Deep venous thrombosis and pulmonary thromboembolism among COVID-19 patients
ORIGINAL RESEARCH ARTICLE
Deep venous thrombosis and pulmonary thromboembolism among COVID-19 patients
Article Summary
- DOI: 10.24969/hvt.2024.463
- CARDIOVASCULAR DISEASES
- Published: 20/02/2024
- Received: 31/01/2024
- Accepted: 01/02/2024
- Views: 4683
- Downloads: 3558
- Keywords: Pulmonary embolism, deep vein thrombosis, Doppler ultrasonography, computed tomography angiography, C-reactive protein
Address for Correspondence: Faranak Salajegheh, Clinical Research Development Unit, Afzalipour Hospital, Kerman University of Medical Sciences, Kerman, Iran
Email: faranaksalajegheh@gmail.com Phone: 034331328325 ORCID: 0000-0003-4368-779X
Mitra Samareh Fekri1, Faranak Salajegheh2*, Mohammad Rezaei Zadeh Ruker3, Mohsen Nakhaie2,3, Seyedeh Mahdieh Khoshnazar3, Reza Sinaei4, Mohammad S. Shahmoradzadeh Miri5, Hanieh Mirkamali6
1Department of Internal Medicine, School of Medicine Physiology Research Center, Neuropharmacology Research Institute, Afzalipour Hospital, Kerman University of Medical Sciences, Kerman, Iran
2Clinical Research Development Unit, Afzalipour Hospital, Kerman University of Medical Sciences, Kerman, Iran; 3Gastroenterology and Hepatology Research Center, Institute of Basic and Clinical Physiology Sciences, Kerman University of Medical Sciences, Kerman, Iran
4School of Medicine, Kerman University of Medical Sciences, Kerman, Iran
5Student Research Committee, School of Medicine, Kerman University of Medical Sciences, Kerman, Iran
Abstract
Objective: Venous thrombosis arises from the formation of clots within the venous wall, precipitating an inflammatory cascade. This study aimed to obtain the statistics of confirmed cases of deep venous thrombosis (DVT) through Doppler ultrasound and pulmonary embolism (PE) via pulmonary computed tomography (CT) angiography within the cohort of COVID-19 patients.
Methods: This cross-sectional study was conducted on 265 COVID-19 patients hospitalized at Afzalipour Hospital in Kerman, Iran, during 2020-2021. The patients' records were examined for Doppler ultrasound of the lower extremities and pulmonary CT angiography. Following the establishment of Doppler ultrasound frequencies, an assessment of DVT frequency was conducted among patients who had undergone Doppler ultrasound, correlating with PE assessments via clinical judgment and pulmonary CT angiography.
Results: The study revealed a thrombosis prevalence of approximately 6.8%, with around 61.1% of thrombosis cases identified in men. The most prevalent underlying conditions within this cohort were diabetes mellitus and hypertension, accounting for approximately 22.2% of the cases. The outcomes of the regression analysis demonstrated a significant association between thrombosis and C-reactive protein (CRP) ( p= 0.02).
Conclusion: In conclusion, venous thromboembolism, encompassing conditions like DVT and PTE, emerges as a heightened occurrence among COVID-19 patients, and this prevalence is notably linked to elevated CRP levels. Acquiring an understanding of the associated risk factors and pertinent symptoms equips physicians with the tools to diagnose individuals at risk, ultimately mitigating avoidable fatalities and curbing treatment expenditures through the effective management and assessment of these risk elements.
Key words: Pulmonary embolism, deep vein thrombosis, Doppler ultrasonography, computed tomography angiography, C-reactive protein
List of Abbreviations
|
ALT – alanine transaminase |
CRP- C-reactive protein |
Plt -platelet counts |
|
AST - aspartate transaminase |
DVT - deep venous thrombosis |
PT - prothrombin time |
|
ARDS - acute respiratory distress syndrome |
ESR - erythrocyte sedimentation rate |
PTE - pulmonary thromboembolism |
|
BS -blood sugar levels |
INR - international normalized ratio |
VTE - venous thromboembolism |
|
Cr - creatinine |
LDH - lactate dehydrogenase |
WBC - white blood cell count |
|
CT - computed tomography |
PE - pulmonary embolism |
Urea - urea nitrogen |
![]()
![]()
Graphical abstract
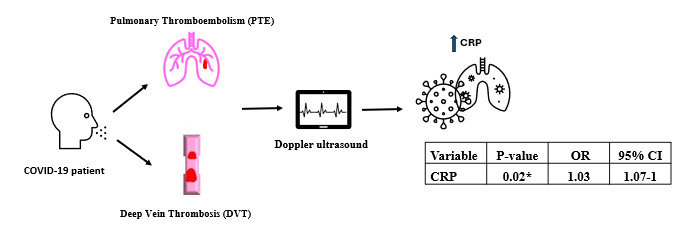 CI- confidence interval, CRP- C-reactive protein, OR – odds ratio
CI- confidence interval, CRP- C-reactive protein, OR – odds ratio
Introduction
Venus thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary thromboembolism (PTE), stands as a significant global contributor to morbidity and mortality (1). PTE ranks among the leading causes of mortality in hospitalized patients, underscoring its clinical significance (2). Reduction in blood flow rate is a significant contributing factor to thrombosis in individuals with long-term hospitalization (3–5). There are several risk factors for VTE where all three components of Virchow's triad, including stasis, hypercoagulation, and endothelial damage, can be affected (6, 7). The coronavirus disease 2019 (COVID-19) has posed unprecedented challenges to the healthcare system. COVID-19 can manifest in various ways, with one notable consequence being an elevated risk of VTE, particularly among individuals who are critically ill and necessitate intensive care unit (ICU) management (8–10). Additionally, coagulopathy is linked to worse outcomes in COVID-19 patients (11). The incidence of VTE in COVID-19 patients varies widely, ranging from 7% to 49%, influenced by diagnostic methods, patient profiles, and prophylactic interventions. The pathophysiology of COVID-19-associated VTE is intricate and multifaceted, encompassing viral-induced endothelial damage, platelet activation, cytokine storms, hypoxia, immobilization, and coagulation aberrations (12, 13). Moreover, COVID-19 has induced alterations in coagulation and inflammatory markers, including platelet counts (Plt), fibrinogen levels, C-reactive protein (CRP), lactate dehydrogenase (LDH), prothrombin time (PT), international normalized ratio (INR), as well as D-dimer concentrations (14, 15). Higher D-dimer levels were associated with an increased risk of acute respiratory distress syndrome (ARDS) and mortality (12, 16–18). As a result, these COVID-19-related abnormalities may portend unfavorable prognoses, leading to outcomes such as ICU hospitalization and the requirement for ventilator support (19, 20).
Nevertheless, the reported rates of PTE and VTE in the literature exhibit significant variability, and the establishment of a clear association between the heightened thrombotic risk in COVID-19 and mortality remains inconclusive, with certain studies indicating no such association (13).
The study aims to gather statistics on DVT and pulmonary embolism in COVID-19 patients at Afzalipur Hospital in Kerman, Iran using Doppler ultrasound and computed tomography (CT) pulmonary angiography.
If the connection is confirmed, implementing effective prevention, diagnosis, and early treatment strategies for VTE disorders could significantly reduce morbidity and mortality. This research seeks to provide insights that may differ from existing studies and enhance the standards of practice in our institution and the broader context of the country.
Methods
Study design and population
This observational (retrospective analysis of prospective study) study, conducted at Afzalipour Hospital in Kerman, Iran, from February 2021 to July 2022, examined DVT and PTE in COVID-19 patients. Overall, 254 patients with established COVID-19 were included in the study. Among them 18 developed thrombosis during follow-up and 236 did not.
Ethical approval
The assessment of frequency of confirmed lower extremity DVT and PE in hospitalized patients with COVID-19 at Afzalipour hospital in 6 months’ period with Reg. No. 99000311 was approved by ethical committee of Kerman University of Medical Sciences. The Ethics Committee of Kerman University of Medical Sciences, Kerman, Iran, approved this study, under ethic number IR.KMU.REC.1399.403. The participants were verbally informed of the study's goal, and each signed informed consent. This study was approved by the institution ethical clearance committee (REC – 05/10/2020).
Baseline variables
We gathered demographic, laboratory and clinical data from patient records, including age, gender, smoking and opium consumption status, disease severity, and other comorbidities such as hypertension, prior thrombotic events, surgical history, pregnancy, and use of contraceptives. Moreover, we documented instances of fractures, surgeries, the ongoing administration of cancer treatment drugs, and the use of blood anticoagulants among the patients. We assessed laboratory data as total blood count, C-reactive protein, D-dimer, lactate dehydrogenase (LDH), alanine (ALT) and aspartate (AST), creatinine, urea nitrogen, and blood sugar (BS) levels.
COVID-19 diagnosis
COVID-19 cases were defined based on national guidelines, including those with common clinical symptoms, recent contact with a confirmed case within 14 days prior to symptom onset, and positive results from either a reverse transcription polymerase chain reaction (RT-PCR) test for COVID-19 or evidence of lung involvement in chest CT scans.
Disease severity assessment
Disease severity was assessed following national guidelines. Patients in stage zero and one, exhibiting mild symptoms, were managed on an outpatient basis and not hospitalized. Patients in stages two and three were admitted for further evaluation. Second-stage patients were categorized as having moderate severity if they presented with shortness of breath, chest pain, or pressure, with or without a fever exceeding 38°C, and their SpO2 levels ranged from 90% to 93%. Those with accelerated respiratory symptoms, tachypnea, SpO2 levels below 90%, PaO2/FiO2 levels of 300 or less, an increased A-a gradient, and over 50% lung involvement on CT scans were classified as severe cases. Patients in the third stage, experiencing respiratory failure with SpO2 levels below 88%, signs of shock, requiring mechanical ventilation, or displaying multi-organ failure, were categorized as having a very severe or critical condition (21).
Doppler ultrasonography
Patient data from medical records formed the study's core data source. We initially examined Doppler ultrasound records and assessed DVT frequency among patients who had undergone these ultrasounds. We employed the HFL38x model from Sonosite M-Turbo, USA machine, for our Doppler ultrasound imaging. This high-frequency linear-array probe was deliberately selected to enhance the resolution and precision of vascular imaging, rendering it particularly well-suited for identifying thrombotic events in patients with COVID-19. This equipment is renowned for its reliability and performance, aligning with the stringent standards set by our institution. Additionally, the selected frequency range and transducer type were carefully considered to ensure adherence to both national and international guidelines for studies investigating thrombotic events among individuals with COVID-19. For PE, we relied on CT pulmonary angiography and clinical symptoms. We meticulously recorded the specific locations of DVT in the limbs and PE sites.
Data analysis was conducted using SPSS version 23.
Data analysis involved both descriptive statistics (mean, standard deviation, median, interquartile range, frequency, and percentage) and inferential statistics. Normality tests, specifically the Kolmogorov-Smirnov method, were first applied to quantitative data, such as age and duration of hospitalization.
If the data met the assumptions of normality, t-tests were employed; otherwise, non-parametric Mann-Whitney U tests were utilized. Categorical data were compared using Chi-square and Fisher's exact tests. Logistic regression analysis was employed to investigate the association between the occurrence of thrombosis (the dependent variable) and various independent variables such as C-reactive protein (CRP) levels, age, gender, comorbidities, and other relevant factors. The aim was to determine if there is a significant relationship between these predictors and the likelihood of developing deep venous thrombosis (DVT) or pulmonary embolism (PE) in COVID-19 patients. A significance level of p < 0.05 was adopted for two-tailed tests.
Results
Our study of 265 COVID-19 patients at Afzalipour Hospital revealed a significant prevalence of thrombosis at 6.8% over a six-month period. A detailed demographic breakdown (Table 1) indicated that 61.1% of patients with thrombosis were male. Additionally, diabetes mellitus and high blood pressure were the most common underlying conditions among patients, both with and without thrombosis. Patients with thrombosis exhibited a more severe disease state compared to those without, as observed in Mayled's condition, though it did not reach significance.
|
Table 1. Demographic and clinical characteristics of COVID-19 patients with and without thrombosis |
||||||
|
Variables |
With thrombosis (n-18) |
Without thrombosis (n=236) |
p |
|||
|
N |
% |
N |
% |
|
||
|
Gender |
Female |
7 |
38.9 |
99 |
40.1 |
0.92 |
|
Male |
11 |
61.1 |
148 |
59.9 |
||
|
Underlying disease |
DM |
4 |
22.2 |
61 |
24.7 |
0.81 |
|
HTN |
4 |
22.2 |
64 |
25.9 |
0.72 |
|
|
IHD |
2 |
11.1 |
32 |
13 |
0.82 |
|
|
Malignancy |
1 |
5.6 |
1 |
0.4 |
0.058 |
|
|
Airway Disease |
2 |
11.1 |
17 |
6.9 |
0.5 |
|
|
Hyperlipidemia |
2 |
11.1 |
18 |
7.3 |
0.55 |
|
|
CKD |
1 |
5.6 |
2 |
0.8 |
0.06 |
|
|
Cigarette Smoking |
Yes |
2 |
11.1 |
16 |
6.5 |
0.45 |
|
No |
16 |
88.9 |
231 |
93.5 |
||
|
Opium Consumption |
Yes |
4 |
22.2 |
38 |
15.4 |
0.44 |
|
No |
14 |
77.8 |
209 |
84.6 |
||
|
COVID-19 severity |
Mild |
5 |
27.8 |
86 |
36.4 |
0.46 |
|
Severe |
13 |
72.2 |
150 |
63.6 |
||
|
CKD - chronic kidney disease, DM - diabetes mellitus, HTN - blood hypertension, IHD - ischemic heart disease Note: "N" represents the number of individuals in each category, and "%" represents the percentage of individuals within the respective group |
||||||
Upon a deeper analysis of the data (Table 2), subjects who experienced thrombosis demonstrated significantly higher mean values for D-dimer (p=0.003) and CRP (p=0.01) compared to subjects without thrombosis, while WBC, urea, chromium, ESR, AST, ALT, and BS did not differ. Although Table 2 results indicated a statistically significant relationship, employing the t-test between two alpha and beta parameters. A more intricate exploration of the relationship between thrombosis and various variables in Table 3 was conducted using logistic regression analysis.
Interestingly, only the CRP variable showed a statistically significant relationship (OR -1.03, 1.0-1.07, p=0.02). Logistic regression analysis, detailed in Table 3 for other parameters, failed to identify a statistically significant association between thrombosis and various factors, including sex, age, COVID-19 severity, smoking, opiate use, and a set of hematological and biochemical markers.
Heart, Vessels and Transplantation 2024; 8: doi: 10.24969/hvt.2023.463
Venous thromboembolism in COVID-19 Fekri et al.
![]()
|
Table 2. Comparison of laboratory parameters Between COVID-19 patients with and without thrombosis |
||||||
|
Variable |
Unit |
With thrombosis (n-18) |
Without thrombosis (n=236) |
p |
||
|
Mean |
SD |
Mean |
SD |
|||
|
WBC |
cells/µL |
6.70 |
0.65 |
6.28 |
0.28 |
0.58 |
|
HB |
g/dL |
12 |
1.15 |
13.25 |
0.31 |
0.18 |
|
PLT |
cells/µL |
237.63 |
36.26 |
244.57 |
11.55 |
0.83 |
|
Urea |
mg/dL |
54.54 |
11.39 |
38.59 |
3.01 |
0.07 |
|
Cr |
mg/dL |
1.69 |
0.34 |
1.36 |
0.09 |
0.38 |
|
ESR |
mm/hr |
39.54 |
6.90 |
39.39 |
1.76 |
0.98 |
|
CRP |
mg/dL |
12.99 |
3.8 |
41.19 |
2.61 |
0.01 |
|
LDH |
IU/L |
312.72 |
50.19 |
329.81 |
16.41 |
0.71 |
|
D-dimer |
µg/mL |
72.56 |
7.76 |
0.56 |
1.65 |
0.003 |
|
AST |
IU/L |
37.18 |
3.47 |
33.22 |
1.86 |
0.43 |
|
ALT |
IU/L |
41.18 |
3.82 |
35.50 |
1.91 |
0.27 |
|
BS |
mg/dL |
155.45 |
18.44 |
146.88 |
6.59 |
0.77 |
|
Note: The table displays the means and standard deviations (SD) for various laboratory parameters among patients with and without thrombosis. |
||||||
|
Table 3. Relationship between thrombosis and various variables |
|||
|
Variables |
OR |
95% CI |
p |
|
Gender |
0.95 |
0.35-2.35 |
0.92 |
|
Age |
0.98 |
0.96-1.01 |
0.39 |
|
COVID-19 severity |
1.03 |
0.33-3.17 |
0.95 |
|
Cigarette Smoking |
0.48 |
0.1-2.32 |
0.36 |
|
Opium Consumption |
0.59 |
0.18-1.9 |
0.37 |
|
WBC |
0.93 |
0.71-1.2 |
0.58 |
|
HB |
1.12 |
0.94-1.34 |
0.2 |
|
PLT |
1 |
0.99-1.0 |
0.83 |
|
Urea |
0.98 |
0.96-1.0 |
0.1 |
|
Cr |
0.89 |
0.66-1.16 |
0.39 |
|
ESR |
1 |
0.97-1.02 |
0.98 |
|
CRP |
1.03 |
1.0-1.07 |
0.02* |
|
LDH |
1 |
0.99-1.0 |
0.71 |
|
D-dimer |
0.87 |
0.72-1.05 |
0.15 |
|
AST |
0.98 |
0.94-1.02 |
0.43 |
|
ALT |
0.97 |
0.94-1.01 |
0.27 |
|
BS |
0.99 |
0.99-1.0 |
0.76 |
|
AST- aspartate transaminase, ALT- alanine transaminase, BS- blood sugar, CRP- C-reactive protein, CI- confidence intervals, Cr- creatinine, ESR- Erythrocyte Sedimentation Rate, HB- Hemoglobin, LDH- Lactate Dehydrogenase, OR- Odds Ratios, PLT- platelet, Urea- urea nitrogen, WBC- white blood cell count Note: The table displays the means and standard deviations (SD) for various laboratory parameters among patients with and without thrombosis.
|
|||
These comprehensive findings indicate that within the scope of this study, demographic factors and independently analyzed blood markers did not show a statistically significant association with thrombosis in COVID-19 patients. This emphasizes the intricate and multifaceted nature of thrombotic events in this specific patient group and underscores the necessity for ongoing research to unravel the complex interplay of contributing factors.
Discussion
COVID-19 is attributed to the SARS-CoV-2 virus, primarily impacting the respiratory system and potentially resulting in systemic coagulation activation (22). The findings of this study yield valuable insights into the frequency, characteristics, and associated risk factors of thrombosis within the examined population. The observed prevalence of thrombosis in this investigation stands at 6.8%, highlighting its significant presence among the participants. It is noteworthy that a notable gender disparity exists, with more than 60% of individuals afflicted by thrombosis being male. This observation prompts further inquiry into the potential gender-related influences on thrombosis risk, necessitating deeper exploration of the underlying causal factors. Among a cohort of 81 critically ill patients in China who did not receive standard thromboprophylaxis, 25% were diagnosed with DVT (23). In a distinct study conducted across three hospitals in the Netherlands, encompassing 184 ICU patients who received routine low-molecular-weight heparin prophylaxis, 37% of patients experienced VTE, with a cumulative incidence reaching 49% (24). In the study conducted by Middeldorp et al., in which thrombosis prophylaxis was standardized for hospitalized patients with COVID-19, VTE occurred in 35 out of 75 ICU patients (47%), with a cumulative incidence of 59% at 21 days. Among COVID-19 patients admitted to the general ward who received thromboprophylaxis, 3% (4 out of 123) were diagnosed with symptomatic VTE (22). According to recently published research, the incidence of PE in individuals diagnosed with COVID-19 who underwent pulmonary CT angiography ranged from 23% to 30% (25). In COVID-19 patients admitted to a prominent healthcare system in New York City, thrombotic events were documented in 16% of patients. Among these cases, 6.2% were venous thrombotic events, with 3.2% being PE and 3.9% DVT. Thrombosis observed in patients with COVID-19 may be attributed to factors such as cytokine storm, hypoxic injury, endothelial dysfunction, hypercoagulability, and increased platelet activity (26).
Furthermore, the study unveiled that patients with thrombosis exhibited D-dimer levels exceeding 72 µg/mL in contrast to those without thrombosis. D-dimer, as a fibrin degradation product, stands as an indicator of ongoing clot formation and dissolution. This discovery reinforces the association between thrombosis and the notable elevation in D-dimer levels among afflicted individuals. Early investigations in China similarly reported elevated D-dimer levels (0.5 mg/L or higher) in 46% to 63% of patients, accompanied by additional indications of coagulation activation, including mild thrombocytopenia and relatively prolonged prothrombin time (20–27). The elevation of D-dimer levels in individuals with COVID-19 may be attributed to prothrombotic coagulation processes or pulmonary microvascular thrombosis, which can extend beyond what, is observable through the chest CT angiography (28). A study revealed that D-dimer levels exhibited a notable elevation in patients with severe COVID-19 when compared to those with a milder form of the disease (median 2.4 vs. 0.5 μg/mL). Furthermore, the study found that D-dimer levels surpassing 1 μg/mL were significantly associated with an increased risk of intensive care unit admission, mechanical ventilation, or mortality (29).
The results of the regression analysis have unveiled a statistically significant relationship between thrombosis and CRP levels, implying that elevated CRP levels may be indicative of an augmented risk for thrombosis. CRP, as a systemic inflammation marker, has been associated with an increased propensity for thrombotic events. Consequently, the monitoring of CRP levels could offer valuable utility as a tool for assessing the risk of thrombotic events and guiding preemptive measures. A study demonstrated that elevated levels of CRP and D-dimer exhibit a notably high positive predictive value for VTE in critically ill COVID-19 patients. Furthermore, a more pronounced state of coagulation activation appears to be closely associated with a more severe disease progression, including ICU admission and mortality (30).
Heart, Vessels and Transplantation 2024; 8: doi: 10.24969/hvt.2023.463
Venous thromboembolism in COVID-19 Fekri et al.
![]()
Based on our study, the most prevalent underlying diseases among individuals with thrombosis were identified as diabetes and hypertension, with a prevalence of 22.2%. This observation underscores the critical importance of effectively managing these conditions, as they appear to significantly contribute to the occurrence of thrombotic events. Additionally, these findings align with prior research indicating that COVID-19 is associated with a heightened risk of DVT and PTE, particularly in severe cases.
Furthermore, these complications have been shown to elevate the mortality rate among COVID-19 patients. Notably, a statistically significant association has been established between thrombosis and CRP levels. Consequently, it is imperative to monitor the coagulation status of COVID-19 patients and implement appropriate prophylactic and therapeutic measures for those at risk of VTE.
Study limitations
The study has some limitations that need to be acknowledged and addressed in future research. The study was based on a retrospective analysis of prospectively collected medical records, which may have introduced selection bias, information bias, and confounding factors. This research relied on the availability and accuracy of the diagnostic tests, which may have varied across different time periods and settings. Moreover, the study did not include a control group of non-COVID-19 patients, which limits the ability to compare the incidence and risk factors of VTE between COVID-19 and other conditions. The retrospective analysis of medical records might introduce biases like selection, information, and confounding factors. Variations in diagnostic tests across time and settings could affect accuracy. Excluding non-COVID-19 patients limited comparisons with VTE incidence and risk factors. The study's small sample size reduced statistical power and precision. Low numbers in Doppler ultrasound and pulmonary CT angiography might have underestimated VTE prevalence. Lack of systematic screening for asymptomatic VTE and assessing patient outcomes limited comprehensive insights. Conducted in a single center in Iran, the study's findings might not apply universally. It didn't delve into genetic, environmental, or behavioral factors impacting VTE severity. Therefore, future research is needed to address these limitations and extend the knowledge on the epidemiology, risk factors, and outcomes of VTE in COVID-19 patients. Larger, multicenter, prospective studies with standardized screening and diagnostic methods are required to obtain more accurate and representative estimates of the prevalence and incidence of VTE in different regions and settings. Randomized controlled trials are also needed to evaluate the efficacy and safety of different anticoagulant regimens and strategies for the prevention and treatment of VTE in COVID-19 patients. Additionally, more studies are needed to investigate the molecular and cellular mechanisms that mediate the interaction between COVID-19 infection, inflammation, and thrombosis, and to identify potential biomarkers and targets for the diagnosis and intervention of this complication.
Conclusions
Pulmonary embolism (PE) and deep vein thrombosis (DVT) represent critical and perilous complications of COVID-19, contributing substantially to morbidity and mortality rates. Our study findings have unveiled a thrombosis prevalence of 6.8% among COVID-19 patients, a figure that closely aligns with prior research. Notably, a statistically significant association has been established between thrombosis and C-reactive protein (CRP) levels.
Ethics: The assessment of frequency of confirmed DVT lower extremity and pulmonary embolism in hospitalized patients with COVID-19 at Afzalipoor hospital in 6 months period with Reg. No. 99000311 was approved by ethical committee of Kerman University of Medical Sciences. The Ethics Committee of Kerman University of Medical Sciences, Kerman, Iran, approved this study, under ethic number IR.KMU.REC.1399.403. The participants were verbally informed of the study's goal, and each signed informed consent. This study was approved by the institution ethical clearance committee (REC – 05/10/2020).
Peer-review: External and internal
Conflict of interests: The authors declare that they have no competing interests.
Authorship: MSF and FS: conception and design of the study. MRZR and MN: interpretation of data, draft and revision of the manuscript. MRZR, MN, SMKH, MSSM, and HM: revision of study protocol, interpretation of data and revision of the manuscript. All authors fulfilled authorship criteria and approved final version.
Acknowledgements and Funding: The authors would like to thank Kerman University of Medical Sciences for considering study. This research was done with the financial support of Kerman University of Medical Sciences.
References
1.Dhall SS, Hadley MN, Aarabi B, Gelb DE, Hurlbert RJ, Rozzelle CJ, et al. Deep venous thrombosis and thromboembolism in patients with cervical spinal cord injuries. Neurosurgery 2013; 72 Suppl 2: 244–54.
2.Saukko P, Knight B. Knight’s forensic pathology. CRC press 2015.
3.Vahdati SS, Marzabadi LR, Sadeghi H, Alikhani M. Evaluating the associated risk factors of pulmonary thrombo-embolism (PTE) in patients coming to emergency department (ED).
4.Cushman M. Epidemiology and risk factors for venous thrombosis. Semin Hematol 2007; 44: 62–9.
5.Lichota A, Szewczyk EM, Gwozdzinski K. Factors Affecting the formation and treatment of thrombosis by natural and synthetic compounds. Int J Mol Sci 2020; 21: 7975.
6.Francis CW. Prophylaxis for thromboembolism in hospitalized medical patients. N Engl J Med 2007; 356: 1438–44.
7.Bahloul M, Chaari A, Kallel H, Abid L, Hamida CB, Dammak H, et al. Pulmonary embolism in intensive care unit: Predictive factors, clinical manifestations and outcome. Ann Thorac Med 2010; 5: 97–103.
8.Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv 2020; 4: 4693–738.
9.Chen S, Zhang D, Zheng T, Yu Y, Jiang J. DVT incidence and risk factors in critically ill patients with COVID-19. J Thromb Thrombolysis 2021; 51: 33–9.
10.Oddi FM, Bernardo OD, Fresilli M, Morosetti D, Ippoliti A. Recovery of baseline renal function after treatment for prolonged in-stent artery thrombosis, in a COVID-19 positive patient: a case report. Heart Vessels Transplant 2023; 7: 130-4.
11.Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JC, Fogerty AE, Waheed A, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood J Am Soc Hematol 2020; 136: 489–500.
12.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med 2020 18; 173: 268–77.
13.Hippensteel JA, Burnham EL, Jolley SE. Prevalence of venous thromboembolism in critically ill patients with COVID-19. Br J Haematol 2020; 190: e134–7.
14.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020; 135: 2033–40.
15.Teimury A, Khameneh MT, Khaledi EM. Major coagulation disorders and parameters in COVID-19 patients. Eur J Med Res 2022; 27: 1–10.
16.Simadibrata DM, Lubis AM. D-dimer levels on admission and all-cause mortality risk in COVID-19 patients: a meta-analysis. Epidemiol Infect 2020; 148: e202.
17.Long X, Zhang Z, Zou W, Ling J, Li D, Jing L, et al. Coagulopathy of patients with COVID-19 is associated with infectious and inflammatory markers. Risk Manag Healthc Policy 2020; 13: 1965–75.
18.Guo H, Sheng Y, Li W, Li F, Xie Z, Li J, et al. Coagulopathy as a prodrome of cytokine storm in COVID-19-infected patients. Front Med 2020; 7: 572989.
19.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18: 844–7.
20.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet Lond Engl 2020; 395: 1054–62.
21.Rahmanzade R, Rahmanzadeh R, Hashemian SM, Tabarsi P. Iran’s Approach to COVID-19: evolving treatment protocols and ongoing clinical trials. Front Public Health 2020; 8: 551889.
22.Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost JTH 2020; 18: 1995–2002.
23.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost JTH 2020; 18: 1421–4.
24.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res 2020; 191: 148–50. 25.Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology 2020; 296: E186–8.
26.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA 2020; 324: 799–801.
27.Guan W jie, Ni Z yi, Hu Y, Liang W hua, Ou C quan, He J xing, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–20.
28.Suh YJ, Hong H, Ohana M, Bompard F, Revel MP, Valle C, et al. Pulmonary Embolism and deep vein thrombosis in COVID-19: A systematic review and meta-analysis. Radiology 2021; 298: E70–80.
29.Yu HH, Qin C, Chen M, Wang W, Tian DS. D-dimer level is associated with the severity of COVID-19. Thromb Res 2020; 195: 219–25.
30.Dujardin RWG, Hilderink BN, Haksteen WE, Middeldorp S, Vlaar APJ, Thachil J, et al. Biomarkers for the prediction of venous thromboembolism in critically ill COVID-19 patients. Thromb Res 2020; 196: 308–12.
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

