AQP4-IgG-seropositive neuromyelitis optica spectrum disorder concurring with anti-N-methyl-D-aspartate receptor encephalitis at pediatric age: a case report
CASE REPORT
AQP4-IgG-seropositive neuromyelitis optica spectrum disorder concurring with anti-N-methyl-D-aspartate receptor encephalitis at pediatric age: a case report
Article Summary
- DOI: 10.24969/hvt.2024.472
- CARDIOVASCULAR DISEASES
- Published: 14/03/2024
- Received: 09/03/2024
- Accepted: 10/03/2024
- Views: 4796
- Downloads: 3263
- Keywords: AQP4-IgG-seropositive neuromyelitis optica spectrum disorder, anti-N-methyl-D-aspartate 4 receptor encephalitis, demyelination, pediatrics
Address for Correspondence: Begimai Kadyrova, International School of Medicine of International University of Kyrgyzstan, April 7 Str, 6, 720065, Bishkek, Kyrgyz Republic
E-mail: begimai.kadyrova@gmail.com
ORCID: Begimai B. Kadyrova 0000-0003-3208-5689 Nurzhan T. Dzhaparalieva - 0000-0003-0443-2639
Asel T. Jusupova 0000-0001-8430-9504 Yulia A. Solodovnikova - 0000-0002-2544-9766 Zarema A. Kadyrova -0009-0002-7955-3531 Kubat Sh. Ibraimov 0000-0001-7753-3938
Nurzhan T. Dzhaparalieva1, Asel T. Jusupova2, Begimai B. Kadyrova1,3, Yulia A. Solodovnikova4, Zarema A. Kadyrova2 , Kubat Sh. Ibraimov5
1Department of Neurology and Neurosurgery, Kyrgyz State Medical Institute of Retraining and Advanced Training named after S. B. Daniyarov, Bishkek, Kyrgyz Republic
2Department of Neurology and Clinical Genetics, Kyrgyz State Medical Academy named after I. K. Akhunbaev, Bishkek, Kyrgyz Republic
3Department of Special Clinical Disciplines, International School of Medicine of International University of Kyrgyzstan, Bishkek, Kyrgyz Republic
4Department of Neurology and Neurosurgery of Odessa National Medical University, Odessa, Ukraine
5Department of Radiology, I.K. Akhunbaev Kyrgyz State Medical Academy, Bishkek, Kyrgyz Republic
Abstract
Objective: Neuromyelitis optica spectrum disorder (NMOSD) and autoimmune encephalitis with anti-N-methyl-D-aspartate receptor (NMDA) receptor antibodies pose distinct autoimmune challenges, yet their clinical symptomatology can overlap. This study explores the combined occurrence of these conditions, recognizing the potential for intensified symptoms due to their shared immune system involvement.
Case presentation: A rare case of an 8-year-old girl diagnosed with both disorders is presented. Initial treatment resulted in improvement, but a subsequent relapse required hospitalization. Laboratory, imaging, and clinical assessments supported the coexistence of NMOSD and autoimmune encephalitis associated with NMDA receptors. Considering the high risk of progression and incomplete response to conventional therapy, rituximab, a monoclonal antibody targeting B-lymphocytes, was administered, showing positive neurological dynamics.
Conclusion: This case underscores the diagnostic challenges posed by overlapping features and emphasizes the importance of prompt antibody testing for accurate treatment and prognosis determination. Understanding the intricate relationship between NMOSD and autoimmune encephalitis is vital for tailored therapeutic approaches, shedding light on the complexity of autoimmune comorbidities in neurology.
Key words: AQP4-IgG-seropositive neuromyelitis optica spectrum disorder, anti-N-methyl-D-aspartate 4 receptor encephalitis, demyelination, pediatrics
Introduction
Autoimmune encephalitis stands out as a significant contributor to non-infectious acute encephalitis. Studies indicate that the incidence of anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis surpasses that of specific viral causes among individuals under 30 years old. Incidence is estimated at around 1.5 per million persons annually (1). This condition primarily affects children and young females, with its onset manifesting as acute, subacute, or potentially evolving into a chronic state.
Neuromyelitis optica spectrum disorder (NMOSD) is an inflammatory condition affecting the central nervous system, commonly characterized by clinical indications of optic nerve impairment and longitudinally extensive transverse myelitis (2). Population-based studies from Europe, Asia, and South America suggest that the incidence of NMOSD ranges from 0.05 to 4/100,000/y and the prevalence from 0.52 to 4.4/100,000 (3). Pediatric onset is relatively rare.
NMOSD and autoimmune encephalitis with antibodies to NMDA receptors are two different severe autoimmune diseases, but can present with similar clinical symptoms. The question of the combination of these two pathologies, which requires serious attention and comprehensive treatment, becomes interesting and medically significant (4). Since both diseases are associated with the immune system, they can interact with each other and increase the clinical manifestations of the patient. Research in this area is still limited, and more work is needed to fully understand the interaction mechanisms.
Understanding their nature and the relationships between them is an important step towards more effective diagnosis and timely treatment. Treatment of the combination of neuromyelitis optica and autoimmune encephalitis is often complex and requires an individual approach. Immunosuppressive drugs are commonly used to suppress the activity of the immune system and reduce inflammation in the nervous system (5, 6).
Below we present a rare case of AQP4-IgG-seropostivie NMOSD concurring with anti-NMDAR antibody-positive encephalitis in a Mongoloid girl. The diagnosis was made according to the diagnostic criteria for NMDAR defined by Graus et al (7) and for NMOSD proposed by Wingerchuk et al (8).
Case report
On February 15, 2023, an 8-year-old girl presented with severe daytime sleepiness, repeated vomiting and catarrh of the upper respiratory tract. This condition was regarded as a manifestation of rotavirus infection. Due to persistent pathological drowsiness, she underwent magnetic resonance imaging (MRI) examination of the brain (February 26, 2023), where an increase in signal intensity in T2 and FLAIR modes was found in the hypothalamus and optic tracts on both sides. An 8 × 6 mm lesion with similar signal characteristics and unclear contours in the left half of the medulla oblongata was also observed. On the following MRI tomograms of the brain with intravenous contrast enhancement (February 27, 2023) – all the lesions were contrast-negative. Radiologists suggested this as a manifestation of neuromyelitis optica with damage to the hypothalamus and medulla oblongata (Fig. 1, 2). The MRI tomograms of the cervical spine showed no pathological changes.
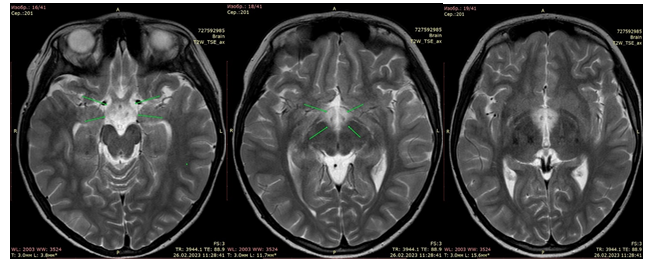
Figure 1. Axial T2-weighted images show an increase in the intensity of the MR signal from chiasm and hypothalamus
![]()
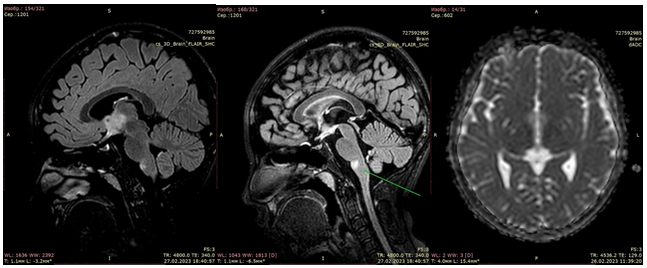
Figure 2. The same changes on sagittal FLAIR images. In addition, a lesion is detected in the medulla oblongata (arrow). On the ICD maps, in the area of changes in the magnetic resonance signal, an increase in the diffusion rate is noted
Neurological status: 3d oriented. The pupils are S=D, round. The impression of a slight convergent strabismus on the left. Movements of the eyeballs are not limited. There is no nystagmus. Trigeminal points are painless on palpation. The face is symmetrical. Tongue in the midline. Swallowing and phonation are not impaired. Tendon reflexes are slightly quicker on the left. There are no pathological foot signs. There is no paresis. Muscle tone is not changed. No sensory deficits were identified. Stable in the Romberg position. Slight dysmetria when performing a finger-nose test. Positive reflexes of oral automatism - Marinescu-Radovich from both sides.
Examination by an ophthalmologist: VOD = 0.9-1.0, VOS = 0.9-1.0. Full movement of the eyeballs. The strabismus angle according to Hirschberg is 0°, cover test is negative. Fundus of the eye: the optic discs are pale pink, the boundaries are clear, the vascular bundle is from the center, the arteries are narrow, the veins are of normal caliber, a:v = 1.25:3, the course of the vessels is not disturbed. The macular reflex is clea, the middle periphery is without features. However, Optical coherent tomography revealed signs of moderate thinning of the macular area both eyes.
Antibodies to aquaporin 4 antigen: 1:10 (normal titer <1:10 negative). Antibodies to myelin < 1:10 (normal titer <1:10 negative).
Electroencephalography (EEG): Video- EEG monitoring during wakefulness reveals pronounced cerebral changes in the form of low activity of the main cortical rhythm. Decreased functional state of the cerebral cortex with a predominance of inhibition processes. There were paroxysms of acute, slow waves with episodes of generalization. The EEG of sleep is characterized by low cyclicity; stages 2 and 3 of deep sleep (slow wave) are not recorded.
An acute disseminated encephalomyelitis was stated as a preliminary diagnosis, a course of steroid therapy (methylprednisolone 500mg) was administered and daytime sleepiness completely resolved.
The MRI of the brain, performed 1 month later (March 26, 2023) showed a reduction in the size of the demyelination foci to 80% (Fig. 3).
On April 8, 2023, steroids were completely withdrawn; the patient's condition remained stable, with occasional nausea. On April 16, 2023, she suffered acute gastroenteritis (nausea, vomiting, diarrhea, febrile fever up to 38.5°C), received treatment on an outpatient basis with positive dynamics.
Repeated exacerbation on May 10, 2023, when the condition worsened: the child began to choke, could not walk, weakness and dizziness intensified, diplopia appeared, convergent strabismus on the left, unsteadiness, repeated increase in muscle weakness, coarse horizontal nystagmus and in the dynamics, increased atactic syndrome, periodic disorientation in space, impossibility of independent walking or verticalization within the bed. Periodic attacks are also observed in the form of a sharp decrease in postural muscle tone and limpness. Speech is dysarthric, slow - progression of speech disorders over the last 7 days. Periodically - imperative urge to urinate.
![]()
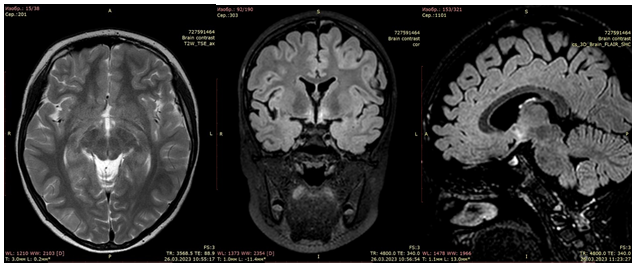
Figure 3. After the course of treatment, after 1 month there is a significant decrease in the area of magnetic resonance signal changes
On the MRI of the brain with intravenous contrast (May 26, 2023) (Fig. 3) – areas of damage to the brain substance are visualized, located supratentorial bilaterally in the area of subcortical structures, more on the left, in the insular lobe on the left, subtentorially in the medulla oblongata and the tegmentum of pons. There were no signs of diffusion limitation, no pathological accumulation of the contrast agent, negative dynamics compared to the MRI dated March 26, 2023. She was referred for a hospital abroad for further examination and treatment.
Neurological status: Conscious, drowsiness, phrasal, dysarthric, slow speech. Emotionally labile, tearful, accessible to examination after a certain period of adaptation. In time and space, one's own personality is oriented. Asthenia, drowsiness, lethargy, and increased fatigue are noted during examination. Slight asymmetry of the palpebral fissures, D less than S, photophobia. The movements of the eyeballs are full and painless. Accommodation and convergence are not impaired. Pupils: round, D=S, reaction to light is direct and friendly. Alternating convergent strabismus. Sensitivity on the face is preserved, the trigeminal points are painless, the movements of the lower jaw are full, the trophism of the chewing muscle is not impaired. The face is symmetrical, facial expressions are lively. The hearing is preserved. No nystagmus is noted. The pharyngeal and palatal reflexes are increased and symmetrical. Phonation is not impaired. Uvula in the midline. The range of active movements is reduced due to diffuse muscle hypotonia, tendon reflexes from the extremities are reduced, regardless of the sides. Muscle strength in the limbs is reduced to 4 points. There are no pathological foot or hand reflexes. Motor skills: walks mainly with support due to severe ataxia, sits with support, does not sit up independently from a lying position. There is marked general weakness. She is not stable in the Romberg position; she performs the finger-nose test with intention, D=S. Progression of ataxic gait over the last 2 days (according to the medical history). Severe hyperesthesia is noted, to a greater extent in the proximal parts of the upper extremities. Periodically - imperative urge to urinate, constipation (stool only after stimulation).
The MRI of the brain with contrast enhancement (June 7, 2023) revealed a focal contrast-negative lesions of the brain substance of the supra- and subtentorial localization (possibly as a manifestation of autoimmune encephalitis). Compared to the MRI result dated May 26, 2023, there is moderate positive dynamics.
The MRI of the spinal cord (June 7, 2023) - no MRI evidence of pathological changes.
In the coagulogram, blood biochemistry, no significant deviations from the reference values were detected.
• Antineuronal antibodies, immunoblot: Antibodies to HU, YO-1, CV2, PNMA2, RI, AMPH - were not detected.
• Anti-MOG (antibodies to myelin oligodendrocyte glycoprotein): 12.7 pg/ml (normal 0 - 15 pg/ml).
• Antibodies to GAD in CM, IgG - 0.62 IU/ml - negative;
• HLA B51 typing Behçet's disease - not detected.
● Culture of cerebrospinal fluid for flora: The liquid is clear, after centrifugation there is no sediment, no blood cells or microbial cells are found under microscopy, no flora was found. General analysis of cerebrospinal fluid: slight cytosis up to 25×106/l (normal 6-10×106/l) with a predominance of lymphocytes (95%) is noted.
● Cellular presentation of aquaporin 4 antigen: Titer > 1:100 (normal titer <1:10 negative).
● Oligoclonal IgG - type 2 synthesis;
● Antineuronal antibodies in the CSF, IgG (CASPR, NMDA, LGI, AMPA, AMPA2, GABAR) - antibodies to NMDA solutions were detected in a titer of 64.
Thus, taking into account the positive results of antibodies to NMDA receptors, and positive AB titer of the cellular presentation of the aquaporin 4 antigen, as well as neuroimaging data (MRI picture of focal contrast-negative lesions of the brain substance of supra- and subtentorial localization), neurological status (pseudobulbar disorders, dysarthria, ataxic syndrome, diffuse muscle hypotonia, decreased muscle strength and tendon reflexes, emotional lability), the child was diagnosed with neuromyelitis optica and autoimmune encephalitis associated with NMDA receptors. Due to the high risk of further progression of neurological symptoms, increasing disability, and incomplete response to hormonal therapy with methylprednisolone, therapy with monoclonal antibodies to the surface receptors of B-lymphocytes CD 20 - Rituximab at a dose of 375 mg/m2 of the body surface was initiated by intravenous drip (first injection – June 14, 2023; second injection – June 22, 2023) with premedication.
She tolerated the infusion satisfactorily. During the therapy, the neurological status showed positive dynamics in the form of a gradual increase in muscle strength, a decrease in symptoms of hyperesthesia, ataxic syndrome, an increase in the duration of the period of verticalization within the bed, a decrease in emotional lability, the frequency of episodes of decreased postural tone, limpness, EDSS 6.0.
On the follow-up MRI of the brain with intravenous contrast (October 3, 2023) there were signal changes in the hypothalamus and in the left insula without accumulation of the contrast agent. Signs of subatrophic changes in the brainstem and cerebellum (Fig. 4).
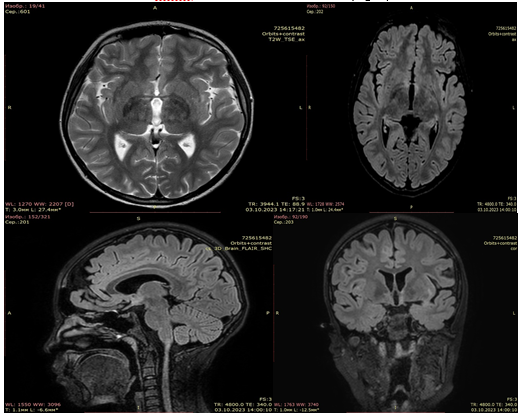
Figure 4. Follow-up MRI image 3 months after of rituximab therapy. Areas of increased MRI signal intensity on FLAIR remain, but the area and signal intensity have decreased over time
Discussion
The coexistence of AQP4-IgG-seropositive NMOSD and anti-NMDAR encephalitis challenges the conventional understanding of autoimmune neurological disorders as singular entities. While the pathogenesis of NMOSD is primarily attributed to the presence of aquaporin-4 antibodies targeting astrocytic water channels (9), anti-NMDAR encephalitis involves antibodies against NMDA receptors, predominantly affecting synaptic function (10). The convergence of these distinct autoimmune responses in a single patient poses intriguing questions about shared immunological pathways and mechanisms underlying the simultaneous emergence of these antibodies.
However, some researchers have suggested that autoimmune encephalitis may be a contributing cause of optic inflammation in some patients with neuromyelitis optica (1, 2). This may mean that patients with this combination of diseases may have more severe and prolonged symptoms than those with only one of these diseases (4, 5). Moreover, the clinical implications of such co-occurrence extend beyond the diagnostic realm, influencing therapeutic strategies and prognostic considerations. The rarity of this double-positive antibody presentation underscores the need for heightened clinical awareness, emphasizing the importance of early and comprehensive antibody testing in cases of atypical neurological symptoms and imaging findings (9).
This case report aims to contribute to the evolving understanding of autoimmune neurological comorbidities by providing insights into the diagnostic intricacies, therapeutic challenges, and potential implications for the disease management. By elucidating the unique clinical course and response to treatment in this patient, we seek to enhance the collective knowledge base surrounding these complex autoimmune disorders and stimulate further investigation into their underlying immunopathogenic mechanisms.
Conclusion
The presented clinical case provides additional evidence of the occurrence of seropositive neuromyelitis optica in combination with autoimmune encephalitis to NMDA receptors in a girl of the Mongoloid race. Antibodies to aquaporin 4 antigen and NMDA receptors are useful biomarkers for diagnosing these diseases. The specific mechanisms of double-positive antibody formation remain unclear, and clinical features and imaging findings of autoimmune neurological comorbidities overlap, leading to diagnostic difficulties. Therefore, it is necessary to remain alert in cases of atypical symptoms and neuroimaging findings, and to test for appropriate antibodies as soon as possible to determine both treatment and prognosis.
Ethics: The informed consent for diagnostic procedures and treatment was obtained from parents of patients
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: N.T. D, A. T. J, B.B. K., Y. A. S., Z. A. K. , and K.Sh.I. equally contribute to the case management and manuscript preparation
Acknowledgement and funding: None to declare
References
| 1.Dalmau J, Armangué T, Planagumà J, Radosevic M, Mannara F, Leypoldt F, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019; 18: 1045-57. doi: 10.1016/S1474-4422(19)30244-3 https://doi.org/10.1016/S1474-4422(19)30244-3 PMid:31326280 |
||||
| 2.Weinshenker BG, Wingerchuk DM. Neuromyelitis spectrum disorders. Mayo Clin Proc 2017; 92: 663-79. doi: 10.1016/j.mayocp.2016.12.014 https://doi.org/10.1016/j.mayocp.2016.12.014 PMid:28385199 |
||||
| 3.Pandit L, Asgari N, Apiwattanakul M, Palace J, Paul F, Leite MI, et al. Demographic and clinical features of neuromyelitis optica: a review. Mult Scler 2015; 21: 845-53. DOI: 10.1177/1352458515572406 https://doi.org/10.1177/1352458515572406 PMid:25921037 PMCid:PMC4463026 |
||||
| 4.Ran Y, Wang L, Zhang F, Ao R, Dong Z, Yu S. Anti-NMDAR encephalitis followed by seropositive neuromyelitis optica spectrum disorder: a case report and literature review. Clin Neurol Neurosurg 2017;.155: 75-82. https://doi.org/10.1016/j.clineuro.2017.02.016 PMid:28282627 |
||||
| 5.Sinani AA, Maawali SA, Alshekaili J, Kindi MA, Ramadhani KA, Khabouri JA, et al. Overlapping demyelinating syndrome (Neuromyelitis optica spectrum disorders NMOSD with anti-NMDA receptor encephalitis); A case report. Mult Scler Relat Disord 2020; 42: 102153. doi: 10.1016/j.msard.2020.102153. https://doi.org/10.1016/j.msard.2020.102153 PMid:32413838 |
||||
| 6.Zhang S, Yang Y, Liu W, Li Z, Li J, Zhou D. Clinical characteristics of Anti-N-Methyl-d-Aspartate receptor encephalitis overlapping with demyelinating diseases: a review. Front Immunol 2022; 13: 857443. doi: 10.3389/fimmu.2022.857443. https://doi.org/10.3389/fimmu.2022.857443 PMid:35837405 PMCid:PMC9273846 |
||||
| 7.Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016; 15: 391-404. doi: 10.1016/S1474-4422(15)00401-9. https://doi.org/10.1016/S1474-4422(15)00401-9 PMid:26906964 |
||||
| 8.Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International Panel for NMO Diagnosis. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177-89. doi: 10.1212/WNL.0000000000001729 https://doi.org/10.1212/WNL.0000000000001729 PMid:26092914 PMCid:PMC4515040 |
||||
| 9.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol ; 6: 805-15. https://doi.org/10.1016/S1474-4422(07)70216-8 PMid:17706564 |
||||
| 10.Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, Parsons TD et al.. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci 2010; 30: 5866-75. https://doi.org/10.1523/JNEUROSCI.0167-10.2010 PMid:20427647 PMCid:PMC2868315 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

