Ejection fraction improvement in left ventricular-only pacing vs. BiVentricular pacing in patients with heart failure
ORIGINAL RESEARCH ARTICLE
Ejection fraction improvement in left ventricular-only pacing vs. BiVentricular pacing in patients with heart failure
Article Summary
- DOI: 10.24969/hvt.2024.465
- Page(s): 191-199
- CARDIOVASCULAR DISEASES
- Published: 27/02/2024
- Received: 27/01/2024
- Revised: 17/02/2024
- Accepted: 17/02/2024
- Views: 4512
- Downloads: 3317
- Keywords: BiVentricular pacing, cardiac resynchronization therapy, heart failure, left ventricular-only pacing
Address for Correspondence: Bernard Abi-Saleh, Section of Electrophysiology and Pacing, Division of Cardiology,
American University of Beirut Medical center, Beirut, Lebanon
Email: Ba47@aub.edu.lb
ORCID: Randa Tabbah - 0000-0003-2987-9568
X Twitter: Randa Tabbah: @22tabbah_randa Bernard Abi-Saleh: @AbiSalehBernard
Facebook: Randa Tabbah: randa.tabbah Bernard Abi-Saleh: bernard.abisaleh
Randa Tabbah1,2,3, Jamil Francis4, Hadi Skouri5, Maurice Khoury5, Bernard Abi-Saleh5,6
1Division of Cardiology, Holy Spirit of Kaslik University, Notre Dame de Secours University Hospital, Beirut, Lebanon
2Division of Cardiology, St John University Hospital, Lebanese American University, Beirut, Lebanon
3Division of Cardiology, Balamand University, Notre Dame du Liban University Hospital, Beirut, Lebanon
4Division of Internal Medicine, the American University of Beirut Medical Center, Beirut, Lebanon
5Division of Cardiology, the American University of Beirut Medical Center, Beirut, Lebanon
6Department of Cardiovascular Medicine, Section of Electrophysiology and Pacing, Cleveland Clinic, Cleveland. OH, USA
Abstract
Objective: Left ventricular (LV) pacing with resynchronization therapy improves ventricular synchrony in patients with decreased LV function and Left Bundle Branch Bock (LBBB). Ventricular activation in these cases may be obtained by recruiting the intrinsic atrioventricular (AV) conduction, over the right bundle branch that causes a multisite activation and a synchronized activity of the right ventricle and part of the septum. Fusion pacing between intrinsic AV conduction and LV capture initiates right ventricular (RV) activation and compensates for LV electrical delay.
The goal of this study is to show that LV-only pacing is superior to BiVentricular (BiV) pacing in patients with LV systolic dysfunction and LBBB.
Methods: This is a retrospective study of 2 different hospitals' registries in Lebanon. 121 consecutive patients were identified between January 2014 and December 2019. Patients with left ventricular ejection fraction (LVEF) ≤ 35%, a QRS interval ≥130 msec, and an LBBB pattern on full medical therapy were included in this study and divided in 2 groups: LV pacing and BiV pacing. All patients had echocardiograms before and 3 to 6 months post-device implantation. The primary endpoint was the change in ejection fraction, and the secondary endpoints were a decrease in systolic pulmonary artery pressure (SPAP), a decrease in LV end-diastolic diameter (LVEDD), and a decrease in LV end-systolic diameter (LVESD). Statistical analysis was done with SPSS software, and a p-value <0.05 was considered significant.
Results: The study population was mostly males (69.4%) (mean LVEF of 26.5%, mean age of 67 years old), with 74 (61.2%) ischemic cardiomyopathy patients and 47 (38.8%) non-ischemic cardiomyopathy patients. Fifty (41.3%) patients were programmed as LV-only pacing. A statistically significant difference in improvement in ejection fraction (EF) was seen between the LV-only pacing arm (9.2%) and the BiV pacing arm (5.5%) (p=0.043, 95% CI (0.12-7.11)). Similarly, we noticed a significant decrease in the LVEDD (p=0.007, 95% CI (0.15-1.4)) and LVESD (p=0.03, 95% CI (0.13-1.8)) in the LV pacing compared to the BiV pacing group. There was a trend in favor of more SPAP improvement in the LV pacing group that did not reach statistical significance.
Conclusion: This study demonstrates that LV-only pacing mode significantly improves EF and LV size compared to BiV pacing mode.
Key words: BiVentricular pacing, cardiac resynchronization therapy, heart failure, left ventricular-only pacing
Introduction
Ventricular dyssynchrony is a frequently observed feature in patients with left ventricular (LV) failure. Delays in ventricular conduction produce suboptimal filling, a decrease in ventricular contractility, prolonged duration of mitral regurgitation (diastolic MR), and a paradoxical septal motion (1-3).
LV pacing with resynchronization therapy improves ventricular synchrony in patients with left bundle branch block (LBBB) and poor LV function (2). Correcting electrical delay in the LV with a coronary sinus (CS) lead placement is especially important, knowing that the right ventricular (RV) electrical activity may be normal in these cases. In BiVentricular (BiV) pacing, RV capture can cause RV dyssynchrony with a prolonged electrical activation. However, in LV pacing, impulses through the right bundle branch (RBB) to the Purkinje fibers activate multiple RV sites and maintain RV synchrony “multisite activation” (5-7). With isolated left univentricular pacing, fusion pacing between intrinsic atrioventricular (AV) conduction and LV capture initiates RV activation with or without pacing and, at the same time, compensates for LV electrical delay. In addition, decreasing RV pacing increases the longevity of the device and improves the current drain, an advantage of LV pacing (8-10). Moreover, studies on LV pacing mechanisms showed that a pre-stretching of the RV-free wall and interventricular septum promote hypercontractility of both balanced by an LV-free wall hypo-contractility leading to a better RV contractility that appeared to enhance cardiac output. Improvement in the output of the RV will sequentially increase the LV output. However, the main issue is the variability of AV delay to provide an optimal fusion due to medications, disease status, and daily activity (8,11).
This study aims to demonstrate that isolated LV pacing is as safe and better than BiV pacing with a superior improvement in echocardiographic parameters.
Methods
Study design and population
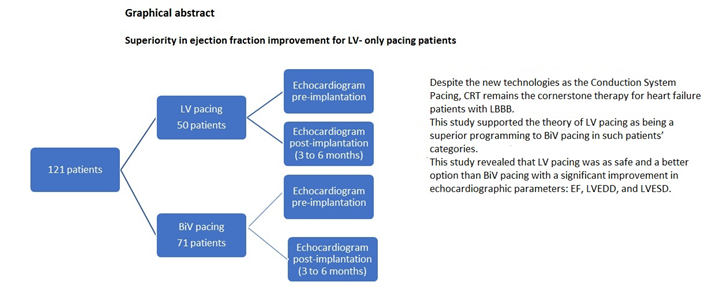
This is a 5-year retrospective observational multicenter study (Fig.1). It has been designed to test the hypothesis that LV pacing is as safe and more effective than BiV pacing. The primary composite endpoint included a change in LVEF after the cardiac resynchronization therapy (CRT) implantation.
Patients were recruited from two different university hospitals between January 2014 and December 2019 and patient files were studied carefully after approval of the Ethic committee. Overall 121 patients with left ventricular ejection fraction (LVEF) ≤ 35%, a QRS interval ≥130 msec, and an LBBB pattern on full medical therapy (Table 1) were included and divided into two groups: LV pacing (50 patients)and BiV pacing (71 patients).
Patients with complete heart block and permanent atrial fibrillation were excluded from this study (Table 1). All local institutional review boards approved the study, and enrolled patients consented to the study.
|
Table 1. Inclusion and exclusion criteria |
|
Inclusion criteria: |
|
LVEF ≤ 35% |
|
QRS ≥130ms |
|
LBBB |
|
Patient on full guideline-directed medical therapy (GMDT) |
|
Exclusion criteria: |
|
Complete heart block |
|
Permanent atrial fibrillation |
|
LBBB – left bundle branch block, LVEF- left ventricular ejection fraction |
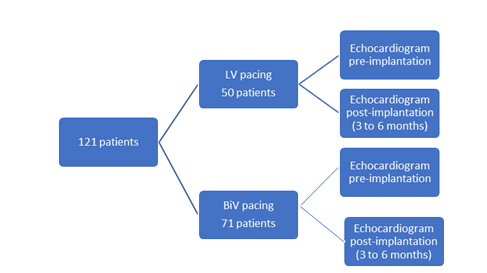
Figure 1. Study design
Baseline variables
Baseline variables studied were: age, sex, type of cardiomyopathy (ischemic or non-ischemic) and device programming (LV vs BiV pacing) (the latter is also a predictor variable).
Outcome variables
Primary endpoint
The primary endpoint was a change in LVEF after the device implantation after 3 months.
Secondary endpoint
The secondary endpoints included mitral regurgitation improvement, a decrease in left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD) and left atrial dimension, and an improvement in hemodynamics with a decrease in systolic pulmonary pressures (SPAP) and amelioration in left ventricular end-diastolic pressures (LVEDP).
Echocardiography
Echocardiography was performed before and 3 to 6 months after cardiac resynchronization therapy-defibrillator (CRT-D) implantation. Echocardiographic parameters were studied including LVEF, LVEDD, LVESD, SPAP, mitral regurgitation (MR), LVEDP, and left atrial volume index (LAVI).These parameters were performed with high technology machines and experts in the field following the guidelines. Responders were defined as defined as of >5 points increase in LVEF.
Mean interval for echocardiographic evaluation between pre and post implantation of the device in both group was 4 months. All parameters noted in this study were measured by cardiac ultrasound with respect to the European and American guidelines for echocardiography and done by certified echocardiographers.
Measurements were performed according to the European Association of Cardiovascular Imaging (EACVI) (12) and the American Society of Echocardiography (ASE) recommendations (13).
CRT-D implantation
All patients included in this study underwent implantation of CRT-D devices with right atrial, RV and LV leads.
The latter is placed transvenously in a posterior, lateral, or posterolateral branch of the coronary sinus. All Boston Scientific devices were programmed to LV-only mode with AV delays programmed according to the SmartDelayTM optimization recommendation with optimal parameters for an optimal CRT response.
Other devices were programmed to BiV pacing with AV delays programmed as per each company’s recommendation.
Follow -up
All patients had echocardiograms before implantation and 3 to 6 months after the procedure (Fig.1).
Statistical analysis
The statistical analysis was done with SPSS software (IBM, New York, USA) for the evaluation of the hypothesis. Data are presented as mean (SD) and number (%). T-test was used for the comparison of the continuous variables. A p value <0.05 was accepted as statistically significant.
Results
Clinical and pacing characteristics (Table 2)
One hundred twenty-one patients were enrolled in this study. The majority of patients studied were in class II-III NYHA (New York Heart association) classification before implantation. The study population included mostly males (69.4%) with a mean age of 67 years old, mainly with ischemic cardiomyopathy (74, 61.2%) and 47 (38.8%) patients with dilated cardiomyopathy. Fifty (41.3%) patients had devices programmed as LV pacing and the rest (71, 58.7%) as BiV pacing. The enrolled patients had a baseline mean LVEF of 26.5% with a mean QRS complex of 156 ms, typically with LBBB morphology on full medical therapy.
The rate of response was better with LV pacing than BiV pacing: 70% were responders with LV pacing whereas 55% responders were in the second arm. The non-ischemic cardiomyopathy patients responded better to the CRT-D than ischemic (75% vs 45%) and they were separated homogenously in both groups.
|
Table 2. Clinical and pacing characteristics (descriptive data of 121 patients) |
||
|
Variables |
n |
% |
|
Age, years (mean) |
67 |
- |
|
Sex |
|
|
|
Male |
84 |
69.4 |
|
Female |
37 |
30.6 |
|
Cardiomyopathy |
||
|
Ischemic |
74 |
61.2 |
|
Dilated |
47 |
38.8 |
|
Device Programming |
||
|
LV pacing |
50 |
41.3 |
|
BiV pacing |
71 |
58.7 |
|
BiV-biventricular, LV-left ventriclar, N-number of patients |
||
Echocardiographic data
The baseline echocardiographic parameters in this population were as follows: a mean LVEF of 26.5% with an LVEDD of 61.4 mm and an LVESD of 49.5mm. The hemodynamic pre-implantation findings suggested a mean SPAP of 47mmHg with 63% of patients with high LVEDP.
All patients had a dilated left atrium, and 54.3% of them had mild MR, 41.3 % had moderate MR, and 4,4% had severe MR.
Three to six months of CRT pacing led to a reduction in LVEDD (57.9 mm) and LVESD (47.47 mm) and an improvement in LVEF (34.1%) in both arms
Improvement was noticed in parameters post-implantation in both programming options: a decrease in SPAP with a mean of 41.7 mmHg, 51% of patients had normal LVEDP, 45.5% had a decrease in LAVi post-implantation and 67% of patients had mild MR, 30.8% with a moderate MR and 2.2% with severe MR(Table 3).
|
Table 2. Clinical and pacing characteristics (descriptive data of 121 patients) |
||
|
Variables |
n |
% |
|
Age, years (mean) |
67 |
- |
|
Sex |
|
|
|
Male |
84 |
69.4 |
|
Female |
37 |
30.6 |
|
Cardiomyopathy |
||
|
Ischemic |
74 |
61.2 |
|
Dilated |
47 |
38.8 |
|
Device Programming |
||
|
LV pacing |
50 |
41.3 |
|
BiV pacing |
71 |
58.7 |
|
BiV-biventricular, LV-left ventriclar, N-number of patients |
||
|
Table 3. Echocardiographic descriptive data of 121 patients before and after CRT-D implantation |
||
|
Variables |
Pre-CRT-D |
Post-CRT-D |
|
LVEF, % |
26.5 |
34.1 |
|
LVEDD, mm |
61.4 |
57.9 |
|
LVESD, mm |
49.5 |
47.5 |
|
SPAP, mmHg |
47.0 |
41.7 |
|
LVEDP, % |
||
|
Normal |
37 |
51 |
|
High |
63 |
48.8 |
|
Mitral regurgitation, % |
||
|
Mild |
54.3 |
67 |
|
Moderate |
41.3% |
30.8 |
|
Severe |
4.4% |
2.2 |
|
LAVI |
All dilated |
45.5% decrease in indexed volume |
|
BiV – biventricular, CRT-D –cardiac resynchronization therapy –defibrillator, LAVI - left atrial volume index , LVEDD -left ventricular end-diastolic diameter, LV - left ventricular, LVEF – left ventricular ejection fraction, LVEDP - left ventricular end-diastolic pressure, LVESD - left ventricular end-systolic diameter, SPAP - systolic pulmonary pressure |
||
Effect of LV and BiV pacing on echocardiographic data (Table 4)
Primary endpoint
In the LV pacing arm (Group 1) the mean LVEF pre-implantation was 26.9% vs 36.1% after implantation. On the other hand, for the BiV pacing (Group 2) the mean LVEF pre-implantation was 26.3% vs 31.8% after implantation. Data analysis showed a significant improvement in LVEF in the LV arm (by 9.2%) when comparing to BiV group (5.5%) (p=0.043, 95% CI (0.12-7.11)).
Secondary endpoints
Moreover, the mean baseline LVEDD was 63.9mm with a reduction of the diastolic diameter post-device implantation with a mean of 57.9 mm for Group 1. For Group 2, a mean baseline LVEDD of 60.4 mm was noticed and after the post-device implantation - 59.2 mm. A significant decrease by 6 mm in the LVEDD was noticed in the LV group with a significant p value of 0,007 and a 95% CI (0.15-1.4), while in BiV group there was no reduction. Furthermore, initial LVESD was 51.8 mm vs 43.3 mm after the procedure for the LV arm. On the other hand, LVESD did not change in BiV arm. A significant decrease in the LVESD was demonstrated in the LV pacing arm of 8.5 mm with a p value of 0.03 and a 95% CI (0.13-1.8). SPAP decreased from 45.4 mmHg at baseline to 36.4 mmHg during follow-up for Group 1. Whereas for Group 2, the baseline SPAP was 47.9 mmHg, and months after - 45.9 mmHg. Improvement in SPAP was noticed in both groups, though not significant, with a larger trend for the LV group as compared to BiV group (decrease by 9 mmHg vs 2mmHg, respectively, p=0.134) ( Table 4).
|
Table 4. Echocardiographic data on superiority of LV pacing vs. BiV pacing |
|||||||
|
Variables |
Pre-CRTD |
Post-CRTD |
Change* |
p |
|||
|
LV pacing |
BiV pacing |
LV pacing |
BiV pacing |
LV pacing |
BiV pacing |
||
|
LVEF, % |
26.9 |
26.3 |
36.1 |
31.8 |
9.18 (9.95) |
5.56 (9.28) |
0.043 |
|
LVEDD, mm |
63.9 |
60.4 |
57.9 |
59.2 |
-6.12 (9.37) |
0.08 (6.98) |
0.007 |
|
LVESD, mm |
51.8 |
48.7 |
43.3 |
48.7 |
08.45 (12.6) |
-0.13 (9.53) |
0.03 |
|
SPAP, mmHg |
45.4 |
47.9 |
36.4 |
45.9 |
- |
- |
0.134 |
|
Data are presented as mean, * - data are presented as mean (SD) BiV – biventricular, CRT-D –cardiac resynchronization therapy –defibrillator, LVEDD -left ventricular end-diastolic diameter, LV - left ventricular, LVEF – left ventricular ejection fraction, LVEDP - left ventricular end-diastolic pressure, LVESD - left ventricular end-systolic diameter, SPAP - systolic pulmonary pressure |
|||||||
Discussion
In BiV pacing, RV capture can cause RV dyssynchrony with prolonged electrical activation. However, in LV pacing, impulses through the RBB to the Purkinjean fibers activate multiple RV sites and maintain RV synchrony "multisite activation"(5, 8, 11).
The very first studies showed that BiV pacing and LV pacing were similar with a trend for LV pacing due to optimal AV delay that provides fusion between the intrinsic RV conduction via a preserved right bundle branch and LV pacing. Several other studies suggested that even when fusion is not reached LV pacing has benefits similar to BiV pacing as was shown and confirmed in this study (14, 15).
Some studies elaborated a comparison between BiV and LV pacing (Table 5). Most notably, BELIEVE (The Bi vs Left Ventricular Pacing: an International Pilot Evaluation on Heart Failure Patients with Ventricular Arrhythmias), conducted in 2006, contains inclusion criteria similar to our study, including New York Heart Association (NYHA) II-IV, LBBB, sinus rhythm, QRS interval>130ms, EF≤35%, LVEDD≥55mm, and a follow up for 12 months). The randomized single-blind study demonstrated an increase in EF of 5.2% in the LV group (9.2% in this study vs 5.5% in the BiV group) with a comparable safety profile with BiV pacing (16). In a pilot study, LOLA ROSE (Cardiac resynchronization therapy: left or left-and-right for optimal symptomatic effect), with 18 patients differentiating the two types of pacing showed no difference in peak VO2, 6 minutes walking distance (6MWD), quality of life (QoL) but a better NYHA in patients with BiV pacing (17). In addition, the DECREASE-HF (the Device Evaluation of CONTAK RENEWAL 2 and EASYTRAK 2: Assessment of Safety and Effectiveness in Heart Failure) trial in 2007 studied 306 patients and divided them into 3 groups: simultaneous BiV, sequential BiV, and LV pacing. It showed during a follow-up of 6 months, similar improvement in LVEDD, left ventricular end-diastolic volume (LVEDV), LVESD, stroke volume (SV), cardiac output (CO), and EF in all groups with a greater decrease in LVESD with simultaneous BiV pacing (18). In 2010, 2 different studies compared BiV and LV pacing. B-LEFT-HF (The Biventricular versus Left Univentricular Pacing with ICD Back-up in Heart Failure Patients) trial, a prospective study involving 176 patients, demonstrated no difference in the primary endpoint between both groups but demonstrated a trend toward a better hemodynamic outcome and EF improvement with the LV pacing arm that seemed to be a plausible alternative to BiV pacing [19). On the other hand, Sedlacek et al. (20) showed a trend in patients with BiV pacing.
Furthermore, a study in 2011, GREATER EARTH (The Greater Evaluation of Resynchronization Therapy for Heart Failure), involving 121 patients, noticed an increase of more than 50% in exercise capacity in both groups with similar improvement in EF, left ventricular end-systolic volume (LVESV), NYHA, 6-minute walk test and a similar incidence of adverse effects (21).
Moreover, in 2011, Thibault et al., in a multicenter trial comparing the effect of LV and BiV pacing in 211 patients, revealed that LV pacing was not superior to BiV pacing but non-responders to BiV may respond to LV pacing (22). In addition, this pacing strategy decreases costs by decreasing the current drain and reduces implantation time and radiation exposure. A trial comparing the hemodynamic effect of BiV pacing vs LV pacing in patients that were first in BiV pacing mode and then all set to LV pacing and assessed their echocardiographic findings. It showed a non-inferiority of LV pacing to BiV pacing with a similar hemodynamic response. Overall, 21% who were non-responders to BiV pacing responded better to LV pacing which is why it is logical that in our study LV pacing patients performed better than BiV (23)(Table 5).
Several benefits from LV pacing were noticed. The transition from BiV to LV pacing increased the longevity of the device with a decrease in costs, avoiding repeated procedures when RV leads were displaced or had high thresholds. In our study, we observed a better improvement in EF and hemodynamics in the LV pacing group vs the traditional biventricular pacing. This led us to think if it is appropriate to program patients to LV pacing in most cases. Further large and prospective studies need to be conducted to clear these issues.
|
Table 5. Summary of the studies comparing LV pacing vs BiV pacing |
||
|
Study |
Study population |
Result |
|
BELIEVE 200616 |
74 patients |
Increase in EF of 5,2% in the LV group with a comparable safety profile with BiV pacing |
|
LOLA ROSE 200717 |
18 patients |
No difference in peak Vo2, 6 minutes walking distance (6MWD), quality of life (QOL) but a better NYHA in patients with BiV pacing |
|
DECREASE-HF 200718 |
306 patients |
Similar improvement in LVEDD, Left ventricular end-diastolic volume (LVEDV), LVESD, stroke volume (SV), cardiac output (CO), and EF in all groups with a greater decrease in LVESD with simultaneous BiV pacing |
|
B-LEFT-HF 201019 |
176 patients |
Demonstrated a trend toward a better hemodynamic outcome and EF improvement with the LV pacing arm |
|
Sedlacek et al 201020 |
40 patients |
A Trend in patients with BiV pacing |
|
GREATER EARTH 201121 |
121 patients |
Increase of more than 50% in exercise capacity in both groups with similar improvement in EF |
|
Thibault et al 201122 |
211 patients |
LV pacing was not superior to BiV pacing but non-responders to BiV may respond to LV pacing |
|
Faghfourian et al 201723 |
44 patients |
Showed a non-inferiority of LV pacing to BiV pacing with a similar hemodynamic response |
|
BiV- biventricular, EF - ejection fraction, LV – left ventricular, LVEDD -left ventricular end-diastolic diameter, LV - left ventricular, LVESD - left ventricular end-systolic diameter |
||
In the end, CRT still has an important place despite the new upcoming technologies and studies on the conduction system pacing (CSP) that needs more studies to prove the safety of these new leads. For the time being CSP could be used as a bailout when there is no optimal lead positioning or difficulty to position a coronary sinus lead. So, programming CRTD in an optimal way is a good option for these patient’s category (24).
Study limitations
This is a retrospective study comparing both LV and BiV pacing. The sample is rather small. The study used mainly a specific algorithm used in only one manufactured company. Further investigations are needed to shed light on these findings with a prospective study involving a bigger sample with several algorithms from different manufacturers.
Conclusion
This study revealed that LV pacing was as safe and a better option than BiV pacing with a significant improvement in echocardiographic parameters: EF, LVEDD, LVESD. Further studies with larger population are needed to shed light on the possibility of programming most devices on an LV-only pacing mode.
Ethics: All local institutional review boards approved the study and enrolled patients consented to the study.
Peer-review: External and Internal
Conflict of interest: The authors declare that there is no conflict of interest
Authorship: R.T., J.F., H., M., and B.A-S. equally contributed to the study and manuscript preparation. All authors have been personally and actively involved in substantial work leading to the paper and will take public responsibility for its content. All authors fulfilled authorship criteria
Acknowledgement and Funding: The authors have not received any funding for this study.
References
| 1.Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl JMed 2010; 363: 2385-95. https://doi.org/10.1056/NEJMoa1009540 PMid:21073365 |
||||
| 2.Abraham WT. Rationale and design of a randomized clinical trial to assess the safety and efficacy of cardiac resynchronization therapy in patients with advanced heart failure: The Multicenter in Sync Randomized Clinical Evaluation (MIRACLE). J Card Fail 2000; 6: 369-80. https://doi.org/10.1054/jcaf.2000.20841 PMid:11145762 |
||||
| 3. Iuliano S, Fisher SG, Karasik PE, Fletcher RD, Singh SN. QRS duration and mortality in patients with congestive heart failure. Am Heart J 2002; 143: 1085-91. https://doi.org/10.1067/mhj.2002.122516 PMid:12075267 |
||||
| 4.Leclercq C, Faris O, Tunin R, Johnson J, Kato R, Evans F, et al. Systolic improvement and mechanical resynchronization do not require electrical synchrony in the dilated failing heart with the left bundle branch block. Circulation 2002; 106: 1760-3. https://doi.org/10.1161/01.CIR.0000035037.11968.5C PMid:12356626 |
||||
| 5. Sade LE, Demir O, Atar I, Muderrisoglu H, Ozin B. Effect of right ventricular pacing lead on left ventricular dyssynchrony in patients receiving cardiac resynchronization therapy. Am J Cardiol 2009; 103: 6957. https://doi.org/10.1016/j.amjcard.2008.11.027 PMid:19231336 |
||||
| 6. Valzania C, Rocchi G, Biffi M, Martignani G, Bertini M, Diemberger I, et al. Left ventricular versus biventricular pacing: a randomized comparative study evaluating mid-term electromechanical and clinical effects. Echocardiography 2008; 25: 141-8. https://doi.org/10.1111/j.1540-8175.2007.00576.x PMid:18269558 |
||||
| 7. Eschalier R, Ploux S, Lumens J, Whinnett Z, Varma N, Meillet V, et al. Detailed analysis of ventricular activation sequences during right ventricular apical pacing and left bundle branch block and the potential implications for cardiac resynchronization therapy. Heart Rhythm 2015; 12: 137-43. https://doi.org/10.1016/j.hrthm.2014.09.059 PMid:25285646 |
||||
| 8. Verbeek XAAM, Auricchio A, Yu Y, Ding J, Pochet T, Vernooy K, et al. Tailoring cardiac resynchronization therapy using interventricular asynchrony. Validation of a simple model. Am J Physiol 2006; 290: H968-77. https://doi.org/10.1152/ajpheart.00641.2005 PMid:16172160 |
||||
| 9. Vatasescu R, Berruezo A, Mont L, Tamborero D, Sitges M, Silva E, et al. Midterm 'super-response' to cardiac resynchronization therapy by biventricular pacing with fusion: insights from electro-anatomical mapping. Europace 2009; 11: 1675-82. https://doi.org/10.1093/europace/eup333 PMid:19880850 PMCid:PMC2780924 |
||||
| 10. Arbelo E , Tolosana JM, Trucco E, Penela D, Borràs R, Doltra A, et al. Fusion-optimized intervals (FOI): a new method to achieve the narrowest QRS for optimization of the AV and VV intervals in patients undergoing cardiac resynchronization therapy. J Cardiovasc Electrophysiol 2014; 25: 283-92. https://doi.org/10.1111/jce.12322 PMid:24237881 |
||||
| 11. Burri H, Prinzen FW, Gasparini M, Leclercq C. Left univentricular pacing for cardiac resynchronization therapy. Europace2017; 19: 912-9. doi: 10.1093/europace/euw179 https://doi.org/10.1093/europace/euw179 PMid:28339579 |
||||
| 12. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Emande L, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J – Cardiovasc Imag 205; 16: 233–71. Doi:1 0.1093/ehjci/jev014 | ||||
| 13. Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2019; 32: 1-64. doi: 10.1016/j.echo.2018.06.004 https://doi.org/10.1016/j.echo.2018.06.004 PMid:30282592 |
||||
| 14. Blanc JJ, Bertault-Valls V, Fatemi M, Gilard M, Pennec PY, Etienne Y. Midterm benefits of left univentricular pacing in patients with congestive heart failure. Circulation 2004; 109: 1741-4. https://doi.org/10.1161/01.CIR.0000124479.89015.64 PMid:15023885 |
||||
| 15. Gold MR, Niazi I, Giudici M, Leman RB, Sturdivant L, Kim MH, et al. A prospective randomized comparison of the acute hemodynamic effects of biventricular and left ventricular pacing with cardiac resynchronization therapy. Heart Rhythm 2011; 8: 685-91. https://doi.org/10.1016/j.hrthm.2010.12.039 PMid:21193063 |
||||
| 16. Gasparini M, Bocchiardo M, Lunati M, Ravazzi PA, Santini M, Zardini M, et al. Comparison of 1-year effects of left ventricular and biventricular pacing in patients with heart failure who have ventricular arrhythmias and left bundle-branch block: The Bi vs Left Ventricular Pacing: an International Pilot Evaluation on Heart Failure Patients with Ventricular Arrhythmias (BELIEVE) multicenter prospective randomized pilot study. Am Heart J 2006; 152: e1-7. https://doi.org/10.1016/j.ahj.2006.04.004 PMid:16824846 |
||||
| 17. Sirker A, Thomas M, Baker S, Shrimpton J, Jewell S, Lee L, et al. Cardiac resynchronization therapy: left or left-and-right for optimal symptomatic effect-the LOLA ROSE study. Europace 2007; 9: 862-8. https://doi.org/10.1093/europace/eum161 PMid:17684066 |
||||
| 18.Rao RK, Kumar UN, Schafer J, Viloria E, De Lurgio D, Foster E. Reduced ventricular volumes and improved systolic function with cardiac resynchronization therapy: a randomized trial comparing simultaneous biventricular pacing, sequential biventricular pacing, and left ventricular pacing. Circulation. 2007; 115: 2136-44. https://doi.org/10.1161/CIRCULATIONAHA.106.634444 PMid:17420340 |
||||
| 19. Boriani G, Kranig W, Donal E, Calo L, Casella M, Delarche N, Fernandez Lozano I, et al. A randomized double-blind comparison of biventricular versus left ventricular stimulation for cardiac resynchronization therapy: The Biventricular versus Left Univentricular Pacing with ICD Back-up in Heart Failure Patients (B-LEFT HF) trial. Am Heart J 2010; 159: 1052-8 https://doi.org/10.1016/j.ahj.2010.03.008 PMid:20569719 |
||||
| 20. Sedlacek K, Burianova L, Mlcochova H, Peichl P, Marek T, Kautzner J. Isolated left ventricular pacing results in worse long-term clinical outcome when compared with biventricular pacing: a single-center randomized study. Europace 2010; 12: 1762-8. https://doi.org/10.1093/europace/euq307 PMid:20729533 |
||||
| 21.Thibault B, Harel F, Ducharme A, White M, Frasure-Smith N, Roy D, t al. Evaluation of resynchronization therapy for heart failure in patients with a QRS duration greater than 120 ms (GREATER-EARTH) trial: rationale, design, and baseline characteristics. Can J Cardiol 2011; 27: 779-86. https://doi.org/10.1016/j.cjca.2011.03.010 PMid:21791363 |
||||
| 22. Thibault B, Ducharme A, Harel F, White M, O'Meara E, Guertin MC, et al. Evaluation of Resynchronization Therapy for Heart Failure (GREATER-EARTH) Investigators Left ventricular versus simultaneous biventricular pacing in patients with heart failure and a QRS complex ≥120 milliseconds / clinical perspective. Circulation 2011; 124: 2874-81. https://doi.org/10.1161/CIRCULATIONAHA.111.032904 PMid:22104549 |
||||
| 23. Faghfourian B, Homayoonfar S, Rezvanjoo M, Poorolajal J, Emam AH. Comparison of hemodynamic effects of biventricular versus left ventricular only pacing in patients receiving cardiac resynchronization therapy: A before-after clinical trial. J Arrhythm 2017; 33: 127-9. doi: 10.1016/j.joa.2016.07.014 https://doi.org/10.1016/j.joa.2016.07.014 PMid:28416979 PMCid:PMC5388040 |
||||
| 24. Indik JH. Introducing the 2023 HRS/APHRS/LAHRS guideline on cardiac physiologic pacing for the avoidance and mitigation of heart failure: Are we entering a new age in pacing? Heart Rhythm O2 2023; 4: 523-5. doi: 10.1016/j.hroo.2023.08.002 https://doi.org/10.1016/j.hroo.2023.08.002 PMid:37744941 PMCid:PMC10513917 |
||||
Copyright

This work is licensed under a No Licensing Information.
AUTHOR'S CORNER

