Predictors of early hospital mortality in patients with ischemic stroke
ORIGINAL RESEARCH ARTICLE
Predictors of early hospital mortality in patients with ischemic stroke
Article Summary
- DOI: 10.24969/hvt.2024.464
- CARDIOVASCULAR DISEASES
- Published: 27/02/2024
- Received: 26/10/2023
- Revised: 15/02/2024
- Accepted: 16/02/2024
- Views: 4344
- Downloads: 3551
- Keywords: ischemic stroke, early period, hospital mortality, predictors
Address for Correspondence: Khalida Musaeva, Department of Neurology and Clinical Genetics named after A.M. Murzaliev, I.K. Akhunbaev Kyrgyz State Medical Academy, Bishkek, Kyrgyzstan
Email: elmiramamytova@yahoo.com
ORCID: Khalida Musaeva- 0000-0003-0334-1700; Elmira M. Mamytova 0000-0002-4322-5555; Nurbek K. Monolov - 0000-0001-7589-5820; Darikha I. Bakaeva - 0000-0002-3016-866X; Damirbek A. Abibillaev-0000-0002-4660-3064
Khalida Musaeva1, Elmira M. Mamytova1,2, Nurbek K. Monolov2, Darikha I. Bakaeva1, Damirbek A. Abibillaev3
¹Department of Neurology and Clinical Genetics named after A.M. Murzaliev, I.K. Akhunbaev Kyrgyz State Medical Academy, Bishkek, Kyrgyzstan
²Department of Clinical Disciplines, Salymbekov University, Bishkek, Kyrgyzstan
3Medical Faculty, International Ala-Too University, Bishkek, Kyrgyzstan
Abstract
Objective: Identifying predictors that forecast hospital mortality among patients with ischemic stroke is crucial for ensuring timely and appropriate treatment and for improving its outcomes.
The aim of this study was to investigate predictors associated with early hospital mortality in patients with ischemic stroke. We examined the relationship between various clinical, laboratory, and neuroimaging factors and adverse clinical outcomes.
Methods: This retrospective, cohort study, included the analysis of medical records of 90 patients aged 18 and older diagnosed with "ischemic stroke," who were admitted to the stroke unit of a large tertiary-level multi-profile hospital. All patients were divided into two groups - the deceased group (n=45) and the surviving group (n=45). The collected data included patients' demographic information, basic clinical and laboratory characteristics upon admission to the hospital, historical data on vascular risk factors, neuroimaging findings (including angiography), as well as cardiological evaluations, consultations with specialists, and autopsies. The association of predictors with clinical outcome of ischemic stroke was determined using multivariate logistic regression analysis.
Results: In the deceased group, hospital mortality was associated with the initial severity of the condition due to reduced consciousness (n=31 (69%), p<0.001), a short hospital stay (n=27 (60%), p<0.001), unstable hemodynamics and respiratory failure (n=25 (55.5%), p<0.001), necessitating the use of mechanical ventilation (n=20 (44.4%), p<0.001) and a urinary catheter insertion (n=45 (100%), p<0.001), hypercreatinemia (238 (232) mmol/L, p<0.05), hyperglycemia (n=23 (51.1%), p<0.001), and hyperthermia (n=20 (44.4%), p<0.001), as well as signs of increasing brain edema with manifestations of displacement (n=17 (37.7%), p<0.001). The results of multiple logistic regression analysis showed that hypoxemia, tachycardia/bradycardia, atherothrombotic stroke, cerebral edema with dislocation of the stredin structures according to neuroimaging data were the most significant predictors of the risk of death in the group of deceased individuals (p<0.05).
Conclusions: The results of multiple logistic regression analysis showed that hypoxemia, atherothrombotic stroke, creatinine level were the most significant predictors of the risk of death in patients with ischemic stroke. Cerebral edema with dislocation of the stredin structures according to neuroimaging data had borderline predictive value.
Our results emphasize the importance of risk stratification of fatal outcomes, monitoring intracranial pressure and its reduction in case of compression threat, vital organ parameters, screening, and preventive treatment of early extracerebral hospital complications (cardiorespiratory and renal).
More aggressive control of vascular risk factors, including hypertension, diabetes, and hyperlipidemia at the pre-hospital level, serves as prevention not only for stroke itself but also for its complicated forms, ultimately affecting the frequency of ischemic stroke and mortality from it.
Key words: ischemic stroke, early period, hospital mortality, predictors.
Introduction
Ischemic stroke is the leading cause of morbidity and mortality worldwide, accounting for approximately 87% of all stroke cases (1). Despite advancements in the treatment of acute stroke, including thrombolysis and endovascular therapy, the overall mortality rate from ischemic stroke remains high, especially in patients with severe strokes (2). Identifying predictors that forecast a fatal outcome in patients with acute ischemic stroke is crucial for ensuring timely and appropriate treatment and for improving treatment outcomes.
Several studies have been conducted to investigate predictors of hospital mortality in patients with ischemic stroke. Age, comorbidities, and stroke subtype have been identified as important predictors of mortality (3-5). Additionally, some studies have shown that certain laboratory values, such as elevated blood glucose levels and leukocytosis, can also predict in-hospital adverse outcomes (6, 7).
However, these studies did not include and fully explore some potentially important predictors. For example, hypercreatinemia, hyperthermia, and cardiopulmonary insufficiency, complications, and the impact of other factors such as hypercoagulability and ethnicity on hospital mortality still require comprehensive investigation.
The aim of this study is to examine predictors associated with early hospital mortality in patients with ischemic stroke. We have studied the relationship between a range of historical, clinical, neuroimaging, and laboratory data and low survival rates in adult individuals during the acute period of ischemic stroke.
Methods
Study design and population
The study design was retrospective, cohort, analytical research, which included 90 patients aged over 18 years diagnosed with "ischemic stroke." These patients were admitted to the stroke department of the largest tertiary-level multi-profile hospital (National Hospital, Ministry of Health of the Kyrgyz Republic), the largest in the Kyrgyz Republic, from January 1, 2021, to July 31, 2022, due to ischemic stroke. The study included 90 patients, with 45 in both the survived (SG) and non-survived (NG) groups.
The Ethics Committee of the I.K. Akhunbaev Kyrgyz State Medical Academy approved the research protocol (Protocol dated 27.04.2022) and all patients provided informed consent for procedures.
Definitions
The diagnosis of stroke was established in accordance with the clinical protocol for the management of patients with acute ischemic stroke and transient ischemic attack (TIA) at the hospital stage in 2017, as well as the updated guidelines of the American Heart Association/American Stroke Association (2023) (www.aha.org). All cases of ischemic stroke included in this study were coded according to ICD-10 in the following categories: I63.3, I63.4, and I63.5. The diagnosis was made after a neurological examination based on the results of neuroimaging studies. The study analyzed socio-demographic, historical, clinical, radiological, and laboratory-instrumental data from the medical records aof all patients
Data Collection
The collected data included patients' demographic information (age, gender, address, and medical history), baseline characteristics upon admission to the hospital (physical examination, neurological examination, laboratory results (e.g., paraclinical, biochemical, hemostasis profile, etc.). Stroke was verified upon admission using computed tomography (CT) or magnetic resonance imaging (MRI), in some cases supplemented with non-contrast MR angiography. The initial volume of brain damage was noted.
The following variables were included in the analysis:
1. Gender
2. Age and age range (WHO)
3.Ethnicity
4. Time of hospitalization(time from onset of stroke to arrival at hospital)
5. Hospitalization duration
6.Time of stroke onset (morning, afternoon, night)
7.Symptoms of stroke onset (onset or debut with general cerebral or focal symptoms)
8.Body temperature on admission to the hospital
9. Vital signs such as respiratory rate, heart rate, blood oxygen saturation, temperature on admission to hospital.
10. Neurological status on admission (level of consciousness, presence of focal and general cerebral, brain stem symptoms)
11.Diagnoses on referral (i.e., diagnosis of referred physician, ambulance)
12.Diagnosis on admission (i.e., primary preliminary diagnosis based on clinical data)
13 Ischemic stroke subtypes (TOAST ischemic stroke subtypes were defined) (https://evidence-neurology.ru/evidentiary-medicine/nosology/ischemic-stroke/patogeneticheskie-podtipi-ishemicheskogo-insulta/)
14.Stroke pool, infarct size and brain infarct area (according to brain neuroimaging techniques, CT or MRI)
15.Stroke complications
16.Need for urinary catheter insertion, for mechanical ventilation
17.Complication of stroke
18.Eelectrocardiogram, Echocardiogram, Chest X-Ray.
19.Laboratory data (baseline total blood count, urinalysis, blood glucose, creatinine, liver tests, total cholesterol, coagulation system such as prothrombin index, fibrinogen according to the patient's medical records)
20. Overweight (body mass index (BMI) based on the formula weight(kg)/height(m2).
21.Systolic and diastolic blood pressure levels
22.Mortality
Statistical Analysis
Statistical analysis was conducted using SPSS 23 statistical software (IBM, New York, USA). Qualitative data are presented as number (%). Normally distributed quantitative data are expressed as mean and standard deviation. Comparisons between groups were made using Student's t-test or Chi square models. Statistical significance was considered at p < 0.05. The association of predictors with clinical outcome of ischemic stroke was determined using multiple logistic regression analysis.
Results
The demographic characteristics of survived and non-survived patients at the time of the initial examination are presented in Table 1. As evident from the provided tables, the study groups of patients are representative.
Key demographic indicators among NG and SG patients were similar, and their numerical values did not statistically differ (in terms of age, gender, and nationality).
As seen in Table 1, the average age in the NG was 71.6 (10.3) years, and males accounted for 48.9% (n=22).
|
Table 1. Clinical characteristics of patients with ischemic stroke
|
|||
|
Variables |
NG (n=45) |
SG (n=45) |
p |
|
Gender, male, n(%) |
22 (48.9)
|
19 (42.2) |
0.52 |
|
Age, years |
71.6 (10.3) 72 (34-90) |
70.0 (9.4) 71 (49-94) |
0.35 |
|
Age Range (WHO), n(%)
|
1 (2.2%) 6 (13.3%) 21(46.7%) 16 (35.6%) 1 (2.2%) |
0 7 (15.6%) 24 (53.3%) 14 (31.1%) 0 |
0.66 |
|
Ethnicity, n(%) 1) Slavic 2) Turkic-speaking 1 |
17 (37.8) 28 (62.2) |
12 (26.7) 33 (73.3)
|
0.17 |
|
Table 1. Clinical characteristics of patients with ischemic stroke (continued from page) |
|||
|
Variables |
NG (n=45) |
SG (n=45) |
p |
|
Overweight n(%) |
18 (40) |
17 (37.8) |
0.83 |
|
BMI Categories, n(%) 1) 16-18.5 (underweight) 2) 18.5-25 (normal) 3) 25-30 (overweight) 4) 30-35 (obesity class 1) 5) 35-40 (obesity class 2) 6) >40 (morbid obesity) |
0 7 (15.6 ) 9 (20 ) 12 (26.7 ) 10 (22.2 ) 7 (15.6 ) |
2 (4.4) 12 (26.7 ) 13 (28.9 ) 12 (26.7 ) 3 (6.7 ) 3 (6.7 ) |
0.09 |
|
Systolic blood pressure, mmHg |
161.3 (26.2) 165 (110-220) |
165.1 (27.7) 170 (130-220) |
0.61 |
|
Diastolic blood pressure, mmHg |
96.1 (13.6) 95 (70-120) |
94.7 (16.7) 100 (70-180) |
0.33 |
|
Blood Pressure Categories According to AHA 2017 Classification, n(%) 1) 120/80 2) 120/129 3)130-139/80-89 4) 140-159/90-99 5) ≥160/≥100 |
5 (11.1 ) 3 (6.7 ) 0 8 (17.8 ) 29 (64.4 ) |
0 0 10 (22.2 ) 9 (20 ) 26 (57.8 ) |
<0.001 |
|
HTN1 |
42 (93.3 ) |
43 (95.6 ) |
0.65 |
|
Kidney Dysfunction2 |
25 (55.6 ) |
6 (13.3 ) |
<0.001 |
|
CHD3 |
39 (86.7 ) |
44 (97.8 ) |
<0.05 |
|
COPD4 |
5 (11.1 ) |
3 (6.7 ) |
0.45 |
|
DM5 |
17 (37.8 ) |
10 (22.2 ) |
0.11 |
|
CKD6 |
16 (35.6 ) |
17 (37.8 ) |
0.83 |
|
Length of hospitalization, days |
|||
|
Up to 1 day Up to 3 days Up to 1 week Up to 2 weeks More than 2 weeks |
4 (8.9 ) 23 (51.1 ) 1 (2.2 ) 7 (15.6 ) 10 (22.2 ) |
0 2 (4.4 ) 0 39 (86.7 ) 4 (8.9 ) |
<0.001 |
|
Note: 1 Turkic-speaking ethnicities included Central Asian descendants: Kyrgyz, Uzbeks, Kazakhs, Uighurs, etc. Note: BMI - Body Mass Index-is the metric currently in use for defining anthropometric height/weight characteristics in adults and for classifying (categorizing) them into groups (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4890841/) AHA - American Heart Association, CHD – coronary heart disease, COPD – chronic obstructive pulmonary disease, CKD – chronic kidney disease; DBP - diastolic blood pressure, DM – diabetes mellitus, HTN – arterial hypertension, NG – non-survivor group, SBP - systolic blood pressure, SG – survivor group 1. Any hypertensive condition, regardless of type (symptomatic or essential), stage, severity (urgent or emergent), onset (first occurrence or chronic), control (controlled or uncontrolled), when BP exceeds 140/90 mm Hg.(The Editorial Team (on behalf of the World Heart Federation). Abstracts from the World Congress of Cardiology/Brazilian Congress of Cardiology 2022. Glob Heart 2023; 18: 10. doi: 10.5334/gh.1165. PMCID: PMC10000335.) 2. Any abnormal case when the creatinine level exceeded 1 mg/dL, regardless of the severity of the condition upon admission for stroke treatment (acute kidney injury and chronic kidney disease were not differentiated). 3 Any form of ischemic heart disease detected during the first visit to a doctor, regardless of the history and documentary data. 4. COPD is diagnosed during clinical examination (rales, crepitations) and documented by visualization, regardless of the cause and severity. 5. Blood glucose level consistent with laboratory definitions in current ADA (American Diabetes Association) recommendations, regardless of treatment and HbA1c status. 6 Chronic kidney disease, determined in accordance with current KDIGO (Kidney Disease: Improving Global Outcomes) recommendations, regardless of therapy. |
|||
As seen in Figure 1, ischemic stroke was observed more frequently in the age group of 60-74 years (elderly individuals) in both the NG (n=21, 46.7%) and the SG (n=24, 53.3%), with no statistically significant difference between the groups. It was slightly less common in the 75-90 years (elderly) and 45-59 years (middle-aged) age groups. The diagram indicates that ischemic stroke in the age category of 18-44 years (young adults) and those over 90 years (longevity) was only encountered in the NG and even then in isolated cases.
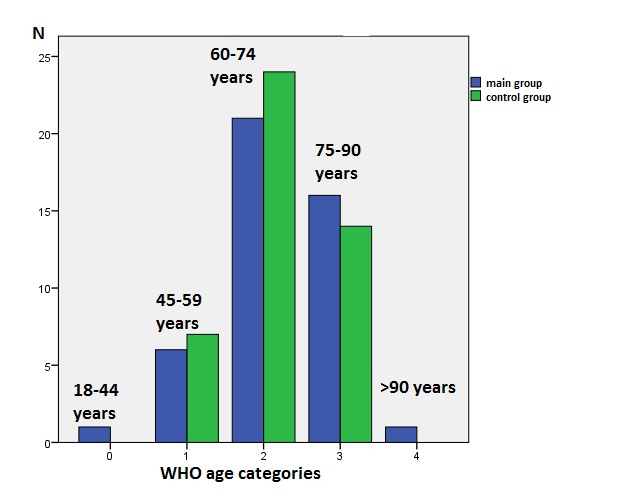
Figure 1. Distribution of stroke cases by WHO age categories
(blue – non-survivor group, green survivor group)
Hypertension, coronary heart disease, and kidney dysfunction were the most common risk factors among both NG and SG individuals. Coronary heart disease, chronic kidney diseases, and chronic obstructive pulmonary disease (COPD) were slightly less common. There was no statistically significant difference between the two study groups regarding these risk factors (Table 1). However, a statistically significant difference was observed between the occurrence of kidney diseases among NG and SG patients. Kidney dysfunction was present in 25 patients (55.6%) among the dead, while in survivors, it was found in 6 patients (13.3%) (p<0.001). In contrast, coronary heart disease was more frequent in surviving patients, with 44 patients (97.8%) compared to 29 cases (86.7%) among the non-survived ones (p<0.05). Regarding the association of COPD, diabetes mellitus (DM), and chronic kidney diseases (CKD) with adverse outcomes, no statistically significant differences were found between the groups.
It's worth noting that there were no differences between the two patient groups in terms of the degree of blood pressure elevation and the difference between systolic and diastolic blood pressure at the time of admission (Table 1).
When comparing the levels of systolic and diastolic blood pressure between the two study groups, it is noteworthy that both deceased and surviving individuals had high average values of systolic blood pressure, within 161.3 (26.2) mm Hg in the deceased and 165.1 (27.7) mm Hg in the surviving, and diastolic blood pressure was also above normal limits, with 96.1 (13.6) mm Hg in the deceased patients and 94.7 (16.7) mm Hg in the surviving patients. In deceased patients, there was a wide range of blood pressure grades according to the AHA classification (2017 - American Heart Association) when compared to survivors. However, as shown in Figure 2, both in the deceased and surviving groups, blood pressure often exceeded 160/100 mm Hg, with no statistically significant difference between the groups. It is worth noting that borderline blood pressure values of 130-139/80-89 mm Hg were not present among the deceased, whereas such values were found in 10 cases (22.2%) among the survivors.
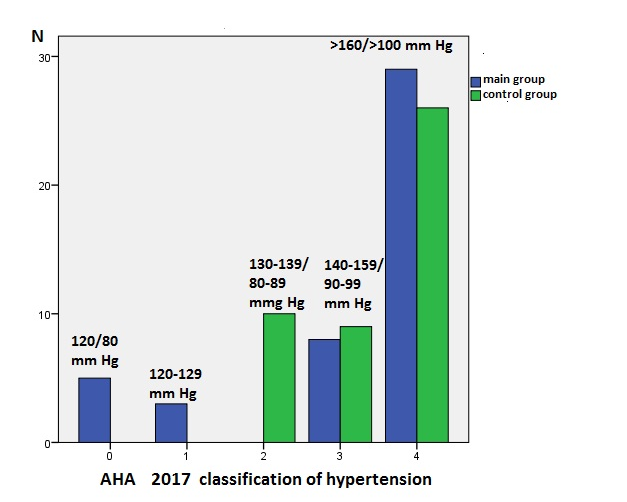
Figure 2. Distribution of stroke cases according to blood pressure levels
Blue – non-survivor group, green – survivor group
We also assessed the length of hospital stay, which indicates the severity of the condition and the course of the disease (Table 1). Patients in whom the acute vascular process ended in a fatal outcome had a relatively short duration of hospitalization, which significantly differed from the duration of patients who were discharged from the department for further rehabilitation (p<0.001). In more than half (51.1% of cases) of the deceased patients with a diagnosis of "ischemic stroke," death occurred within the first 3 days from the moment of admission to the department, in 8.9% - within 1 day, and in 2.2% - within the first 7 days. Only 37.8% of deceased patients were able to survive the 14-day threshold. As for the living individuals, 4 out of 5, namely 95.6% (n=43), survived the 2-week period.
Hyperergic reaction in the form of fever at the onset of the disease was observed in half (44.4%, n=20) of deceased patients, among whom 14 patients (31.1%) had body temperature rise to subfebrile levels, 5 (11.1%) deceased patients had feverish temperatures, and 1 patient (2.2%) had hectic temperatures. In deceased patients, normal body temperature was observed in the remaining half (55.6%) of the cases. In contrast, none of the surviving patients had an increase in body temperature. There is a highly significant difference between the groups in this examined parameter (Table 2).
The functions of vital organs were also significantly impaired in deceased patients with ischemic stroke, and the degree of deviation in hemodynamic and respiratory parameters was noticeably different from the degree of disturbances in the surviving group (p≤0.001). Symptoms of respiratory and cardiovascular insufficiency, such as tachypnea (11.1%, n=5), hypoxemia (55.6%, n=25), and tachycardia (44.4%, n=20), were observed 5-6 times more frequently in the deceased group than in the surviving group (Table 2).
|
Table 2. Distribution of patients by subtypes, indicators of vital organ function, characteristics of neurological symptoms in non-survovor (NG )and survivor (SG) groups of patients diagnosed with "ischemic stroke" |
|||
|
Variables |
NG (n=45) |
SG (n=45) |
p |
|
Ischemic stroke subtype according to TOAST, n(%) |
|||
|
Atherothrombotic |
37 (82.2 ) |
23 (51.1 ) |
<0.05 |
|
Cardioembolic |
3 (6.7 ) |
9 (20 ) |
|
|
Lacunar |
4 (8.9 ) |
12 (26.7 ) |
|
|
Cryptogenic |
1 (2.2 ) |
1 (2.2 ) |
|
|
Temperature upon admission, ºС n(%) 1) Subfebrile (37-38) 2) Febrile (38.1-39) 3) Hectic (over 40) |
14 (31.1 ) 5 (11.1 ) 1 (2.2 ) |
0 0 0 |
<0.001 |
|
Tachypnea, n(%) |
5 (11.1 ) |
0 |
<0.05 |
|
Hypoxemia, n(%) 1) Normal values (≥95) 2) Less than 95 |
25 (55.6 ) 20 (44.4 ) |
43 (95.6 ) 2 (4.4 ) |
<0.001 |
|
Symptomatic tachycardia, n(%) |
20 (44.4 ) |
5 (11.1 ) |
<0.01 |
|
Neurological symptoms, n(%) |
|||
|
Focal symptoms |
7 (15.6 ) |
28 (62.2 ) |
<0.001 |
|
Cerebral + focal symptoms |
25 (55.6 ) |
17 (37.8 ) |
|
|
Cerebral + focal + meningeal symptoms |
12 (26.7 ) |
0 |
|
|
Cerebral + Focal + Secondary Brainstem Symptoms |
1 (2,2 ) |
0 |
|
|
Need for urinary catheter insertion |
45 (100 ) |
9 (20 ) |
<0.001 |
|
Need for mechanical ventilation, n(%) |
|
|
|
|
MV Not used |
25 (55.6 ) |
45 (100 ) |
|
|
On the day of admission |
5 (11.1 ) |
0 |
|
|
Within the first 3 days |
4 (8.9 ) |
0 |
|
|
Within 3-7 days |
8 (17.8 ) |
0 |
|
|
After 7 days |
3 (6.7 ) |
0 |
|
|
Stages of altered consiousness Glasgow Coma Scale, points |
|||
|
15 – clear consciousness 13-14 – obtundation 12-9 – stupor 8-3 – coma |
10.3 (2.4) 10 (5-14) |
13.1 (1.9) 14 (5-15) |
<0.001 |
In the neurological status of patients in the two observation groups, four types of symptom complexes were observed: cerebral, focal, meningeal, and secondary brainstem symptoms. There is a statistically significant difference in the frequency of development of various neurological symptoms between deceased and surviving patients. It is noteworthy that NG patients more frequently exhibited a combination of two neurological symptom complexes – focal and cerebral, such a clinical picture was observed in over half of the patients in the main group (55.6% - n=25), whereas in the group of survivors, the most common symptom complex was the focal brain damage syndrome, which was observed in 62.2% (n=28) of patients. It is worth noting that meningeal and secondary medullary signs were not encountered at all in the group of survivors (Table 2).
One of the important indicators of the severity of the condition and the deepening of impaired consciousness was the need for a urinary catheter, which was inserted in all deceased patients (100% - n=45), while in the surviving group, only 20% (n=9) of patients required its insertion. There was a significant difference between the groups in this parameter (p≤0.001) (Table 2).
An even more critical indicator of the patient's life-threatening condition was the need for mechanical ventilation. It is worth noting that 44.4% (n=20) of deceased patients had respiratory failure due to the severity of their condition, necessitating connection to the ventilator. However, the timing of its installation varied. As for the group of survivors, none of the patients required mechanical ventilation, demonstrating a high degree of difference between the groups in this parameter (<0.001) (Table 2).
The duration of MV in deceased patients was divided into four grades: on the day of admission, within the first 3 days, within 3-7 days, and after 7 days. Most often, worsening of the condition in deceased patients occurred within 3-7 days, leading to the need for mechanical ventilation in 17% of deceased patients. The worsening of the condition with subsequent mechanical ventilation at other times also occurred but was more prolonged and 1.5-2 times less frequent in the deceased group (Table 2).
The level of consciousness, as an important indicator of stroke severity, showed a statistically significant difference between the groups. Deceased patients had severe impairment of consciousness to the level of stupor (10.3(2.4)) at the onset of the disease, while in survivors, signs of impaired consciousness were observed but to a milder extent, at the level of obtundation (13.1(1.9)) (p ≤ 0.001).
Regarding the frequency of occurrence of different subtypes of ischemic stroke according to the TOAST classification (years) among deceased and surviving patients, it is worth noting that the most common subtype was atherothrombotic stroke, which occurred in 37 deceased patients (82.2%) and half of the surviving patients (51.1% - n=23). The second most common was lacunar stroke, which was the reason for hospitalization in 4 deceased patients (8.9%) and 12 surviving patients (26.7%). The third most common was cardioembolic stroke, which occurred in 3 deceased (6.7%) and 9 surviving patients (20%). Cryptogenic stroke was found in 1 patient (2.2%) in both the NG and SG patient groups. Since the frequency of the main subtypes of stroke in those who had a stroke was different, the comparison between the groups showed a statistically significant difference in this parameter (p < 0.05) (Table 2).
Comparison of infarct sizes and their numbers according to vascular territories in patients from the study groups did not reveal a statistically significant difference between the groups in this parameter (Table 3). Both deceased and surviving patients most commonly had large areas of cerebral ischemia, with 48.8% of deceased patients and 42.2% of surviving patients having large infarcts. It's worth noting that in the deceased group, there were either 2 large areas of ischemia (17.8%) or 1 large area and 1 small (lacunar) area of ischemia (13.3%). In the living group, lacunar infarcts were also found in 40% of cases, similar to the deceased group. In general, no statistically significant difference was observed between the groups in this parameter.
|
Table 3. Neuroimaging and laboratory findings of non-survivor (NG) and survivor (SG) groups diagnosed with "ischemic stroke" |
||||
|
Variables |
NG (n=45) |
SG (n=45) |
p |
|
|
Infarct size and number of infarcts, n(%) |
||||
|
1 lacunar infarct (size up to 1.5 cm²) |
3 (6.7 ) |
10 (22.2 ) |
<0.01 |
|
|
2 or more lacunar foci |
6 (13.3 ) |
8 (17.8 ) |
||
|
Infarct size more than 1.5 cm² |
11 (24.4 ) |
8 (17.8 ) |
||
|
2 large infarcts in 2 different territories |
8 (17.8 ) |
0 |
||
|
1 lacunar infarct and 1 large infarct |
6 (13.3 ) |
0 |
||
|
1 large infarct |
11 (24.4 ) |
19 (42,2 ) |
||
|
Complications, n(%) |
||||
|
Cerebral edema |
17 (37.8 ) |
2 (4.4 ) |
<0.001 |
|
|
Hemorrhagic transformation of the infarct |
7 (15,6 ) |
2 (4.4 ) |
||
|
Combined complications |
18 (40 ) |
0 |
||
|
Absence of complications |
4 (8.9 ) |
41 (91.1 ) |
||
|
Acute kidney injury |
2 (4.4 ) |
0 |
||
|
Septic complications |
1 (2.2 ) |
0 |
||
|
Acute coronary syndrome |
3 (6.7 ) |
2 (4.4 ) |
||
|
Serum glucose, mmol/L |
8.7 (3.5) 7.9 (2.8-18) |
6.1 (2.1) 5.5 (3-14) |
< 0.001 |
|
|
Glycemic profile, n(%) |
Moderate hyperglycemia |
11 (24.4 ) |
5 (11.1%) |
< 0.001 |
|
Severe hyperglycemia |
9 (20 ) |
9 (20%) |
||
|
Lipid metabolism parameter, n(%) |
||||
|
Total cholesterol, mmol/L |
5.2 (1.5) 5 (2-8) |
4.6 (1.1) 4.5 (2.7-7.5) |
<0.05 |
|
|
Hypercholesterolemia, n(%) |
21 (46.7) |
10 (22.2) |
< 0.05 |
|
|
Creatinine, µmol/L |
256.6 (232.1) 200 (45-970) |
96.6 (43.5) 83.2 (51-268) |
<0.05 |
|
|
Fibrinogen, g/L |
4950.1 (1697.8) 3999 (2095-9888) |
3793.2 (964.5) 3888 (2004-7540) |
<0.001 |
|
|
Prothrombin index,% |
98.8 (10.8) 98 (68-110) |
91.6 (8.7) 94 (72-106) |
<0.001 |
|
|
Hypercoagulation, n(%) |
20 (44.4 ) |
4 (8.9 ) |
<0.001 |
|
|
Hypocoagulation, n(%) |
3 (6.7 ) |
11 (24.4 ) |
||
Regarding the analysis of stroke complications, there was a statistically significant difference between the groups (p<0.001). In deceased patients, the most common complications were combined complications (intracerebral + extracerebral), cerebral edema, and hemorrhagic transformation of the infarction. Acute kidney injury or decompensation of chronic kidney disease (4.4% n=2), such as in patients with diabetes (diabetic nephropathy), septic complications (pulmonary or urinary tract infections) (2.2% n=1), and acute coronary syndrome (6.7% n=3), were less frequently observed. The most common complications in the NG were cerebral edema (37.8% n=17) and combined complications (40% n=18). Strikingly, two patients (4.4%) in the NG were not presented with complications. In the surviving group, 91.1% (n=41) had no complications during the course of the disease. Only 4 patients in the surviving group experienced complications, including cerebral edema (n=2) and acute coronary syndrome (n=2) (Table 3).
We analyzed as well, the carbohydrate metabolism parameters. The blood glucose level in peripheral blood was significantly elevated in the deceased group, with an average value of 8.7 (3.5), while in living individuals, the average glucose level remained within the reference range at 6.1 (2.1) (p< 0.001).
Heart, Vessels and Transplantation 2024; 8: doi: 10.24969/hvt.2023.464
Predictors of ischemic stroke mortality Musaeva et al.
![]()
The glycemic profile also showed a significant difference in values between the two study groups, with moderate hyperglycemia (n=11 - 24.4%) being more pronounced in deceased individuals and severe hyperglycemia, observed in half of the deceased group (n=25 - 51.1%), which was 2.5 times more common than in the comparison group (n=6 - 20.7%) (p<0.001). In contrast, moderate (n=5 - 11.1%) and severe hyperglycemia (n=9 - 20%) were observed significantly less frequently in living individuals (Table 3).
Lipid profile was also assessed based on the level of total cholesterol, which was significantly higher in deceased individuals compared to surviving patients ( (p<0.05). Hypercholesterolemia was also significantly more common in the main group (n=21 - 46.7%), i.e., in half of the deceased patients, whereas in the surviving group, it was observed in 1/5 of the individuals in the comparison group (n=9 - 20%) (p≤0.05), as reflected in Table 3.
One of the important indicators of kidney function, creatinine, was also included in the study. The serum creatinine level in deceased patients was 2.6 times higher than that in patients from the surviving group (p <0.05) (Table 3).
The blood clotting profile was also examined based on fibrinogen, prothrombin index (PTI), and other parameters. When comparing with the deceased group, the level of fibrinogen and PTI in these patients significantly differed from those in the group of living individuals. For instance, in deceased patients, the level of fibrinogen A was 1.3 times higher than in surviving patients (p<0.001). The PTI level in deceased individuals was 99.2(17.3)%, whereas in surviving individuals, it was 91.2(11.9)% (p<0.001). When analyzing deviations in the coagulogram, it is noteworthy that hemostasis profile in deceased individuals (n=20 - 44.4%) were skewed towards hypercoagulation and occurred 5 times more often than in the surviving group, whereas hypocoagulation was observed 3.5 times more often among surviving individuals (n=11 - 24.4%) (p<0.001). These shifts in blood coagulation parameters are reflected in Table 3.
Predictors of death in the early post-stroke period in patients with ischemic stroke
Hypoxemia, tachycardia/bradycardia, pathological changes on the electrocardiogram, atherothrombotic stroke, brain dislocation, cerebral edema, leukocyte level, % segmented leukocytes, platelets, blood glucose, total cholesterol, creatinine, prothrombin index, fibrinogen were defined as independent variables (Table 4).
|
Table 4. Assignment of independent variables – multiple logistic regression analysis |
|||
|
Independent variable |
Assignment |
Independent variable |
Assignment |
|
Hypoxemia |
Yes =1, no =0 |
Leukocytes |
Continuous variable |
|
Arrhythmia |
Yes =1, no =0 |
Segments |
Continuous variable |
|
Atherothrombotic stroke |
Yes =1, no =0 |
Platelets |
Continuous variable |
|
Dislocation syndrome |
Yes =1, no =0 |
Glucose |
Continuous variable |
|
Cerebral edema |
Yes =1, no =0 |
Cholesterol |
Continuous variable |
|
Electrocardiogram |
Yes =1, no =0 |
Creatinine |
Continuous variable |
|
Fibrinogen |
Continuous variable |
Prothrombin index |
Continuous variable |
Multiple logistic regression was performed to analyze the adverse clinical outcome (death) in patients with ischemic stroke as the dependent variable (no =0, yes =1). The results of logistic regression analysis showed that hypoxemia, atherothrombotic stroke and creatinine level were the most significant predictors of hospital mortality: (p-0.003, p=0.04 and p=0.043. respectively). Meaning that in-hospital mortality in ischemic stroke patients was 39.4 time more likely in patients with hypoxemia, 8.1 time higher in patients with atherothrombotic stroke and 2 times higher in patients with kidney dysfunction.
While cerebral edema with dislocation of the stredin structures according to neuroimaging data had a borderline predictive value.
|
Table 5. Multiple regression analysis of predictors of in-hospital morality in ischemic stroke |
|||||
|
Variable |
OR |
Standard error |
z/χ2 value |
p |
95% CI |
|
Hypoxemia |
39.4 |
48.69 |
2.98 |
0.003 |
3.51 – 443.1 |
|
Arrythmia |
1.67 |
.81 |
1.06 |
0.288 |
0.64 – 4.34 |
|
Atherothrombotic stroke |
8.10 |
8.25 |
2.05 |
0.040 |
1.09 – 59.7 |
|
Dislocation syndrome |
2.5 |
5.09 |
0.45 |
0.653 |
0.04 – 135.9 |
|
Changes in electrocardiogram |
0.89 |
.202 |
-0.47 |
0.637 |
0.57 – 1.39 |
|
Cerebral edema |
7.8 |
10.12 |
1.60 |
0.109 |
0.63 – 98.01 |
|
Leukocytes |
1.12 |
.19 |
0.70 |
0.487 |
0.80 – 1.58 |
|
Segments |
0.97 |
.04 |
-0.45 |
0.656 |
0.88 – 1.07 |
|
Platelets |
1.01 |
.006 |
1.81 |
0.071 |
0.99 – 1.02 |
|
Glucose |
0.95 |
.17 |
-0.25 |
0.804 |
0.66 – 1.36 |
|
Cholesterol |
0.83 |
.31 |
-0.48 |
0.631 |
0.40 – 1.73 |
|
Creatinine |
1.01 |
.006 |
2.02 |
0.043 |
1.00 – 1.02 |
|
Prothrombin index |
1.09 |
.057 |
1.79 |
0.074 |
0.99 – 1.21 |
|
Fibrinogen |
1.00 |
.0002 |
1.01 |
0.312 |
0.99 – 1.00 |
Discussion
The aim of this study was to investigate predictors associated with in-hospital mortality in patients with ischemic stroke. Our results indicate a significant relationship between the extent of damage and an increased risk of early in-hospital mortality. Deceased patients were significantly more likely than surviving patients to have larger infarct volumes, such as one large infarct with one lacunar infarct or two large infarcts in two different territories (p< 0.01). Previous research has shown that larger lesion volumes are associated with more severe neurological deficits, delayed recovery, and unfavorable functional outcomes (8). Additionally, patients with stroke and larger lesion volumes have a higher likelihood of developing complications such as hemorrhagic transformation and increased intracranial pressure (9).
In our study, deceased patients more frequently exhibited severe neurological symptoms, characterized by a combination of global, focal, and even meningeal symptoms, as well as altered consciousness, when compared to the control group (p<0.001). In contrast, surviving patients exhibited neurological symptoms characterized by focal neurological deficits without consciousness impairment or with mild impairment (stupor) (p< 0.001). According to the results of our study, individuals with a lethal outcome of stroke more frequently developed complications such as a combination of intracerebral and extracerebral complications, cerebral edema, and hemorrhagic transformation (p < 0.001).
Our study's results showed that 44.4% of deceased patients had significantly more hyperthermia from the first day of observation, while all surviving patients had body temperatures within the normal range during hospitalization (p<0.001). It has been demonstrated that an increase in brain temperature before and after ischemic stroke increases the total infarct volume. Elevated body temperature may have an "all-or-nothing" effect with a certain threshold beyond which temperature elevation worsens ischemic damage (10).
Clinical studies report hyperthermia in 18-61% of patients after ischemic stroke (11).
Previous research has shown that factors such as age, stroke type, lesion location and volume, stroke severity, infections, and a systemic inflammatory response (secondary to the infarction) can be determining factors in body temperature elevation after a stroke (12). Therefore, in the presence of all these factors in patients with acute ischemic stroke, aggressive measures for hyperthermia prevention and treatment may improve clinical outcomes.
Alongside hyperthermia, signs of respiratory failure were very frequently observed in deceased patients, clinically manifested as hypoxemia, tachypnea, and accompanying tachycardia. According to literature data, the development of pathological breathing patterns as described by other authors was also associated with high hospital mortality rates, which remained unchanged and ranged from 50% to 90% (13). The concomitant low level of consciousness on the Glasgow Coma Scale during or before intubation, loss of medullary reflexes and signs of neurological deterioration invariably predicted death.
A decreased level of consciousness also makes stroke patients more susceptible to aspiration pneumonia, impaired mucus clearance from the airways, and the development of ventilator-associated pneumonia compared to patients without impaired consciousness (14). In our study, 44.4% of deceased patients required mechanical ventilation due to the severity of their condition. Recent research has shown that early tracheostomy in patients with infratentorial lesions can help reduce the duration of artificial lung ventilation and the length of stay in the intensive care unit (15).
Another important predictor of extremely unfavorable clinical outcomes in ischemic stroke, the role of which in stroke outcomes is not yet well-studied, is hypercreatininemia. In our study, deceased patients had a statistically significant 2.5-fold increase in creatinine levels compared to control group patients. An analysis of the literature indicates that 27% of patients develop kidney damage after acute stroke, a condition influenced by stroke severity, baseline creatinine levels, heart failure, and stroke subtypes (16). Therefore, to prevent kidney damage (both de-novo and worsening of pre-existing injury), intensive monitoring, abstention from all potential nephrotoxic drugs and interventions, adequate hydration, blood pressure control, correction of any electrolyte disturbances, and acid-base balance are crucial.
Lastly, one cannot ignore the role of laboratory predictors such as hyperglycemia, hypercholesterolemia, and hypercoagulation. Medical records of deceased patients showed significantly elevated levels of glucose in peripheral blood and glycemic profiles (p<0.001). Recent studies have shown that a large proportion of patients who have had an acute stroke can develop hyperglycemia, even in the absence of a pre-existing diabetes diagnosis. Both human and animal studies demonstrate that hyperglycemia is not a benign condition and is associated with a high risk of mortality (17). Moreover, recent data suggest that lowering glucose levels with insulin reduces ischemic brain injury in animal stroke models, suggesting that stroke-induced hyperglycemia may be a modifiable risk factor for brain injury (18). Furthermore, it has been shown that fibrinogen was mostly elevated in non-survived ones, whereas hypocoagulation was frequently observed in survived patients. According to literature, both low and high fibrinogen levels hinder the post-stroke recovery processes (18).
Study limitations
Due to single-center study the data partially highlights the real clinical scenarios of stroke patients in Kyrgyz Republic. It is well-known that hospital mortality is also closely related to the level of medical care - the availability of high-precision therapeutic and diagnostic equipment, medications with proven efficacy, and the appropriate training of medical personnel in the management of stroke patients. At least patients were able to take tertiary care in republican center. The conditions in rural areas cannot be estimated in accordance with these findings. Furthermore, study design was limited to retrospective manner with limited patient data. Due to scarcity of patient records the sample size also suboptimal. Further research is needed to investigate potential mechanisms underlying these associations and to develop targeted interventions to improve patient outcomes. The sample sizes were small, so the data from multiple logistic regression analysis need to be supplemented in subsequent studies with larger numbers of patients or in a prospective study.
Conclusions
The results of multiple logistic regression analysis showed that hypoxemia, atherothrombotic stroke, creatinine level were the most significant predictors of the risk of death in patients with ischemic stroke. Cerebral edema with dislocation of the stredin structures according to neuroimaging data had borderline predictive value.
In summary, our results demonstrate the importance of risk stratification for fatal outcomes, monitoring intracranial pressure and reducing it in case of impending herniation, vital organ parameters, and screening and preventive treatment of early extracranial hospital complications (cardiorespiratory and renal).
More aggressive control of cerebrovascular risk factors, including hypertension, diabetes, and hyperlipidemia at the pre-hospital level, serves as prevention not only for stroke itself but also for its complications, ultimately affecting stroke incidence and mortality.
Ethics: The Ethics Committee of the I.K. Akhunbaev Kyrgyz State Medical Academy approved the research protocol (Protocol dated 27.04.2022) and all patients provided informed consent for procedures.
Peer-review: External and Internal
Conflict of interest: None to declare.
Authorship: K.M., E.M.M., N.K.M, D.I.D., and D.A.A. equally contributed to the study and manuscript preparation.
Acknowledgment and Funding: None to declare
References
| 1.Johnson CO, Nguyen M, Roth GA, Nichols E, Alam T, Abate D, et al. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 439-58. https://doi.org/10.1016/S1474-4422(19)30034-1 PMid:30871944 |
||||
| 2.Feigin VL. Anthology of stroke epidemiology in the 20th and 21st centuries: Assessing the past, the present, and envisioning the future. Int J Stroke 2019; 14: 223-37. https://doi.org/10.1177/1747493019832996 PMid:30794102 |
||||
| 3.Furlan NE, Luvizutto GJ, Hamamoto Filho PT, Zanati Bazan SG, Modolo GP, Ferreira NC, et al. The Impact of age on mortality and disability in patients with ischemic stroke who underwent cerebral reperfusion therapy: A Brazilian Cohort Study. Front. Aging Neurosci 2021; 13: 649902. https://doi.org/10.3389/fnagi.2021.649902 PMid:34295238 PMCid:PMC8291127 |
||||
| 4.Iluţ S, Vesa ŞC, Văcăraș V, Mureșanu D-F. Predictors of short-term mortality in patients with ischemic stroke. Medicina. 2023; 59: 1142. https://doi.org/10.3390/medicina59061142 PMid:37374346 PMCid:PMC10304635 |
||||
| 5.Kortazar-Zubizarreta I, Pinedo-Brochado A, Azkune-Calle I, Aguirre-Larracoechea U, Gomez-Beldarrain M, Garcia-Monco JC. Predictors of in-hospital mortality after ischemic stroke: A prospective, single-center study. Health Sci Rep 2019; 2: e110. https://doi.org/10.1002/hsr2.110 PMid:31049417 PMCid:PMC6482326 |
||||
| 6.Schmidt M, Schmidt SAJ, Adelborg K, Sundbøll J, Laugesen K, Ehrenstein V, et al. The Danish health care system and epidemiological research: From health care contacts to database records. Clin Epidemiol 2019; 11: 563-91. https://doi.org/10.2147/CLEP.S179083 PMid:31372058 PMCid:PMC6634267 |
||||
| 7.Yang J, Yin P, Zhou M, Ou CQ, Li M, Li J, et al. The burden of stroke mortality attributable to cold and hot ambient temperatures: Epidemiological evidence from China. Environ Int 2016; 92-3: 232-8. https://doi.org/10.1016/j.envint.2016.04.001 PMid:27107228 |
||||
| 8.Laredo C, Zhao Y, Rudilosso S, Renú A, Pariente JC, Chamorro Á, et al. Prognostic significance of infarct size and location: the case of insular stroke. Sci Rep 2018; 8: 94-8. https://doi.org/10.1038/s41598-018-27883-3 PMid:29934530 PMCid:PMC6015086 |
||||
| 9.Spronk E, Sykes G, Falcione S, Munsterman D, Joy T, Kamtchum-Tatuene J, et al.. Hemorrhagic transformation in ischemic stroke and the role of inflammation. Front Neurol 2021; 12: 661955. https://doi.org/10.3389/fneur.2021.661955 PMid:34054705 PMCid:PMC8160112 |
||||
| 10.Chen, L, Xu S, Yang X. et al. Association between cooling temperature and outcomes of patients with heat stroke. Intern Emerg Med 2023; 18: 1831-42. https://doi.org/10.1007/s11739-023-03291-y PMid:37133728 PMCid:PMC10504196 |
||||
| 11.Yokobori S, Koido Y, Shishido H, Hifumi T, Kawakita K, Okazaki T, et al. Feasibility and safety of intravascular temperature management for severe heat stroke. Crit Care Med 2018; 46: e670-6. https://doi.org/10.1097/CCM.0000000000003153 PMid:29624537 |
||||
| 12.Liu SY, Song JC, Mao HD, Zhao JB, Song Q. Expert consensus on the diagnosis and treatment of heat stroke in China. Mil Med Res 2020; 7: 1. https://doi.org/10.1186/s40779-019-0229-2 PMid:31928528 PMCid:PMC6956553 |
||||
| 13. Frisvold S, Coppola S, Ehrmann S, et al. Respiratory challenges and ventilatory management in different types of acute brain-injured patients. Crit Care 2023; 27: 247. https://doi.org/10.1186/s13054-023-04532-4 PMid:37353832 PMCid:PMC10290317 |
||||
| 14.Ziaka M, Exadaktylos A. Brain-lung interactions and mechanical ventilation in patients with isolated brain injury. Crit Care 2021; 25: 1-10. https://doi.org/10.1186/s13054-021-03778-0 PMid:34645485 PMCid:PMC8512596 |
||||
| 15.Robba C, Poole D, McNett M, Asehnoune K, Bösel J, Bruder N, et al. Mechanical ventilation in patients with acute brain injury: recommendations of the European Society of Intensive Care Medicine consensus. Intensive Care Med 2020; 46: 2397-410. https://doi.org/10.1007/s00134-020-06283-0 PMid:33175276 PMCid:PMC7655906 |
||||
| 16. Snarska K, Kapica-Topczewska K, Bachórzewska-Gajewska H, Małyszko J. Renal function predicts outcomes in patients with ischaemic stroke and haemorrhagic stroke. Kidney Blood Press Res 2016; 41: 424-33. https://doi.org/10.1159/000443444 PMid:27467276 |
||||
| 17.Mi D, Wang P, Yang B, Pu Y, Yang Z, Liu L. Correlation of hyperglycemia with mortality after acute ischemic stroke. Ther Adv Neurol Disord 2017; 11: 1756285617731686. https://doi.org/10.1177/1756285617731686 PMid:29399044 PMCid:PMC5784549 |
||||
| 18.Donkel SJ, Benaddi B, Dippel DWJ, Ten Cate H, de Maat MPM. Prognostic hemostasis biomarkers in acute ischemic stroke. Arterioscler Thromb Vasc Biol 2019; 39: 360-72. https://doi.org/10.1161/ATVBAHA.118.312102 PMid:30700129 PMCid:PMC6392207 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

