced. In addition, the condition of the patient with replaced valve could be worsening not only because of prosthetic valve dysfunction but also due to other reasons including progressive left or right ventricular failure, arrhythmia, pulmonary hypertension etc. Any of the four native valves can be replaced, moreover the patient may have two or even three valves replaced (1).
There are three main types of mechanical valves, which differ primarily by the construction and functioning of occluders: tilting disk, bileaflet, and ball-and-cage. Monoleaflet tilting disk valves consist of a circular plain occluder which usually opens to the angle 60-80°. Bileaflet valves have occluders presenting by two semilunar disks attached to a rigid ring by tiny hinges (Fig. 1). The opening angle of the discs relative to the ring plane achieves 75-90°, and the orifice of this valve has three parts: the smallest one at the center between the two opened cusps and two bigger semicircular orifices laterally. Caged-ball valves consist of a silastic ball with a circular metal ring and a cage formed by two or three metal arches (2).
Three different categories of biologic artificial valves are used in cardiac surgery practice: stented, unstented and homograft. These prostheses are produced from biologic tissues and they are less thrombogenic than mechanical valves (3). Valve bioprosthesis consists of flexible leaflets, after their opening a single orifice is formed with a larger area than mechanical valves could create. Stented bioprosthesis consists of three cusps produced from the porcine aortic valve or bovine pericardium, mounted on a metal or polymeric ring. Unstented bioprosthesis are also manufactured from porcine or bovine tissues but do not have rigid stents. Homografts are prepared from cryopreserved human valves.
During the last decade the transcatheter valves are more widely used. They are essentially bioprosthetic valves of special construction allowing implantation in aortic and pulmonary positions using a percutaneous transfemoral or transapical approach (4, 5).
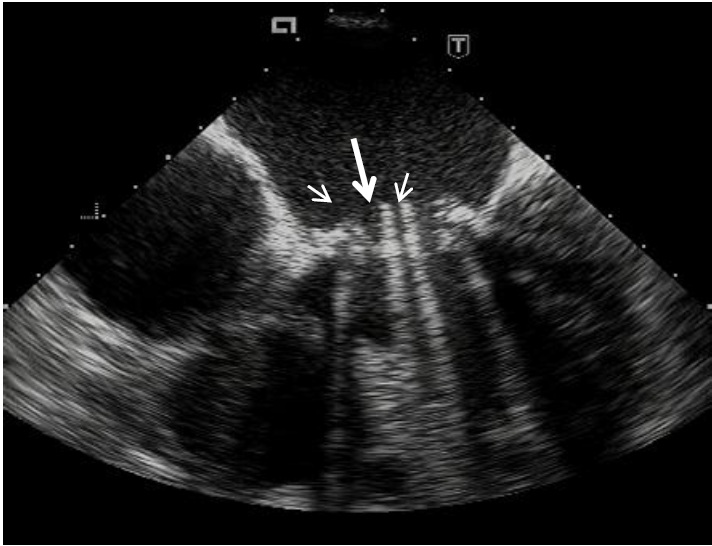
Fig. 1. Transesophageal visualization of prosthetic bileaflet valve opening. Clearly seeing two disks in opened position as a parallel lines with intensive shadowing down. It is also visible three parts of prosthetic orifice: the smallest one at the center between the two opened cusps ( long arrow) and two bigger orifices laterally (short arrows).
Valve prostheses may have different sizes, which affects the echocardiographic parameters characterizing the functioning of the valve. The size of the prosthesis is determined by the outer diameter of its ring and is indicated in millimeters. Therefore, this diameter is not the diameter of the opening area of an artificial valve, which is usually smaller.
Dysfunction of the valve prosthesis is a specific pathology that requires a special knowledge regarding the functioning of prosthetic valves, methods of their examination and the causes of prosthetic function disorders (6).
Thrombosis is the main cause of prosthetic heart valve dysfunction. According to various data it occurs in 0.1 – 5.7% of patients per year. In addition, disorders of the prosthetic functioning may occur due to the vegetation growth, the development of ring abscess or pannus formation (7). A reverse flow (regurgitation) through the prosthetic orifice or outside the valve ring (paravalvular regurgitation) could be detected. Extremely rarely a deformation of the closing disk may occur with a violation of its operation.
Because a clinical examination is not sufficient to evaluate a prosthetic valve, several diagnostic methods have been proposed to assess its functional status. The main echocardiographic characteristics of the prosthetic valve functioning are the pressure gradient and the effective orifice area determined by the Doppler method (8). The pressure gradient depends not only on the normal leaflet or disc opening but also on other factors, in particular hemodynamic situation. Therefore, it is necessary to take into account without excessive anxiety the elevated gradient numbers in a patient with no complains and symptoms of heart function worsening. Flow velocities through prosthetic valves and calculated gradients usually are significantly higher than through healthy native valves (9). These parameters also depend on the blood flow volume, cardiac output, heart rate, the presence of concomitant regurgitation, the size and type of prosthesis, the size of a chamber or vessel the prosthesis is opening in. Even "normal" numbers should be treated with some caution (10). The best approach to avoid serious mistakes is to compare the obtained numbers with the results of previous examinations. It is important for all patients to have measured prosthetic valve function parameters immediately after implantation and before their discharge from the hospital indicated in the medical records (11).
In practice, the mean pressure gradient is more useful than peak valve gradient, which is more hemodynamically dependent. The mean Doppler pressure gradient obtained by echocardiographic examination correlates closely with the mean gradient obtained by the direct measurement during heart catheterization. This applies to both mitral and aortic valve prostheses (12, 13).
One more quantitative parameter used for prosthetic valve evaluation is DVI which is the ratio between the velocity time integral (VTI) of the prosthetic transvalvular flow and the VTI of the left ventricular outflow tract (LVOT): DVI =VTIPrV / VTILVOT. Prosthetic valve dysfunction is suspected when the DVI ratio is reduced (less than 0.3) in a case of prosthetic aortic valve, or increased (greater than 2.2 m/s) in a case of prosthetic mitral valve (13).
The mitral prosthetic opening area is obtained calculating the pressure half-time by the same way as mitral stenosis evaluation. It is called the "effective orifice area" because it reflects the true prosthetic lumen taking into account that opened closing disks and supporting hinges create a certain obstacle to the blood flow (14, 15). The prosthetic mitral valve effective orifice area should be not less than 2.0 cm2. Bileaflet mechanical valves have a larger area than single-tilting disk valves. As a rule the biological prostheses have a larger open area than mechanical prostheses. The prosthetic aortic valve orifice area calculated by continuity equation method should not be less than 1 cm2. The main echocardiographic sings indicating mitral valve prosthetic obstruction are listed in the Table 1.
|
Table 1. Echocardiographic parameters of the significant prosthetic mitral valve obstruction (7, 8, 13, 15, 47) |
|
Thickening or limited mobility of cusps or occluding discs |
|
Narrowed diastolic colour flow through the valve |
|
Pressure half-time >200 ms with diastolic peak velocity ≥2.5 m/s |
|
Change in measurements more than 25% on serial studies |
|
Effective orifice area <1.0 cm2 |
|
Mean gradient >10 mm Hg |
|
Difference in EOA from normal >0.35 cm2 less than expected (in comparison with normal values for implanted type of the prosthesis) |
|
VTIMV/VTILVOT >2.5 |
|
Increase in pulmonary artery pressure |
|
EOA – effective orifice area, LVOT – left ventricular outflow tract, MV – mitral valve, VTI – time-velocity integral |
The main cause of prosthetic valve thrombosis is insufficient anticoagulant administration (16). Instrumental diagnostic criteria of the mechanical valve thrombosis include:
1) limited opening or completely blocked disc movement on the fluoroscopic examination;
2) elevated Doppler gradients on the prosthetic valve on transthoracic echocardiography (TTE);
3) detection of the thrombus during transesophageal echocardiography (TEE) (Fig. 2).
A thrombus on a prosthetic valve could be rarely visualized during transthoracic examination due to the presence of intense artifacts and ultrasound signal reverberation (17). In most cases prosthetic thrombosis develops rapidly with a dramatic increasing of heart failure manifestations.
TTE examination in these patients reveals an elevated pressure gradient on the prosthesis and a reduced effective orifice area, and occluding clots should be detected using transesophageal examination (18).
Prosthetic mitral insufficiency (regurgitation) is often difficult to diagnose during a transthoracic echocardiography because the ultrasound beams pass through a prosthetic metal structures and discs giving intensive artifacts. There is need to use non-standard scanning planes (19). Performing TTE examination the clinically significant mitral prosthetic regurgitation could be suspected in the presence of next findings: a dilated and hyperkinetic LV; systolic flow convergence on the ventricular side of the prosthesis; increased mitral diastolic E wave velocity (≥2 m/s); mean pressure gradient >6 mm Hg; DVI >2.2; unexplained and/or increasing pulmonary arterial hypertension.
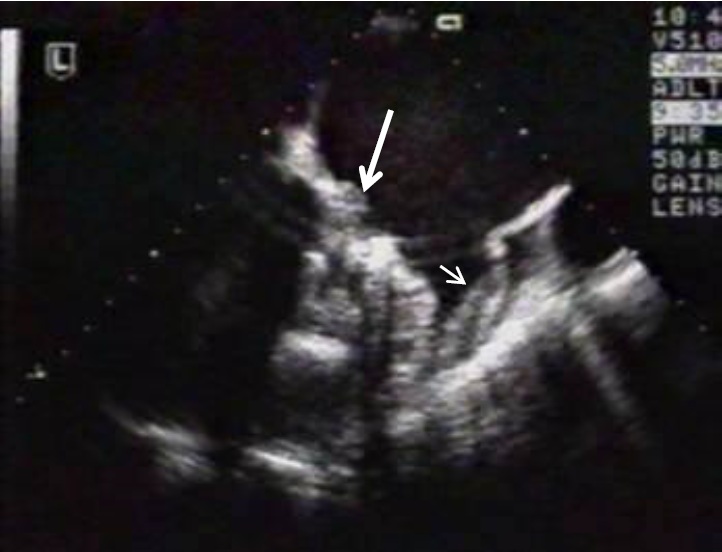
Fig. 2. Transesophageal echocardiography of thrombus obstructing the orifice of mitral valve prosthesis (long arrow). At the same time the thrombus in left atrial appendix is visualized (short arrow).
In cases of technically difficult transthoracic examination it is useful to perform TEE. It is possible to identify by TEE the origin and underlying mechanism of the regurgitant jets, such as prosthesis dehiscence, pannus formation, thrombus, vegetations, abscess formation or paraprosthetic fistula (20, 21). Color Doppler allows to detect a reverse flow on mitral valve prosthesis at the parasternal left ventricle long-axis view. Continuous-wave Doppler (CW) can give an idea of the presence of a reverse flow, but one must be careful not to confuse it with the flow through the aortic valve. Color Doppler allows detecting whether the reverse flow occurs on the prosthetic valve itself or outside its ring (paraprosthetic regurgitation).
Semi-quantitative way of the estimation of the mitral prosthetic or paraprosthetic regurgitation degree is to measure the ratio of maximum area of the regurgitation jet in the left atrium to the left atrium cross-sectional area: the mild degree is less than 20%; moderate degree - 20-40%; severe degree - more than 40%. It is simple but often not accurate. Generally, the estimation of the severity of prosthetic valve regurgitation can be performed similarly to native valve (22). The main Doppler parameters of prosthetic mitral valve regurgitation are presented in Table 2.
|
Table 2. Doppler criteria for severity of prosthetic mitral regurgitation (15, 47) |
|||
|
Parameters |
Mild |
Moderate |
Severe |
|
Color flow jet area |
Small, central jet (<4 cm2 or <20% of LA area) |
Variable |
Large central jet (>8 cm2 or >40% of LA area) or variable size wall-impinging jet |
|
Flow convergence |
None or minimal |
Intermediate |
Large |
|
Jet density: CW Doppler |
Incomplete or faint |
Dense |
Dense |
|
Jet contour: CW Doppler |
Parabolic |
Usually parabolic |
Early peaking, triangular |
|
Pulmonary venous flow |
Systolic dominance |
Systolic blunting |
Systolic flow reversal |
|
Quantitative parameters |
|||
|
Vena contracta width, cm |
<0.3 |
0.3-0.59 |
≥0.6 |
|
Regurgitant volume, mL/beat |
<30 |
30-59 |
≥60 |
|
Regurgitant fraction, % |
<30 |
30-49 |
≥50 |
|
Effective regurgitant orifice area, cm2 |
<0.20 |
0.20-0.49 |
≥0.50 |
|
LA – left atrium |
|||
However, limited data exist on the validation and application of different quantitative echo-parameters such as the effective regurgitant orifice area, width of the regurgitant jet or the vena contracta and calculated regurgitant volumes in the context of valvular prosthesis (23). That is why a multiparametric echocardiographic approach should be reasonable in connection with clinical presentation.
There are many observations that 3D-Echo is superior to two-dimensional TEE in the assessment of mitral paravalvular fistulas and regurgitant jets providing detailed visual information regarding precise localization and analysis of the leak size and shape (24, 25).
The main echocardiographic parameters indicating the severe aortic valve prosthetic obstruction are listed in the Table 3.
|
Table 3. Echocardiographic indices of the severe obstruction of replaced aortic valve (7, 8, 13, 15, 47) |
|
Thickening or limited mobility of cusps or occluding discs |
|
Calculated by Doppler method effective orifice area (EOA) >0.35 cm2 less than expected (in comparison with normal values for implanted type of prosthesis) |
|
Narrowed systolic colour flow through the valve |
|
Change in measurements more than 25% on serial studies |
|
In a case of the previous data absence:
|
Aortic prosthetic regurgitation could also be evaluated by the ratio of the proximal jet width to the LVOT diameter at the same level: a mild degree - 1-24%; moderate degree - 25-46%; moderate-to-severe degree - 47-64%; severe degree - over 65%. The reverse flow through the aortic valve prosthesis can be clearly seen in color Doppler mode on a short-axis plane at the level of the left ventricular outflow tract. The neck of the jet visualization in a short-axis view allows determination of the circumferential extent of paravalvular regurgitation. A regurgitant jet area occupying less than 10% of the sewing ring or stent circumference corresponds to mild, 10-20% - moderate, and more than 20% suggests severe reverse flow. Prosthetic rocking visible on biplane image is usually associated with dehiscence more than 40% (26, 27). In a case of multiple jets, the estimation of regurgitation severity becomes a difficult task. The recommended Doppler parameters for evaluation of the severity of prosthetic aortic valve regurgitation are presented in Table 4.
|
Table 4. Doppler parameters for evaluation of the severity of prosthetic aortic valve regurgitation (15, 47) |
|||
|
Parameters |
Mild |
Moderate |
Severe |
|
Jet width in central jets (%LVOT diameter): Color |
Narrow (≤ 25%) |
Intermediate (26-64%) |
Large (≥65%) |
|
Jet density: CW Doppler |
Incomplete or faint |
Dense |
Dense |
|
Jet deceleration rate (PHT, ms): CW Doppler |
Slow (>500) |
Variable (200-500) |
Steep (<200) |
|
LVOT flow vs pulmonary flow: PW Doppler |
Slightly increased |
Intermediate |
Greatly increased |
|
Diastolic flow reversal in the descending aorta: PW Doppler |
Absent or brief early diastolic |
Intermediate |
Prominent, holodiastolic |
|
Quantitative parameters |
|||
|
Regurgitant volume, mL/beat |
<30 |
30-59 |
≥60 |
|
Regurgitant fraction, % |
<30 |
30-49 |
≥50 |
|
CW – continuous wave, LVOT – left ventricular outflow tract, PHT – pressure half-time, PW- pulse wave |
|||
The most common drawback of the transcatheter implanted prosthetic aortic valve is regurgitant flow. First of all paravalvular leakage could be a consequence of incomplete apposition of the valvular prosthesis to the aortic annulus. Transvalvular regurgitation occurs as a result of restricted leaflet motion or leaflet destruction and improper sizing or overdilatation of the valve during the implantation. If the prosthesis has been implanted too low in the aortic position a special form of regurgitation termed supraskirtal could develop (28-31).
Infective endocarditis could be a cause of prosthetic valve dysfunction (Fig. 3). Its main echocardiographic manifestations include vegetation, thrombi on the replaced valve structures, dehiscence of the prosthetic ring, abscesses, pseudoaneurysms, fistulas and paravalvular regurgitation (32, 33). It should be kept in mind that insignificant paravalvular jets often happen even in the absence of endocarditis. The sensitivity of TEE in the detection of thrombi and vegetation in prosthetic valve infective endocarditis is much more higher (almost 90%) than transthoracic method (not more than 40%) (34-36).
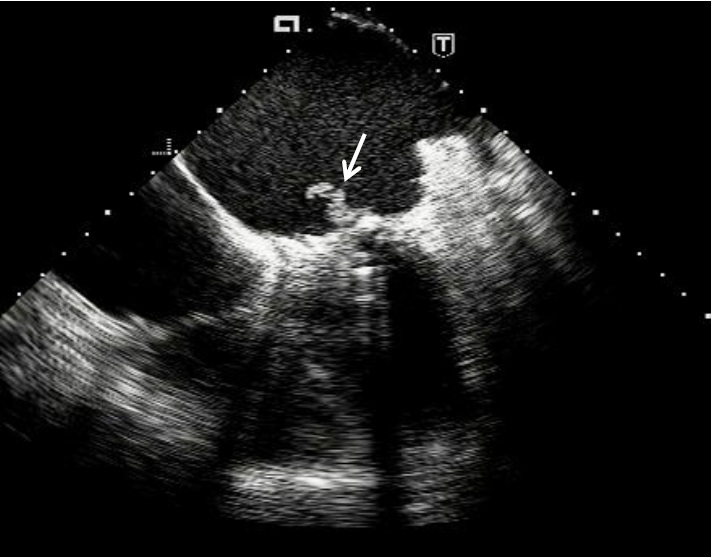
Fig. 3. A long vegetation (arrow) attached to the atrial surface of mitral valve prosthesis visible by transesophageal echocardiography
One more cause of prosthetic valve dysfunction is pannus formation. Pannus is a connective tissue progressively growing into the orifice of the prosthesis and gradually obstructing it (1, 37). The pathophysiology seems to be explained by a chronic inflammation that results in the gradual and late clinical presentation looking like a thrombosis. But the correct diagnosis of pannus is critically important for choosing a treatment method since fibrinolytic agents could resolve thrombosis and have no effect on pannus (38). Cases of pannus are possible to diagnose mostly by TEE (Fig. 4). According to the published data, the incidence of this complication is 1.6%-2% in the different series and occurs exclusively in mechanical prostheses more often in the aortic position (39, 40).
A tricuspid valve prosthetic stenosis may be suspected in the presence of abnormal morphology and limited mobility of the leaflets in combination with a transvalvular peak velocity greater than 1.7 m/sec, mean gradient exceeding 6 mm Hg and a pressure halftime more than 230 msec (41).
Mostly prosthetic pulmonary valves have been implanted in pediatric patients with congenital heart disease. Usually for this purpose surgeons use different homo- and heterografts. When a prosthetic stenosis develops marked thickening or immobility of the cusps, high transvalvular peak velocity, the presence of a depressed right ventricular function or elevated right ventricular systolic pressure could be revealed by echocardiographic examination. In the presence of severe pulmonary prosthetic valve insufficiency, it is possible to identify a right ventricle volume overload signs (15).
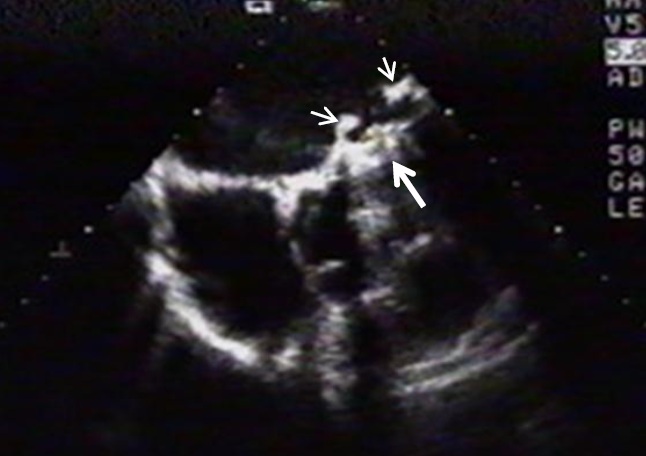
Fig. 4. Pannus formation from the atrial side of mitral valve prosthesis (short arrows) visualized by transesophageal echocardiography. Partially opened prosthetic disk is limited in its movement by thrombus (long arrow).
In a patient with a prosthetic aortic valve, a high peak systolic pressure gradient on it may also indicate that the size of the prosthesis is not consistent with the cardiac output (too small diameter of the prosthesis – patient-prosthesis mismatch – PPM). In these cases, TEE allows to find out that the prosthesis itself operates normally and there are no signs of thrombosis, pannus growing or infectious lesions. The widely used parameter for PPM identification is the indexed effective orifice area (calculated prosthetic EOA divided by the patient's body surface area). A value of indexed EOA <0.6 cm2/m2 for aortic prosthetic valve and <0.9 cm2/m2 for mitral valve suggests a severe PPM (42, 43).
In patients with a narrow ascending aorta (diameter <3cm), the moderately elevated pressure gradient by Doppler could be explained by pressure recovery phenomenon (44). By fact, the net gradient is lower and to approve this calculation of energy loss index is useful (45). Therefore, in patients with small aorta it could be possible an overestimation of prosthetic valve stenosis (46).
The deterioration of the clinical condition of a patient with a prosthetic heart valve may occur not only due to the prosthesis dysfunction but also due to other cardiac causes. Therefore, an echocardiographic examination should include the assessment of native valves, the size of the heart chambers and ventricular function, pulmonary artery pressure, the presence of left atrium thrombosis and fluid in the pericardial cavity.
In all cases suspicious for prosthetic valve dysfunction the transesophageal echocardiography is indicated. This method is highly sensitive in detecting of small clots and vegetations, in addition, it helps to localize regurgitation jets properly and assess the severity of regurgitation more precisely (47).
Therefore, the complete echocardiographic evaluation of prosthetic valves includes:
1) a two-dimensional examination, which does not provide enough information, but can show clear a prosthetic dehiscence;
2) spectral Doppler examination allowing to calculate the pressure gradients in cases of prosthetic obstruction (thrombus, pannus, large vegetations) and calculate the effective orifice area;
3) color Doppler helping in detection of valvular and paravalvular regurgitation;
4) transesophageal echocardiography as a particularly valuable and sensitive method allowing the visualization of thrombi, vegetations, pannus and regurgitant jets.
Peer-review: Internal and external
Conflict of interest: None to declare.
Authorship: Y.I.
Acknowledgement and funding: None to declare.
References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

