Expert opinion on conduction system pacing implantation: A new approach to cardiac pacing
EDITORIALS
Expert opinion on conduction system pacing implantation: A new approach to cardiac pacing
Article Summary
- DOI: 10.24969/hvt.2024.466
- CARDIOVASCULAR DISEASES
- Published: 04/03/2024
- Received: 23/02/2024
- Accepted: 24/02/2024
- Views: 6198
- Downloads: 3680
- Keywords: pacing, His bundle pacing, left bundle branch area pacing, conduction system pacing, guidelines
Address for Correspondence: Narendra Kumar, HeartbeatsZ Academy, United Kingdom
Email: naren.cep@gmail.com
ORCID: 0000-0002-4197-5133 Scopus: 55969944400
Narendra Kumar
HeartbeatsZ Academy, United Kingdom
Abstract
The document titled "Expert Opinion on Conduction System Pacing Implantation" provides expert insights and guidelines for the implementation of conduction system pacing (CSP). CSP offers a more physiologically sound alternative to conventional right ventricular pacing, and it is increasingly utilized not only for pacing but also for cardiac resynchronization therapy.
In this article, we aim to encapsulate the EHRA Clinical Consensus Statement on CSP implantation, offering healthcare practitioners a standardized procedure while underscoring key recommendations.
Key words: pacing, His bundle pacing, left bundle branch area pacing, conduction system pacing, guidelines
Introduction
In recent times, conduction system pacing (CSP) has emerged as a promising alternative to traditional right ventricular pacing. Advancements in tools and techniques have boosted CSP's popularity, expanding its use from mere pacing to cardiac resynchronization therapy. This editorial seeks to summarize the EHRA Clinical Consensus Statement on CSP implantation (1), equipping healthcare professionals with a uniform approach and emphasizing critical guidelines.
CSP comprises various pacing techniques, primarily His bundle pacing (HBP) and left bundle branch area pacing (LBBAP). HBP, introduced more than two decades ago, has gained attraction due to enhanced implantation tools. LBBA pacing, a newer development, provides a broader target area and excellent electrical parameters. However, the precise technique is imperative to ensure the safe and effective administration of therapy.
Key Recommendations and Implications (Table 1, 2)
1. General Considerations for CSP Implantation:
- Essential 12-lead ECG recording during implantation, ideally with an electrophysiology recording system for concurrent endocardial and ECG signals.
- Display of endocavitary electrograms with minimal signal filtration, showcasing current of injury.
- In cases where an EP recording system is unavailable, employing a 12-lead ECG with a pacing system analyzer for mapping endocardial signals.
- CSP implantation must not proceed without displaying a minimum set of ECG leads, including I, II, III, V1, and V5 or V6.
|
Table 1. Summary of main techniques for His bundle pacing and left bundle branch area pacing during implantation |
||
|
Technique |
His Bundle Pacing |
Left Bundle Branch Area Pacing |
|
Mapping |
-Unipolar mapping to locate sharp His potential, -Pacemap if His potential not seen |
-Unipolar mapping to locate His bundle/tricuspid annulus summit -Contrast injection to delineate anatomy -Pacemap for discordant paced QRS in leads II and III |
|
Positioning |
-Position lead slightly above or at His bundle site -Rotate lead to elicit His injury potential |
-Insertion 15-35 mm from His at -10° to +30° towards apex -Penetrate septum at 10-40° angle superiorly in LAO view |
|
Monitoring |
-His potential morphology -Unipolar impedance -Lead stability |
-Fluoroscopy for lead progression -Unipolar paced QRS morphology -Impedance, template beats, injury potential |
|
Thresholds |
-Acceptable: <2.5 V @ 0.5 ms -Ideal: ≤1.5 V @ 0.5 ms |
-Acceptable: <1.5 V @ 0.5 ms -Ideal: < 1 V @ 0.5 ms |
|
LAO – left anterior oblique view The table summarizes the main techniques used for His bundle pacing and left bundle branch area pacing during implantation. It provides guidance on mapping, positioning, monitoring, and threshold values for successful implementation of each pacing technique. |
||
2. His Bundle Pacing:
- Mandatory confirmation of His bundle capture during implantation and follow-up, employing validated criteria such as changes in QRS morphology with decreasing output/programmed stimulation and comparing intervals from His potential and pacing stimulus to V6 R-wave peak.
- Continued screwing of the lead until significant torque buildup is felt, ensuring lead stability.
- Advisable routine assessment of lead stability.
- In instances of infranodal conduction delay or block, testing pacing at a cycle length of 400 ms or shorter, demonstrating 1:1 conduction without aberration.
- Persistence in lead rotations until the observation of a His current of injury or deep negative morphology, predictive of favorable electrical parameters.
- Unipolar His capture thresholds should be < 2.5 V / 0.5ms, aiming for ≤ 1.5V / 0.5ms. Bipolar sensing amplitude should exceed 2 mV (without atrial/His oversensing).
- In specific scenarios such as poor sensing, pacemaker-dependency, high-grade aitrioventricular (av) block, infranodal block, high pacing threshold, and planned AV junction ablation, a backup ventricular lead can enhance safety.
3. Left Bundle Branch Area Pacing:
- Crucial localization of the His bundle or tricuspid annulus summit in the right anterior oblique view 20-30° fluoroscopic view, employing unipolar sensing via the pacing lead.
- Skillful advancement of the guiding catheter into the right ventricle while keeping the lead within the catheter to prevent snagging on the tricuspid valve. Applying counterclockwise torque to position the catheter against the septum, with a slight extension of the lead to indicate proper contact.
- Pacemapping and lead positioning adjustments should strive for discordant QRS in leads II and III, displaying a W' pattern and a notch at the nadir of V1.
- In the left anterior oblique view 30-40° view, the guiding catheter should orient the lead at a superior angle to the horizontal plane, perpendicular to the septal curve.
- While continuously monitoring lead progression in the septum, the lead should be screwed in while applying forward pressure and maintaining the guiding catheter in place. Evaluation of coaxial orientation and lead advancement is possible through continuous screening.
- Essential testing of lead stability and threshold assessments.
- If necessary, additional leads can be implanted, and pacing QRS morphology and thresholds should be evaluated before removing the guiding catheter.
- The necessity of conduction system captures with LBBA pacing for achieving favorable clinical outcomes remains uncertain.
|
Table 2. Comparison of advantages and limitations between His Bundle Pacing vs. Left Bundle Branch Area Pacing |
||
|
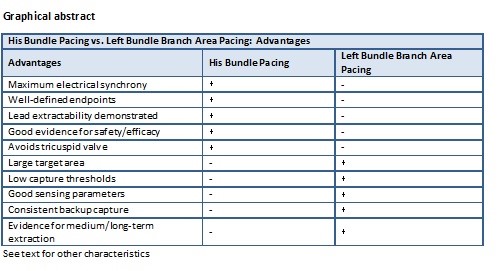
Advantages |
His Bundle Pacing |
Left Bundle Branch Area Pacing |
|
Maximum electrical synchrony |
✓ |
- |
|
Well-defined endpoints |
✓ |
- |
|
Lead extractability demonstrated |
✓ |
- |
|
Good evidence for safety/efficacy |
✓ |
- |
|
Avoids tricuspid valve |
✓ |
- |
|
Large target area |
- |
✓ |
|
Low capture thresholds |
- |
✓ |
|
Good sensing parameters |
- |
✓ |
|
Consistent backup capture |
- |
✓ |
|
Evidence for medium/long-term extraction |
- |
✓ |
|
Limitations |
His Bundle Pacing |
Left Bundle Branch Area Pacing |
|
Small target area |
✓ |
- |
|
High thresholds potentially |
✓ |
- |
|
Sensing issues |
✓ |
- |
|
Limited conduction correction |
✓ |
- |
|
Risk if infra-nodal block develops |
✓ |
- |
|
Conduction capture difficult to confirm |
- |
✓ |
|
Requires digital calipers for diagnostics |
- |
✓ |
|
Less electrical synchrony |
- |
✓ |
|
Complications of transseptal route |
- |
✓ |
|
Significant lead revisions |
- |
✓ |
|
This table compares the advantages and limitations of His Bundle Pacing and Left Bundle Branch Area Pacing. It highlights the unique benefits and potential drawbacks of each pacing technique, aiding in the selection of the most appropriate approach for individual patients. |
||
Comment
As CSP gains broader acceptance, the training of new implanters becomes paramount. While the expert consensus offers a robust foundation for teaching proper techniques, hands-on mentoring proves invaluable in shortening the learning curve. The use of procedure simulators mimicking septal anatomy can also accelerate proficiency.
To enhance access, an "introduction to CSP" module could be integrated into general EP training programs and fellowships. Brief, focused workshops during scientific meetings can generate interest and provide initial exposure. Leveraging social media and webinars can further disseminate expertise globally, extending the benefits of physiological pacing to more patients worldwide.
Furthermore, facilities initiating CSP must possess the necessary equipment—specialized delivery tools, mapping systems, backup leads, etc. Industry partners should persist in enhancing catheter designs and stabilization techniques. The inclusion of radiopaque markings indicating penetration depth may enhance safety. Smaller caliber lumenless leads with extendable/retractable helices can facilitate repositioning.
Additionally, cost reduction measures would promote broader CSP adoption. Increased production volumes can drive down lead prices over time. Bulk procurement agreements can alleviate initial financial barriers for new facilities. Payers should recognize the long-term advantages of avoiding heart failure risks associated with right ventricular (RV) pacing and provide appropriate reimbursement.
In closing, this new expert consensus (1) offers a timely and authoritative guide for implanters, enabling them to effectively administer physiological CSP. With expanded training opportunities, improved tools, and reduced costs, this significant advancement could become the standard of care for patients requiring ventricular pacing.
Beyond implantation, proper follow-up and troubleshooting of CSP systems are vital to ensure ongoing patient benefits. The expert consensus statement provides guidance on confirming intact His/left bundle capture during routine checks, which is crucial for preventing regression to ventricular pacing. If selective His capture is lost, any loss of CSP capture necessitates lead revision to restore physiological pacing.
Optimal device programming is also critical, as suboptimal AV delays or pacing outputs can lead to excessive ventricular pacing or loss of conduction system capture. The threshold search function is a useful tool to avoid phrenic nerve stimulation and excessive energy consumption with high outputs. Nonetheless, excessively low outputs can risk intermittent loss of capture. A pacing safety margin of 0.5-1 V above measured thresholds is a reasonable programming approach.
Troubleshooting elevated His thresholds may require lead repositioning, additional screw-ins, or changes in pacing vectors. In some instances, laser lead extraction with reimplantation may be necessary. For LBBAP, finding a new site on the septum is preferred over repeated lead manipulations if thresholds increase. Caution should be exercised to avoid septal hematomas, and monitoring for late perforations is advisable post-implant.
Patient selection plays a pivotal role in maximizing the benefits of CSP. The most significant advantages are realized in patients with normal baseline QRS and AV block, where ventricular dyssynchrony can be entirely prevented. The benefits are less certain in patients with pre-existing conduction disorders, although LBBAP may still normalize QRS duration.
Both HBP and LBBAP offer solutions to avoid RV pacing's electrical dyssynchrony. While HBP achieves maximum synchrony throughout the Purkinje system, LBBAP provides a broader anatomical target, more consistent backup capture, and facilitates left ventricular lead implants. Long-term comparative studies are needed to determine whether direct His or left bundle branch capture is necessary for maximum benefit.
In summary, the new expert consensus statement offers a timely and practical reference for implanting physicians seeking to initiate or optimize their CSP practice. The EHRA Clinical Consensus Statement on CSP implantation provides a comprehensive guide to the standardized procedure and technique for HBP and LBBAP. The document underscores the importance of proper implantation technique and highlights key recommendations for each pacing modality. By adhering closely to these recommendations, physicians can ensure safe and effective pacing therapy delivery, offering a more physiologically sound alternative to traditional pacing methods.
Wider adoption of CSP may be expected as implanters become more comfortable with the intricacies of these techniques. Ultimately, large randomized trials with long-term follow-up are necessary to fully define the roles of His bundle versus left bundle branch pacing.
Peer-review: Internal
Conflict of interest: None to declare
Authorship: N.K.
Acknowledgement and Funding: None to declare
References
- 1.Burri H, Jastrzebski M, Cano O, Curila K, de Pooter J, Huang W, et al. EHRA clinical consensus statement on conduction system pacing implantation: endorsed by Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS) and Latin American Heart, Rhythm Society (LAHRS). Europace 2023; 25: 1208-36.
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

