Ablation outcomes and quality of life in patients with atrial flutter and concomitant paroxysmal atrial fibrillation
ORIGINAL RESEARCH ARTICLE
Ablation outcomes and quality of life in patients with atrial flutter and concomitant paroxysmal atrial fibrillation
Article Summary
- DOI: 10.24969/hvt.2024.473
- Page(s): 200-207
- CARDIOVASCULAR DISEASES
- Published: 20/03/2024
- Received: 25/01/2024
- Revised: 24/02/2024
- Accepted: 24/02/2024
- Views: 6341
- Downloads: 3501
- Keywords: atrial flutter, atrial fibrillation, quality of life, cavotricuspid isthmus ablation, pulmonary veins isolation
Address for Correspondence: Anastasia Aker, Danylo Halytskyy Lviv National Medical University, Lviv, Ukraine
Email: anastasia.aker@gmail.com
ORCID: Anastasia Aker - 0009-0007-6426-5381 Ulyana Chernyaha-Royko - 0000-0003-0986-0325
Mykhaylo Sorokivskyy - 0000-0003-0872-4145 Borys Kravchuk - 0000-0002-4535-7797
Yuriy Ivaniv - 0000-0002-2153-9262 Oleg Zharinov - 0000-0002-4089-9757
Facebook: Anastasia Aker - nastja.akerkryvenjka Ulyana Chernyaha-Royko - ulyana.chernyaha
Mykhaylo Sorokivskyy - msorokivskyy Yuriy Ivaniv - yivaniv
Anastasia Aker1 , Ulyana Chernyaha-Royko1, Mykhaylo Sorokivskyy1, Borys Kravchuk2 , Igor Tumak1, Yuriy Ivaniv1, Oleg Zharinov3
1Danylo Halytskyy Lviv National Medical University, Lviv, Ukraine
2National M.M. Amosov Institute of Cardiovascular Surgery, National Academy of the Medical Sciences of Ukraine, Kyiv, Ukraine
3Shupyk National Healthcare University of Ukraine, Kyiv, Ukraine
Abstract
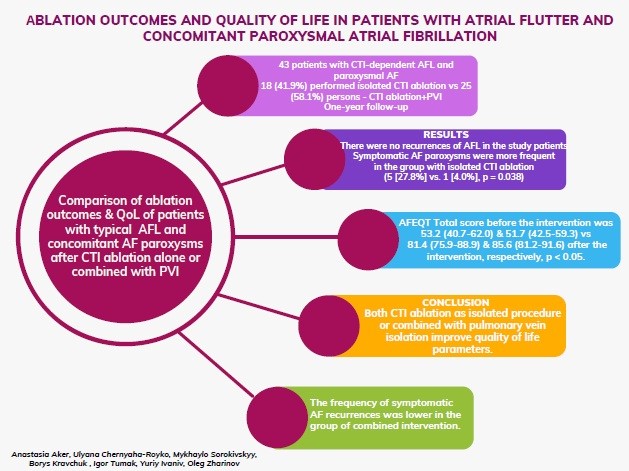
Objective: To compare ablation outcomes and quality of life (QoL) of patients with typical atrial flutter (AFL) and concomitant atrial fibrillation (AF) paroxysms after cavotricuspid isthmus (CTI) ablation alone or combined with pulmonary vein isolation.
Methods: The single-center study included 43 patients with CTI-dependent AFL and paroxysmal AF. We compared QoL, clinical data concerning the course of arrhythmia recurrence in 18 (41.9%) patients with isolated CTI ablation versus 25 (58.1%) persons with both CTI ablation and pulmonary vein isolation (PVI) after one-year of follow-up.
Results: The compared groups did not differ by gender, anthropometric parameters, and concomitant diseases. Patients from the group of isolated CTI ablation were older (64 [54-68] versus 54 [52-60] years, p = 0.006). During the one-year follow-up, there were no recurrences of AFL in the study patients. Symptomatic AF paroxysms were more frequent in the group with isolated CTI ablation (5 [27.8%] vs. 1 [4.0%], p = 0.038). Paroxysms of non-sustained atrial tachycardia were often recorded in both groups during Holter ECG monitoring (11 [61.1%] vs. 10 [40.0%]); for paroxysmal AF, the numbers were 13 (72.2%) vs. 9 (36.0%). Regardless of the extent of the intervention, there were significant QoL improvements in both groups of patients: the AFEQT Total score before the intervention was 53.2 (40.7-62.0) and 51.7 (42.5-59.3) versus 81.4 (75.9-88.9) and 85.6 (81.2-91.6) after the intervention, respectively (p < 0.05).
Conclusions: The frequency of symptomatic AF recurrences was lower in the group of combined intervention. In patients with typical AFL and concomitant paroxysmal AF, both CTI ablation as isolated procedure or combined with pulmonary vein isolation improve quality of life parameters.
Key words: atrial flutter, atrial fibrillation, quality of life, cavotricuspid isthmus ablation, pulmonary veins isolation
Introduction
Atrial flutter (AFL) and atrial fibrillation (AF) are frequently coexisting rhythm disorders with rising prevalence and detection during recent 30 years. According to the epidemiological data, in 2019 AF and AFL affected about 60.0 million people worldwide (1). Both arrhythmias impair the quality of life (QoL) and increase the risk of thromboembolic events, heart failure, and death (2, 3). Cavotricuspid isthmus (CTI) ablation is a first-line treatment in the management of patients with typical AFL (4). It is a highly effective procedure with successful results in 97% of patients and a low recurrence rate, approximately 10%, during 14 months follow-up (5).
Graphical abstract
Atrial fibrillation is often registered in patients with AFL before and after ablation of CTI (4, 6, 7). Some studies revealed AF episodes after isolated CTI ablation in 82% patients during 5-years follow-up (8). Therefore, the prevention of AF recurrences might become an important special goal during catheter treatment of AFL.
The result of the survey by EHRA (European Heart Rhythm Association) and Canadian Heart Rhythm Society shows variability of ablation approaches in patients with coexisting AFL and AF (CTI, pulmonary vein isolation (PVI), or both). Lone CTI would be performed in 53% cases with CTI-dependent AFL, in 15% cases operators use CTI as a first step in AFL cases even if the AFL is not considered to be the predominant arrhythmia, while 32 % respondents would perform a combined CTI and AF ablation regardless of AF symptoms (5).
There are no clear recommendations concerning patient management with CTI-dependent AFL and coexisting AF episodes. Due to these data, the hypothesis of our study was that combination of CTI ablation and PVI during the same procedure may be an effective treatment strategy for patients with AFL combined with AF.
Thus, our study aimed to compare ablation outcomes and QoL of patients with typical AFL and AF paroxysms after AFL ablation alone and combined CTI ablation and pulmonary vein isolation.
Methods
Study design and population
This prospective cross-sectional observational single-center study included 43 patients with symptomatic persistent CTI-dependent AFL and concomitant paroxysmal AF, who were referred to CTI ablation. Patients were consecutively enrolled in the study during the period 2019-2022 when they met the following criteria: 1) being at least 18 years old, 2) having a documented history of symptomatic CTI-dependent AFL, 3) having at least one AF episode lasting over 30 s documented by the electrocardiogram (ECG) or Holter monitoring. Patients with contraindications to catheterization or anticoagulant therapy were not included.
Other exclusion criteria were previous ablations, implantable devices, a recent cardiovascular procedure, such as heart surgery or a cardiac interventional procedure, coronary angioplasty within the past 30 days, valvular heart disease requiring surgery, pregnancy, severe liver and renal failure, and coexisting medical conditions, that could independently influence quality of life. All patients were divided into two groups: the first group consisted of 18 (41.9%) patients with isolated CTI ablation and the second group - of 25 (58.1%) persons who underwent with both CTI ablation and PVI.
The study received ethical approval by local Research Ethics committees and all participants provided informed consent.
Baseline variables
During patient enrollment, demographic and clinical variables were registered, such as age, body mass index, concomitant diseases and CHA2DS2-VASc score. Additionally, echocardiography parameters were studied, including the left ventricular ejection fraction (LVEF), left atrial (LA) size, and LA volume index. Data about the clinical course of arrhythmia were also collected, such as AFL duration before the intervention, frequency of paroxysms, and percentage of patients with sinus rhythm.
Quality of life
Quality of life was analyzed by Atrial Fibrillation Effect on QualiTy-of-life (AFEQT) - AF-specific self-administered questionnaire (21). The 18 questions are grouped into 3 functional subscales: symptoms, daily activities, treatment concerns.
AFEQT was scored on a 1 to 7 Likert scale (from no limitation/symptoms to the most severe limitation/symptoms).
Then raw scales 1 - 7 were standardized to a score of 0-100, from 0 (worst health-related HRQoL) to 100 (best health-related HRQoL). The global sum of the score and its components was calculated using the following formula:
Overall AFEQT score:
[100 - (sum of severity for all questions answered - number of questions answered) X 100 (total number questions answered X 6)].
Symptoms
For the analysis of symptoms related to AFL, we used the EHRA score. Symptoms were categorized into four classes: no symptoms – “I”, mild symptoms – “II”, severe symptoms- “III”, and disabling symptoms –“IV”(23).
Electrophysiology procedure and ablation
Using the traditional technique, a CTI ablation with a bidirectional block was performed. All patients were offered pulmonary vein isolation in terms of CTI ablation procedure after the interview with a physician before ablation. In patients in whom it was decided to perform additional PVI, the procedure technique included the following transseptal puncture with double access to the LA. Mapping catheter (Inquiry Optima; Abbott, Minneapolis, MN, USA) and ablation catheter (FlexAbility; Abbott, Minneapolis, MN, USA) were placed in the left atrium. The next step was a 3D anatomic map reconstruction of left atrium with pulmonary veins ostium using catheters mentioned above and 3D navigation system EnSite Precision. Afterwards, antrum circumferential radiofrequency ablation isolation of the left and right pulmonary veins was performed. Endpoints of ablation were entrance and exit block of conduction in pulmonary veins following by a 20 min waiting period after isolation.
All patients were further divided into two groups: the first group consisted of 18 (41.9%) patients with isolated CTI ablation and the second group - of 25 (58.1%) persons with both CTI ablation and PVI performed.
Post-ablation follow-up
All patients were followed up for one year. There were no cases of significant complications associated with the intervention. The standard set of clinical examinations, echocardiography and 48 or 72-hour Holter ECG monitoring was performed. The re-examination included a questionnaire of the QoL, the collection of clinical data concerning the course of arrhythmia. Symptoms were evaluated according to the EHRA scale. Quality of life was analyzed according to the AFEQT questionnaire.
After one year of follow-up, Holter ECG monitoring was performed, in order to detect the following outcome parameters: paroxysms of nonsustained atrial tachycardia, paroxysms of AF lasting less or more than 5 hours. Symptomatic AF paroxysms were documented during follow-up period as well.
Statistical analysis
Categorical variables are summarized as frequencies and percentages, and compared using Fisher’s exact test between groups and using McNemar's test for paired data (before and after procedure). Continuous variables are summarized as medians and upper and lower quartiles due to the non-Gaussian distribution of many of them (testing using the Shapiro-Wilk test). Continuous and ranked variables were compared using Mann–Whitney test between groups and using Wilcoxon paired test for paired data (before and after procedure) (22). All statistical analyses were performed with Statistica for Windows 6.0 software package (StatSoft, USA).
Results
The study cohort included 28 (65.1%) men and 15 (34.9%) women, median age - 59 (lower - upper quartile 53-64) years. The median risk on the CHA2DS2-VASc scale was 2 (quartiles 1-3) points. Arterial hypertension was present in 28 (65.1%) patients and was well-controlled in all cases. Ischemic heart disease was diagnosed in 9 (20.9%), including 2 (18.6%) patients after earlier myocardial infarction. Non-obstructive hypertrophic cardiomyopathy was present in 2 (4.7%) patients. Type 2 diabetes mellitus (DM) was present in 5 (11.6%) cases. Systolic dysfunction of the left ventricle (LVEF <40%) was diagnosed in 4 (9.3%) patients. Non-vitamin K oral anticoagulants were administered in all patients for at least 1 month before ablation procedure.
The compared groups did not differ by gender, anthropometric parameters, and concomitant diseases. Patients from the group of isolated CTI ablation were older (Table 1). A more frequency of male gender patients was revealed in both study groups, which is consistent with epidemiological data, especially regarding combination of AF and AFL.
There were no differences between groups regarding the risk of thromboembolic events and echocardiography parameters. Patients of both groups had a moderate thromboembolic risk according to the CHA2DS2-VASc scale. The study groups were characterized by a preserved LVEF and a slightly dilated LA.
|
Table 1. Comparison of clinical characteristics and echocardiography parameters in patients of the study groups |
|||
|
Variables |
RFA of CTI (n= 18) |
PVI and RFA of CTI (n= 25) |
p |
|
Age, years |
64 (54-68) |
54 (52-60) |
0.006 |
|
Male gender, n(%) |
12 (66.7 ) |
16 (64.0 ) |
NS |
|
CHA2DS2-VASc scale |
2 (1-3) |
2 (1-3) |
NS |
|
LVEF % |
60 (55-60) |
55 (53-60) |
NS |
|
LA size, cm |
4.1 (4.0-4.3) |
4.0 (3.8-4.3) |
NS |
|
LA volume index (ml/m2) |
36 (34-39) |
32 (29-38) |
NS |
|
Data are presented as n(%) and median (upper and lower quartiles) LA – left atrium, LVEF – left ventricular ejection fraction, PVI - pulmonary veins isolation, RFA of CTI – radiofrequency ablation of the cavotricuspid isthmus |
|||
The groups were similar regarding the duration of arrhythmia before the intervention. The majority of patients had arrhythmia for a period of 1-5 years before catheter treatment.
Symptom manifestation and parameters of life QoL didn`t differ between the study groups before the intervention. A significant part of patients was highly symptomatic (72.3% and 80.0% III-IV EHRA class in both groups, respectively). The parameters of the QoL showed a significant improvement in most of the compared indicators in both groups. Arrhythmia symptoms also decreased significantly (Table 2). Patients in the CTI and PVI group had significantly less anxiety (p<0.05) and more frequently better physical tolerance (p=0.005) possibly due to younger age.
Antiarrhythmic drugs were rarely required, and beta-blockers were used most often.
![]()
|
Table 2. Comparison of symptoms and parameters of quality of life (AFEQT scale) in patients of the studied groups before and after the intervention |
||||||||
|
Variables |
before the intervention |
p* |
after the intervention |
p* |
||||
|
RFA of CTI (n= 18) |
PVI and RFA of CTI (n= 25) |
|
RFA of CTI (n= 18) |
PVI and RFA of CTI (n= 25) |
|
|||
|
AFEQT score, median (lower-upper quartile) |
||||||||
|
Total score |
53.2 (40.7-62.0) |
51.7 (42.5 -59.3) |
NS |
81.4 (75.9-88.9)** |
85.6 (81.2-91.6)** |
NS |
||
|
Symptoms |
43.5 (29.3 – 54.2) |
45.8 (29.2 – 54.2) |
NS |
91.6 (83 -95.8)** |
91.6 (83.3-95.8)** |
NS |
||
|
Daily activity |
45.6 (35.4 -54.2) |
37.5 (31.3 – 65.5) |
NS |
73.9 (62.5-83.3)** |
79.1 (72.5-89.5)** |
NS |
||
|
Treatment |
70.7 (50.0-86.0) |
63.9 (44.5 – 72.7) |
NS |
86.1 (72.0 – 91.6)** |
86.2 (80.5-92.5)** |
NS |
||
|
EHRA symptoms, detection frequency, n (%) |
||||||||
|
I-II III IV |
5 (27.8 %) 9 (50.0 %) 4 (22.3 %) |
5 (20.0 %) 15 (60.0 %) 5 (20.0 %) |
NS |
8 (44.4 %) 9 (50.0 %) 1 (5.6 %) ** |
19 (76.0 % ) 6 (24.0 %) 0** |
0.07 |
||
|
Symptoms, detection frequency, n (%) |
||||||||
|
Palpitations |
13 (72.2 %) |
20 (80.0 %) |
NS |
4 (22.2%)** |
4 (16.0 %) ** |
NS |
||
|
Chest pain |
14 (77.8 %) |
9 (36.0 %) |
0.01 |
0** |
0 ** |
|
||
|
Decreased tolerance to physical activity |
15 (83.3 %) |
16 (64.0 %) |
NS |
10 (55.6 %) |
2 (12.0 %) ** |
0.005 |
||
|
Dizziness |
7 (38.9 %) |
10 (40.0 %) |
NS |
0 |
0** |
NS |
||
|
Anxiety |
8 (44.4 %) |
11 (44.0 %) |
NS |
6 (33.3 %) |
2 (8.0 %)** |
0.05 |
||
|
* Significant difference between groups (p<0,05). ** Estimation of dynamics: significant difference before and after intervention in the group (p<0.05) NS- nonsignificant, PVI – pulmonary valve isolation, RFA of CTI- radiofrequency ablation of cavotricuspid isthmus |
||||||||
At the one-year follow-up visit (Table 3), the predominant finding was the maintenance of sinus rhythm among the majority of patients. Symptomatic AF paroxysms were less in patients in group of CTI and PVI (p=0.038). There were no cases of recurrent typical AFL during the follow-up period. However, the recurrences of AF, including the episodes of AF, which were recorded during Holter ECG monitoring, were observed in 13 (72.2 %) patients in the first group and in 9 (36.0%) patients in the second group, with significant differences in AF of <5 hours of duration (p=0.042.)
|
Table 3. Clinical course of arrhythmia and parameters of Holter ECG monitoring in the study groups one year after the intervention |
|||
|
Variables |
RFA of CTI (n= 18) |
PVI and RFA of CTI (n= 25) |
p |
|
Sinus rhythm, n(%) |
16 (88.9 %) |
23 (92.0 %) |
NS |
|
Symptomatic AF paroxysms, n(%) |
5 (27.8 %) |
1 (4.0 %) |
0. 038 |
|
Frequency of symptomatic AF paroxysms, n(%) - once a year - once a months - once a weak |
3 (16.7 %) 1 (5.6 %) 1 (5.6 %) |
1 (4.0 %) 0 0 |
NS |
|
Paroxysm of atrial tachyarrrhythmias detected by Holter monitoring, n(%) |
|||
|
Paroxysms of nonsustained atrial tachycardia |
11 (61.1 %) |
10 (40.0 %) |
NS |
|
Paroxysm of AF (< 5 hours) |
9 (50.0 %) |
5(20.0 %) |
0. 042 |
|
Paroxysm of AF (> 5 hours) |
4 (22.2 %) |
4 (16.0 %) |
NS |
|
Data are presented as n(%) AF – atrial fibrillation, NS- nonsignificant, PVI – pulmonary valve isolation, RFA of CTI- radiofrequency ablation of cavotricuspid isthmus |
|||
Discussion
Our study showed, that both CTI ablation as isolated procedure or combined with PVI improves quality of life in patients presenting with symptomatic CTI-dependent AFL and concomitant AF, who were referred to CTI ablation alone (first group) or both CTI ablation and pulmonary vein isolation (second group). We demonstrated that at 1-year follow-up there was no recurrence of AFL in both groups. Symptomatic AF reduced significantly in CTI and PVI ablation group and AF episodes on Holter monitoring were significantly less in combined procedure group as compared to RFA of CTI group.
This fact corresponds to the results of other studies, where maintenance of sinus rhythm after RFA did not correlate with indicators of quality of life (9). Despite paroxysms of AF occurred frequently after the intervention, they were mostly short-term. This can explain the symptom reduction in both groups.
Symptomatic AF paroxysms were not common, but they were significantly more frequent in the group of isolated CTI ablation. Therefore, the distribution between EHRA classes differed in the compared groups. Thus, the combined intervention was shown to be more effective. Similarly, the study CABANA demonstrated a reduction in the number of arrhythmia episodes and overall arrhythmia duration (arrhythmia burden), which led to reduction of the symptoms in the patients with AF (10).
It is known that the majority of AFL patients have paroxysmal AF before performing catheter interventions for AFL and after successful treatment of this arrhythmia (4, 11). Previous studies have demonstrated an incidence of AF in patients with typical AFL after CTI ablation is 25% - 82% (12, 13). In a nation-wide cohort undergoing first-time CTI ablation for AFL, 13.5% of patients underwent ablation for AF during mean follow-up of 4.0 (1.7) years (14). According to the present guidelines, rhythm control therapy including ablation is recommended for symptoms and QoL improvement in symptomatic patients with AF and AFL (11, 15).
Despite the expected decrease in the main symptoms (palpitations, dizziness) in both groups, with the combined intervention, patients less often complained at a decrease in exercise tolerance that may be related also to younger age.
The results of our study indicate that the majority of patients with AFL and concomitant paroxysmal AF were symptomatic before the intervention but symptoms were predominantly caused by persistent AFL. There are no data showing the unquestionable benefit of the combined performance of the isthmus block and isolation of the pulmonary veins in such category of patients.
Although, AF ablation is a relatively safe procedure, the complication rate varies between 2-3.5% and life-threatening complications can occur. In addition, it is associated with longer procedure time and fluoroscopy usage (16). That is why the choice of management strategy for such patients with CTI-dependent AFL and paroxysmal AF depends on the clinic experience and the decision of the patient. Arrhythmia-related symptoms are a priority in the decision approach (4, 11, 15). In our study, the ablation strategy was chosen by younger patients, other clinical and functional parameters did not differ significantly in the study groups.
The results of our study demonstrated that, regardless of the volume of the intervention, in two groups of patients, with only an isolated isthmus block and a combined isthmus block and PVI procedure, a significant improvement in the QoL was achieved. During one-year follow-up, the patients had no recurrences of AFL, which indicates the high efficiency of this procedure, and the obtained data are the same as in previous studies (5). However, AF paroxysms were registered in both groups after one-year observation. In the group of patients with the combined isthmus block and PVI, AF episodes were registered in 9 (36.0 %) patients, but they were shorter and less symptomatic which generally improved the quality of patients’ life. These data are similar to the data of long-term follow-up studies, which indicated that the incidence of late arrhythmia recurrence after PVI is up to 30% (17) but they are less symptomatic (18). In our study symptomatic AF paroxysms were significantly more frequent in the group of isolated CTI ablation (5 (27.8 %) vs 1 (4.0 %), p =0.038) after one year of observation.
It is also important to note that paroxysms of non-sustained atrial tachycardia were often recorded in both groups during Holter monitoring (11 (61.1%) vs 10 (40.0 %)), however, this did not significantly affect the QoL indicators. Until now, data on the relationship between QoL and the presence of atrial tachycardia are controversial (18, 19).
Study limitations
Several limitations of these data should be kept in mind, including the small sample sizes in the groups and age differences between the study groups. Yet, the group division largely reflects the choice of catheter interventions in patients with AFL and concomitant AF in clinical practice. Randomized and larger studies are needed to determine the optimal patient selection criteria and compare complications between strategies of CTI ablation alone or combined with PVI.
Conclusion
Thus, the frequency of symptomatic AF recurrences was lower in the group of combined intervention. In patients with typical AFL and concomitant paroxysmal AF, both CTI ablation as isolated procedure or combined with pulmonary vein isolation improve quality of life parameters.
Ethics: The study received ethical approval by local Research Ethics committees and all participants provided informed consent
Peer-review: External and Internal
Conflict of interest: None to declare
Authorship: A. A., U. C.-R., M. S., B. K., I. T., Y. I., and O. Z. equally contributed to preparation of the manuscript and fulfill authorship criteria
Acknowledgement and funding: None to declare
References
| 1.Dong XJ, Wang BB, Hou FF, Jiao Y, Li HW, Lv SP, et al. Global burden of atrial fibrillation/atrial flutter and its attributable risk factors from 1990 to 2019. Europace 2023; 25: 793-803. doi: 10.1093/europace/euac237 https://doi.org/10.1093/europace/euac237 PMid:36603845 PMCid:PMC10062373 |
||||
| 2.Piccini JP, Todd DM, Massaro T, Lougee A, Haeusler KG, Blank B, et al. Changes in quality of life, cognition and functional status following catheter ablation of atrial fibrillation. Heart 2020; 106: 1919-26. doi: 10.1136/heartjnl-2020-316612 https://doi.org/10.1136/heartjnl-2020-316612 PMid:33046527 PMCid:PMC7719908 |
||||
| 3.Rahman F, Wang N, Yin X, Ellinor PT, Lubitz SA, LeLorier PA, et al. Atrial flutter: Clinical risk factors and adverse outcomes in the Framingham Heart Study. Heart Rhythm 2016; 13: 233-40. doi: 10.1016/j.hrthm.2015.07.031 https://doi.org/10.1016/j.hrthm.2015.07.031 PMid:26226213 PMCid:PMC4698205 |
||||
| 4.Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomström-Lundqvist C, et al. ESC Scientific Document Group. 2019 ESC Guidelines for the management of patients with supraventricular tachycardiaThe Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J 2020; 41: 655-720. doi: 10.1093/eurheartj/ehz467 https://doi.org/10.1093/eurheartj/ehz467 PMid:31504425 |
||||
| 5.Glover BM, Chen J, Hong KL, Boveda S, Baranchuk A, Haugaa KH, et al. Catheter ablation for atrial flutter: a survey by the European Heart Rhythm Association and Canadian Heart Rhythm Society. Europace 2016; 18: 1880-5. doi: 10.1093/europace/euw402 https://doi.org/10.1093/europace/euw402 PMid:28130373 |
||||
| 6.Musat DL, Milstein NS, Pimienta J, Bhatt A, Preminger MW, Sichrovsky TC, et al. Incidence, duration, pattern, and burden of de novo atrial arrhythmias detected by continuous ECG monitoring using an implantable loop recorder following ablation of the cavotricuspid isthmus. Cardiovasc Digit Health J 2020; 1: 114-22. doi: 10.1016/j.cvdhj.2020.10.003 https://doi.org/10.1016/j.cvdhj.2020.10.003 PMid:35265883 PMCid:PMC8890330 |
||||
| 7.Spector P, Reynolds MR, Calkins H, Sondhi M, Xu Y, Martin A, et al. Meta-analysis of ablation of atrial flutter and supraventricular tachycardia. Am J Cardiol 2009; 104: 671-7. doi:10.1016/j.amjcard.2009.04.040 https://doi.org/10.1016/j.amjcard.2009.04.040 PMid:19699343 |
||||
| 8.Ellis K, Wazni O, Marrouche N, Martin D, Gillinov M, McCarthy P, et al. Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: left-atrial size as an independent predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol 2007; 18: 799-802. doi: 10.1111/j.1540-8167.2007.00885.x https://doi.org/10.1111/j.1540-8167.2007.00885.x PMid:17593230 |
||||
| 9.Wokhlu A, Monahan KH, Hodge DO, Asirvatham SJ, Friedman PA, Munger TM, et al. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol 2010; 55: 2308-16. doi: 10.1016/j.jacc.2010.01.040. PMID: 20488300 https://doi.org/10.1016/j.jacc.2010.01.040 PMid:20488300 |
||||
| 10.Poole JE, Bahnson TD, Monahan KH, Johnson G, Rostami H, Silverstein AP, et al. CABANA Investigators and ECG Rhythm Core Lab. Recurrence of atrial fibrillation after catheter ablation or antiarrhythmic drug therapy in the CABANA Trial. J Am Coll Cardiol 2020; 75: 3105-18. doi: 10.1016/j.jacc.2020.04.065 https://doi.org/10.1016/j.jacc.2020.04.065 PMid:32586583 PMCid:PMC8064404 |
||||
| 11.Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM et al. 2023 ACC/AHA/ACCP/HRS Guideline for the diagnosis and management of atrial fibrillation: A report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation 2023. doi: 10.1161/CIR.0000000000001193 https://doi.org/10.1161/CIR.0000000000001193 PMid:38033089 |
||||
| 12.Li JH, Xie HY, Chen YQ, Cao ZJ, Tang QH, Guo XG, et al. Risk of new-onset atrial fibrillation post-cavotricuspid isthmus ablation in typical atrial flutter without history of atrial fibrillation. Front Physiol 2021; 12: 763478. doi: 10.3389/fphys.2021.763478 https://doi.org/10.3389/fphys.2021.763478 PMid:34916957 PMCid:PMC8669788 |
||||
| 13.Fujimoto Y, Yodogawa K, Oka E, Hayashi H, Yamamoto T, Murata H, et al. Significance of fragmented QRS complexes for predicting new-onset atrial fibrillation after cavotricuspid isthmus-dependent atrial flutter ablation. Heart Rhythm 2020; 17: 1493-9. doi: 10.1016/j.hrthm.2020.04.021. https://doi.org/10.1016/j.hrthm.2020.04.021 PMid:32325199 |
||||
| 14.Giehm-Reese M, Kronborg MB, Lukac P, Kristiansen SB, Nielsen JM, Johannessen A et al. Recurrent atrial flutter ablation and incidence of atrial fibrillation ablation after first-time ablation for typical atrial flutter: A nation-wide Danish cohort study. Int J Cardiol 2020; 298: 44-51. doi: 10.1016/j.ijcard.2019.07.077 https://doi.org/10.1016/j.ijcard.2019.07.077 PMid:31521436 |
||||
| 15.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. Corrigendum to: 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021; 42: 4194. doi:10.1093/eurheartj/ehab648 https://doi.org/10.1093/eurheartj/ehab648 PMid:34520521 |
||||
| 16.Deshmukh A, Patel NJ, Pant S, Shah N, Chothani A, Mehta K, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93,801 procedures. Circulation 2013; 128: 2104-12. doi:10.1161/CIRCULATIONAHA.113.003862 https://doi.org/10.1161/CIRCULATIONAHA.113.003862 PMid:24061087 |
||||
| 17.Erhard N, Metzner A, Fink T. Late arrhythmia recurrence after atrial fibrillation ablation: incidence, mechanisms and clinical implications. Herzschrittmacherther Elektrophysiol 2022; 33: 71-6. doi: 10.1007/s00399-021-00836-6 https://doi.org/10.1007/s00399-021-00836-6 PMid:35006336 PMCid:PMC8873127 |
||||
| 18.Rohrer U, Manninger M, Zirlik A, Scherr D. Impact of catheter ablation for atrial fibrillation on quality of life. J Clin Med 2022; 11: 4541. doi: 10.3390/jcm11154541 https://doi.org/10.3390/jcm11154541 PMid:35956155 PMCid:PMC9369868 |
||||
| 19.Stempfel S, Aeschbacher S, Blum S, Meyre P, Gugganig R, Beer JH, et. al. Swiss-AF study investigators. Symptoms and quality of life in patients with coexistent atrial fibrillation and atrial flutter. Int J Cardiol Heart Vasc 2020; 29: 100556. doi: 10.1016/j.ijcha.2020.100556 https://doi.org/10.1016/j.ijcha.2020.100556 PMid:32577496 PMCid:PMC7303549 |
||||
| 20.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017; 14: e275-e444. doi:10.1016/j.hrthm.2017.05.012 https://doi.org/10.1016/j.hrthm.2017.05.012 PMid:28506916 PMCid:PMC6019327 |
||||
| 21.Spertus J, Dorian P, Bubien R, Lewis S, Godejohn D, Reynolds MR, et al. Development and validation of the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) Questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol 2011; 4: 15-25. doi: 10.1161/CIRCEP.110.958033 https://doi.org/10.1161/CIRCEP.110.958033 PMid:21160035 |
||||
| 22.Glantz SA. Primer of Biostatistics. McGraw-Hill, Health Professions Division; 1997. 473 p. | ||||
| 23.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016; 18: 1609-78. doi: 10.1093/europace/euw295 https://doi.org/10.1093/europace/euw295 PMid:27567465 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

