Etiopathogenesis, diagnosis and treatment strategies for stroke-associated pneumonia
REVIEW
Etiopathogenesis, diagnosis and treatment strategies for stroke-associated pneumonia
Article Summary
- DOI: 10.24969/hvt.2024.477
- Page(s): 293-301
- CARDIOVASCULAR DISEASES
- Published: 08/04/2024
- Received: 14/12/2023
- Revised: 26/02/2024
- Accepted: 26/02/2024
- Views: 8541
- Downloads: 3725
- Keywords: Acute stroke, stroke-associated pneumonia, etiopathogenesis, diagnostic criteria, risk factors, treatment strategies, systematic review
Address for Correspondence: Elmira M.Mamytova, Department of Neurology and Clinical Genetics named after A.M. Murzaliev of the I. K. Akhunbayev Kyrgyz State Medical Academy, Bishkek, Kyrgyz Republic
E-mail: elmiramamytova@yahoo.com
ORCID: Turat S. Kadyrov - 0000-0003-4670-242Х , Elmira M. Mamytova - 0000-0002-4322-5555 Aina Dj. Mamytova –
0009-0007-1637-6984, Anara U. Toktomametova -0000-0003-4679-4750, Мaksatbek A. Batyrov –
0009-0003-0533-5264, Nurzhan T. Dzhaparalieva -0000-0003-0443-2639
Turat S. Kadyrov1, Elmira M.Mamytova1,2, Aina Dj. Mamytova 3, Anara U. Toktomametova,1 Мaksatbek A. Batyrov 4, Nurzhan T. Dzhaparalieva5
1Department of Neurology and Clinical Genetics named after A.M. Murzaliev of the I. K. Akhunbayev Kyrgyz State Medical Academy, Bishkek, Kyrgyz Republic.
2Department of Clinical Disciplines, Salymbekov University, Bishkek, Kyrgyz Republic.
3Department of Internal Diseases of the Kyrgyz State Medical Institute of Retraining and Advanced Studies named after S.B. Daniyarov, Bishkek, Kyrgyz Republic.
4Department of Special Clinical Disciplines, International University of Kyrgyzstan, Bishkek, Kyrgyz Republic.
5Department of Neurology and Neurosurgery of the Kyrgyz State Medical Institute of Retraining and Advanced Studies named after S.B. Daniyarov, Bishkek, Kyrgyz Republic
Abstract
Objective: The stroke-associated pneumonia (SAP) is considered an important risk factor for death following a stroke. It is important for clinicians to be able to recognize the causes of its occurrence, characteristic clinical and radiological signs and to be aware of treatment and prophylaxis in the early post-stroke period.
This review article is devoted to the analysis and presentation of modern data on etiopathogenesis, modern strategies for early diagnosis and management of patients with stroke-associated pneumonia in the early post-stroke period.
Design: Review of observational studies.
Methods: We used following databases: PubMed, Web of Science, Scopus for the period of time 2019-2023 for studies search. Data from selected studies were extracted, including study design, data source, outcome definition, sample size, etiopathogenesis, diagnostic criteria and treatment strategies for stroke-associated pneumonia.
Results: Totally 354 studies were reviewed, and after the selection process, 37 studies were included in this article. The incidence of stroke-associated pneumonia in patients with acute stroke ranged from 6.7 % to 37.98 %. Despite the abovementioned practical statements, there is currently no «gold standard» or generally accepted criteria for the diagnosis and treatment of SAP. As a rationale, detection of patients with the highest risk of SAP facilitates both prevention and treatment. The highest risks are associated with the age over 65, brain stem infarction with dysphagia, decreased throat and cough reflexes, impaired consciousness.
Conclusion: The identification of high-risk patients requires knowledge of the modern criteria for diagnosing SAP. Since most SAP consists of aspiration pneumonia, hospital-acquired pneumonia, healthcare-associated pneumonia, pneumonia caused by artificial ventilation, partial diagnosis, treatment, rehabilitation, are related to the provision of medical care.
Key words: Acute stroke, stroke-associated pneumonia, etiopathogenesis, diagnostic criteria, risk factors, treatment strategies, systematic review
Introduction
Cerebrovascular diseases, including various forms of cerebral stroke, are among the leading causes of mortality among the population (1). In addition, these diseases carry a significant socio-economic burden and present frequent long-term complications (2).
![]()
Graphical abstract
Respiratory and urogenital infections, congestive heart failure, dysphagia, hyperthermia, deep vein thrombosis with pulmonary embolism, bedsores, falls and depression are most commonly reported in clinical practice.
Adequate prevention and early detection of these complications and the use of specific treatment tactics at the inpatient and outpatient levels are considered to be the guarantee to good clinical outcomes (3).
As one of the most common infectious complications of stroke-patients the incidence of stroke-associated pneumonia (SAP) ranges from 6.7% to 37.98%, and sometimes up to 47%. The SAP is considered an important risk factor for death following a stroke. Studies have shown that the development of SAP is associated with an increased risk of 30-day and annual mortality, resulting in longer hospitalization times and higher health care costs (4). Strikingly, SAP significantly increases the risk of other non-infectious complications in patients with acute ischemic stroke, such as gastrointestinal bleeding and recurrent stroke (4). Therefore, it is important for clinicians to be able to recognize the causes of its occurrence, characteristic clinical and radiological signs and to be aware of treatment and prophylaxis in the early post-stroke period.
The purpose of this review article was to focus on the analysis of current data regarding pathophysiological mechanisms, as well as diagnostic criteria and treatment strategies of stroke-associated pneumonia in early post-stroke period.
Methods
Search strategy
English scientific citation database of PubMed, Web of Science, Scopus for the period of time 2019-2023 were included. Data from selected studies were extracted, including study design, data source, outcome definition, sample size, etiopathogenesis, diagnostic criteria, treatment strategies. The most common demographic, clinical and/or laboratory markers for predicting stroke-associated were analyzed. We used the following keywords for search of references: “Acute stroke”, “Stroke”, “Cerebral stroke”, “Stroke-associated pneumonia”, “Etiopathogenesis”, “Diagnostic criterium”, “Treatment strategies”, “Systematic review”.
For our review, we utilized the PRISMA system (Preferred Reporting Items for Systematic review and Meta-Analysis Protocols). This system helps framing the review's objective, search strategy, and study inclusion and exclusion criteria.
![]()
The key items of our review are described below:
• P (Population): Patients with acute cerebral stroke in early stage.
• I (Intervention model): Etiopathogenesis, diagnostic criteria, risk factors, treatment strategies for SAP in acute cerebral stroke patients that were developed and published (predictors ≥ 2).
• C (Comparator): No competing group.
• O (Outcome): The outcome is connected with stroke associated pneumonia rather than its subgroups.
• S (Setting): To characterize the etiopathogenesis, diagnostic criteria, risk factors, treatment strategies of SAP in patients with acute cerebral stroke, facilitating the implementation of preventive measures to prevent adverse events.
Inclusion and exclusion criteria
The inclusion criteria were: (1) studies involving patients with acute cerebral stroke; (2) a prospective single- and multicenter cohort studies; (3) retrospective cross-over studies; (4) reported a etiopathogenesis, diagnostic criteria, risk factors, treatment strategies; (5) the outcome of interest was SAP; (6) full text articles; (7) studies published within period from 2019-2023.
The exclusion criteria were: (1) studies published out of period from 2019-2023; (2) not written in English; (3) not full text articles.
Study selection
Preliminary, duplicate studies were excluded, and then the rest studies were reviewed based on their titles and abstracts to determine their eligibility. The inclusion and exclusion criteria were assessed, full texts articles were looked through, and the reference lists of all eligible studies were examined to identify any potentially relevant studies.
Data collection
The collected information from the selected studies was categorized into two groups: (1) Basic information: included details such as the authors, years of publication, research design, participants, scientific data source, and sample size. (2) Predictors information: encompassed information about etiopathogenesis, diagnostic criteria, risk factors, treatment strategies.
We performed analysis and synthesis of selected sources: observational studies, randomized controlled studies and systematic reviews and meta-analysis.
Results
Figure 1 demonstrates the PRISMA 2020 flowchart results of the search process.
In the beginning of search, 354 indexed records were yielded totally. Overall, 148 duplicate records were identified and removed across all databases, 202 titles and abstracts were screened for eligibility. The next was reviewing for further evaluation - 86 articles were removed based on title and abstract. After the subsequent evaluation, 184 studies were excluded as they did not focus on acute stroke. Additionally, 31 studies were excluded because of not acute period of stroke, 35 studies were found to be inconsistent due to mixed population of the review, 13 studies had limited early developed pneumonia. Ultimately, 37 studies were included in this review.
Study characteristics
Studies of comparing SAP and without stroke-associated pneumonia were included concerning to patients in acute phase stroke. The acute phase stroke is typically defined as ≤ 72 h from admission. All articles were published between 2019 and 2023. Of the included studies, 12 were prospective (one-centered and multi-centered study), 7 were retrospective cohort, 5 prospective (observational), 7 were randomized control trials, narrative reviews - 6. Regarding the study subjects, all patients were with acute cerebral (ischemic or hemorrhagic) stroke. The sample sizes ranged from 239 to 146,062 participants across the studies.
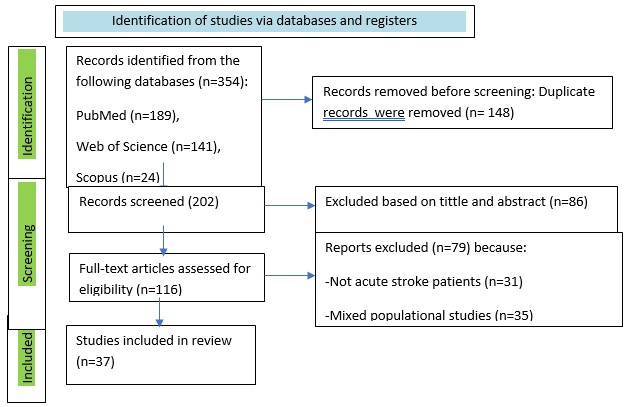
Figure 1. Flowchart of literature search and selection (PRISMA)
Stroke and SAP
Stroke is the leading cause of disability worldwide and the second leading cause of death. The Global Stroke Newsletter, published in 2022, shows that the lifetime risk of stroke has increased by 50% over the past 17 years, and it is now estimated that 1 in 4 people will have a stroke during their lifetime. From 1990 to 2019, the incidence of stroke increased by 70%, stroke mortality increased by 43%, stroke prevalence increased by 102% and the number of disability-adjusted life years increased by 143% (DALY). The most striking feature is that the bulk of the global stroke burden (86% of stroke deaths and 89% of DALYs) falls on low- and lower-middle-income countries. This disproportionately heavy burden faced by low- and middle-income countries has created an unprecedented problem for families with fewer resources (5).
In the Eurasian region, Kyrgyzstan ranks first in terms of standardized mortality according to the Republican Medical Information Center of the Ministry of Health of the Kyrgyz Republic, more than 185 deaths per 100,000 people are registered annually (6, 7). Meanwhile, 34.6% of victims die of stroke within a month, and every second patient dies within a year. Only 20% of the survivors recover almost completely (they are not assigned as a disability group) (8).
Various types of medical complications occur during hospitalization of stroke patients. These medical complications result in prolonged hospital stay, high morbidity and mortality, as well as increased costs of care of this patient population (9).
Pneumonia is a group acute infectious diseases predominantly non-specific bacterial etiology, which is characterized by localized lesions of lungs with intra-alveolar exudation, detected physically and radiologically. Clinically pneumonia is classified into following major types: community-acquired pneumonia (CAP), hospital-acquired pneumonia (HAP), healthcare-associated pneumonia (HCAP), pneumonia caused by artificial ventilation (VAP); aspiration pneumonia; pneumonia caused by opportunistic organisms; and others (9).
According to available current evidence, post-stroke pneumonia is often caused by aspiration. The upper segments of the lower lungs, as well as the posterior segments, are most vulnerable to aspiration content when it drains there primarily from the patient lying on the back.
This occurs more frequently on the right side than on the left, as the right main trunk of the bronchial tree is more aligned with the trachea. However, stroke patients undergo regular change of their position, so that any lung region can be affected (10).
Mostly SAP is related to conditions in health-care system and usually seen in predisposed patient groups, such as in older individuals and chronically debilitated patients. Most hospitalized patients are colonized with hospital flora within 48 hours. The features of HAPS, HAP and VAP are similar and interrelated, but have a number of differences. Indeed, HAP is pneumonia that occurs in a hospital more than 48 hours after hospitalization, when the patient did not suffer prior to admission to stroke. Contrarily HCAP is pneumonia seen in a patient who was closely connected to the health system and exposed to its flora (for example, hemodialysis, wound care over the last 30 days, recent intravenous antibiotic administration, chemotherapy, nursing home or pre-hospital care within 2 days over the last 3 months). VAP is a term used for pneumonia occurring more than 48-72 hours after intubation (11).
Moreover, HAP and VAP are further classified as early and late forms. This difference is important because HAP or VAP with a delayed start (5 days after hospitalization) are more often associated with pathogens that already have multidrug resistance (MDR), unlike HAP or VAP with an early (first 4 days after hospitalization) start. These important differences determine the empirical treatment of the patient at the beginning of the antibiotic therapy course (before the results of the culture). However, if a patient develops an early HAP, it means that he/she has come to the hospital with previous risk factors associated with staying in the health care system and should be treated as an HAP with a late start from previous environmental effects (10).
As already mentioned, SAP occurs in about 6.7%-37.98% of patients, as shown by the above figures with variable frequencies by different authors (4). The main reasons for these differences are unclear, but their development may be related to potentially modifiable and unmodifiable factors.
It is important to know what factors are relevant to the modifiable ones. This can lead to prevention of excessive or insufficient SAP treatment, better antibiotic treatment strategy, reduced antibiotic resistance and improved clinical outcomes.
Pathogenesis and risk factors of SAP
Several factors have been identified in the development of SAP, but the pathophysiological mechanisms are not well understood. Aspiration is one of the most common causes of SAP development, considering the weakened pharyngeal reflex in stroke patients (12). Other factors of SAP include advanced age, uncontrolled severe hypertension, female sex, malnutrition, prolonged recumbent position, and being fed through a nasogastric tube (13).
Although the nasogastric tube is used to protect against aspiration with large volume, its presence does not exclude the possibility of aspiration with a smaller volume of gastric or oropharyngeal contents. In addition, the nasogastric probe can prevent adequate coughing - the most important mechanism for protecting against aspiration and pneumonia development. On the other hand, too severe cough considered as an important predictor of SAP in acute phase of stroke. Conclusively, weakening of cough and pharyngeal reflexes is an important indicator of increased risk of pneumonia in patients with dysphagia due to stroke (14).
Another suggested mechanism for the development of pneumonia after a stroke is the weakening of the cholinergic pathways. There were studies on the role of the parasympathetic nervous system in post-stroke immunosuppression. According to the data obtained, inhibition of cholinergic signaling by vagotomy or the use of mice with nicotinic acetylcholine receptor deficiency stimulated an increased immune response and prevented pneumonia after stroke. These results showed an association between the cholinergic pathway and the risk of developing pneumonia (15).
Due to reduced chewing, salivation, swallowing and oral hygiene, patients with acute stroke are particularly vulnerable to disruption of the microbial ecology of the oral cavity. The presence of pathogenic oral bacteria in stroke patients is associated with a poor prognosis, which leads to aspiration pneumonia (16). Other studies on experimental models have shown that stroke-induced immunodeficiency increases the risk of developing aspirational pneumonia. Experiments show that immunosuppression is a necessary component that leads to the progression of bacterial aspiration to pneumonia (17).
Acute stroke weakens the peripheral immune system, which is mediated by excessive activation of the sympathetic nervous system and the hypothalamic-pituitary-adrenal system. Suppression of peripheral cellular immune responses is characterized by transient lymphopenia and deactivation of monocytes, which increases susceptibility to infection (18). In a mouse model of human stroke, stroke-affected mice developed panlymphocytopenia and lymphocyte apoptosis in lymphoid tissues, which was reversed either by blockade of β-adrenergic receptors or inhibition of glucocorticoid receptors. It was shown that a change in the tracheal epithelium caused by stroke immunomodulation worsens pulmonary clearance. Decreased pulmonary clearance and impaired mobility associated with reduced access to the respiratory tract and impaired outflow of secretions from the lungs may contribute to the development of pneumonia (12).
A number of comorbid conditions may be associated with an increased risk of SAP. These associated conditions include iron deficiency anemia, epilepsy, alcohol abuse, heart failure, respiratory disorders including vasoconstrictive conditions, diabetes, electrolytic disturbances, kidney failure and coagulopathies (19).
Among hospitalized patients with ischemic stroke, other identified risk factors for pneumonia include non-invasive and invasive mechanical ventilation, prolonged hospital stay, and hemorrhagic transformatıon of brain infarction (19).
In addition, bacteria such as Streptococcus mitis, Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa were also associated with aspiration pneumonia. Patients with acute stroke are at increased risk of colonization by respiratory pathogens during recovery. The number of pathogens, such as the Pseudomonas aeruginosa, Klebsiella pneumoniae and Escherichia coli, may increase in saliva 1 month after acute stroke that allows assuming the concomitant presence respiratory infections in this patient population (20).
SAP diagnostics
The diagnosis of pneumonia after an acute stroke remains difficult, and chest X-rays diagnostic value may be limited in the early stages. The diagnostic criteria for CAP, HAP and VAP are clearly defined in clinical protocols that are available for routine clinical practice (21- 23). Despite the lack of established criteria or gold standards to diagnose pneumonia in acute stroke, the multidisciplinary consensus launched the clinical recommendations for SAP (Table 1) (24).
|
Table 1. Recommended diagnostic criteria for a definite and probable SAP in patients not receiving artificial ventilation based on CDC7 criteria24 |
|
At least two of the followings |
|
New onset of purulent sputum, or change in character of sputum over a 24 h period, or increased respiratory secretions, or increased suctioning requirements |
|
New onset or worsening cough, or dyspnea, or tachypnea (respiratory rate>25/min) |
|
Rales, crackles, or bronchial breath sounds |
|
Worsening gas exchange (eg, O2 desaturation (eg, PaO2/FiO2≤240), increased oxygen requirements1) |
|
And ≥2 serial chest radiographs2 with at least 1 of the following: |
|
New or progressive and persistent infiltrate, consolidation, or cavitation |
|
Note: In patients without underlying pulmonary or cardiac disease, definitive chest radiograph is acceptable |
|
Probable SAP: all CDC criteria met, BUT initial CXR and serial/repeat CXR nonconfirmatory (or not undertaken), and no alternative diagnosis or explanation. |
|
Definite SAP: ALL CDC criteria met, including diagnostic CXR changes (on at least one). |
|
1Category of increased ventilator demand removed. 2CDC recommendation is to repeat CXR at days 2(7) if initial CXR negative. CDC - Center for Disease Control and Prevention, CXR – chest X-Ray; FiO2- fraction of inspired oxygen, PaO2 - partial pressure oxygen, SAP- stroke-associated pneumonia, WBC - white blood cell count (Republished under CC BY license from reference 24) |
To diagnose the SAP, it is recommended to check its compliance with the modified CDC criteria (Table 2) (25).
|
Table 2. Modified CDC criteria25 |
|
The presence of lower respiratory tract and pleural infection must meet at least one of the following criteria: |
|
The patient has an organism (s) identified by microscopy of pulmonary tissue or pleural fluid by Gram or it (they) is identified by microscopy of pulmonary tissue or pleural fluid* (if the pleural fluid was obtained during thoracocentesis or within 24 hours of the thoracic tube insertion) by method of a microbiological examination based on with/without culture, which were conducted for clinical diagnosis or treatment, for example, without active culture/investigation. |
|
In general anatomical or histopathological examination, the patient has an lung abscess or other signs of infection (e.g. empyema). |
|
The patient has evidence of an abscess or infection (pneumonia) through imaging, which, if questionable, is supported by a clinical findings, in particular, the doctor’s documentation on antimicrobial treatment of pulmonary infection. |
|
CDC - Center for Disease Control and Prevention (Republished under CC BY license from reference 25) |
Management of SAP
Medical interventions include nasogastric tube (NGT) feeding, oral care, and preventive measures, such as immunosuppression screening, antibiotics, treatment of gastroesophageal reflux, and the use of angiotensin converting enzyme (ACE) inhibitors, which have been suggested to reduce the risk of pneumonia (26, 27). Care processes included placement, mobilization, staff competence, and adherence to safe swallowing techniques.
The main result of interest was SAP. SAP is defined as the spectrum of lower respiratory tract infections during the first 7 days after the onset of stroke (27). However, given the differences in post-stroke pneumonia reports and the difficulty in establishing the onset of stroke in some patients, studies that reported pneumonia during hospitalization and within < 30 days after stroke onset were included for the purposes of this review.
The incidence of all types of nosocomial infections could be reduced by hand-washing according to CDC Guidelines for the Prevention of Hospital-acquired Pneumonia (28). According to a study conducted in 2016, systemic oral hygiene was strongly associated with a lower incidence of SAP. This approach can reduce oral colonization by pathogenic bacteria and consequently reduce the risk of developing pneumonia after a stroke (29).
In hospitalized patients with post-stroke dysphagia, a change in body position can prevent aspiration and thus play a pivotal role in the prevention of SAP. Studies have shown that the different positions of the head and neck can both hamper and facilitate swallowing, which can affect the risk of aspiration. More specifically, the semi-recumbent position of the body can facilitate the passage of food under the influence of gravity and is therefore considered as a common position to prevent aspiration (30).
Usually, the initial selection of antibiotics remains empirical, because of lack of availability of microbial culture. Therefore, the choice of treatment should be determined by the risk of MDR of bacteria in the patient. In cases where earlier development of pneumonia in patients without MDR-bacteria or risks of HAP, antibiotic therapy can be initiated by drugs of limited spectrum, such as, ceftriaxone or levofloxacin, ampicillin/sulbactam or ertapenem (31).
Treatment depends on the nature of local pathogens, i.e. streptococcal pneumonia, Haemophilus bacilli and Staphylococcus aureus sensitive to methyllin, and gram-negative bacteria are sensitive to antibiotics. In patients with apparent aspiration due to stroke, antibiotic therapy can be recommended with at least one anaerobic-coated drug (32). Antibiotic therapy for MDR pathogens must consider the resistance, virulence, and pathogenicity of normal organisms. Combined antibiotic therapy is indicated. There should be the combination of two drugs for Pseudomonas and other drug-resistant Gram-negative pathogens (i.e. Legionella) and an additional drug for methicillin-sensitive Staphylococcus aureus, such as vancomycin or linezolid (33).
There are currently standardized guidelines for the treatment and prevention of stroke-related pneumonia with antibiotics (34).
Randomized trials on antibiotic treatment of pneumonia complicating stroke have not been found. Antibiotic treatment of pneumonia complicating the course of stroke is based on the recommendations of the consensus group on pneumonia in stroke (PISCES). Consensus was reached on the following issues: (1) Stroke-related pneumonia can be caused by microorganisms associated with both community-acquired and nosocomial pneumonia; (2) Treatment of early stroke-related pneumonia (<72 hours after the onset of stroke) should cover community-acquired pneumonia microorganisms; (3) Treatment of late stroke-related pneumonia (≥72 hours and within seven days after the onset of stroke) should cover community-acquired pneumonia as well as coliforms +/- Pseudomonas spp. if there are risk factors; (4) No additional antimicrobial therapy is required for patients with dysphagia or aspiration; (5) Pneumonia that occurs seven days after the onset of stroke should be treated in the same way as nosocomial pneumonia; (6) Treatment should last at least seven days for each of these scenarios (34).
The estimated duration of treatment was evaluated, and it was concluded that antibiotic therapy scheduled for eight days found equivalent for fifteen days’ therapy of VAP. Delay in treatment of VAP and HAP can significantly alter the patient survival, and well-timed antibiotic therapy improves clinical outcomes. Since the 6% of ischemic and 30% of hemorrhagic stroke patients are intubated, a certain population of patients’ post-stroke pneumonia is at risk of VAP superinfection (35).
The optimal approach of reducing the VAP is to decrease the need for mechanical ventilation as achievable as possible. Non-invasive positive pressure ventilation (NIPPV) can be useful for treating patients with heart or lung diseases, but perhaps less for treating neurological diseases. As a rule, NIPPV is routinely not used in patients with impaired consciousness. Scheduled sedation interruption and prescribed daily extubation attempts should be encouraged. However, the desired balance between adequate analgesia and sedation remains challenging for severe neurological patients, especially those with psychomotor agitation. Short-acting agents such as Midazolam or Propofol for sedation and Fentanyl for analgesia are likely to allow physicians to assess neurological status more frequently (36).
According to literature, prophylactic antibiotic therapy for the prevention of SAP is controversial with evidence gaps. Although prophylactic antibiotics help to reduce the incidence of infection, especially urinary tract infection, long-term outcomes are not particularly affected. Anyway, for patients with stroke, the preventive use of antibiotic therapy was found reasonable option in the clinical setting (36).
There is a risk of selection bias. Studies were identified based on the selection criteria and searching system. We acknowledge that there are other studies that include SAP patients within unselected trial populations. There may also be the possibility that non-infective causes of lung inflammation (e.g. pneumonitis) may have been reported as pneumonia.
Conclusion
Stroke affects a significant number of patients every year. Patients who develop SAP have a three-fold increase in the risk of death in the first 30 days and within one year of the onset of stroke. As a rationale, detection of patients with the highest risk of SAP facilitates both prevention and treatment. The pathophysiology of SAP is multifactorial. The combination of stroke-induced immunodeficiency and aspiration of oropharyngeal secretions and gastric contents into the lungs related to impaired consciousness and dysphagia predisposes patients to SAP in the first few days post stroke. Respiratory tract infections may also precede stroke thereby contributing to stroke etiopathogenesis. The identification of high-risk patients requires knowledge of the modern criteria for diagnosing SAP. Since most SAP consists of aspiration pneumonia, HAP, HP and/or VAP, partial diagnosis, treatment, rehabilitation, are related to the provision of medical care.
Microbiology of these pneumonias varies from less virulent organisms with less drug resistance to more virulent MDR pathogens, which are now colonized by many hospitals and intensive care units. Early aggressive broad-spectrum empirical antibiotic therapy is necessary to reduce mortality from SAP. Prevention of SAP is based on prevention of aspiration, frequent hand washing and decrease the use of intubated ventilation.
Peer-review: Internal
Conflict of interest: None to declare
Authorship: T.S.K., E.M.M., A.Dj. M., A.U.T., М.A.B., and N.T. D. equally contributed to the study and fulfilled authorship criteria
Acknowledgement and funding: None to declare
References
| 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. heart disease and stroke statistics-2021 update: A Report From the American Heart Association. Circulation 2021; 143: e254-e743. doi: 10.1161/CIR.0000000000000950. https://doi.org/10.1161/CIR.0000000000000950 PMid:33501848 |
||||
| 2.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, B et al. American Heart Association Stroke Council. 2018 Guidelines for the early management of patients with acute ischemic stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018; 49: e46-e110. doi: 10.1161/STR.0000000000000158 https://doi.org/10.1161/STR.0000000000000158 PMid:29367334 |
||||
| 3.Verma R. Stroke-associated pneumonia: management issues. J Neurosci Rural Pract 2019; 10: 472-3. doi:10.1055/s-0039-1696743 https://doi.org/10.1055/s-0039-1696743 PMid:31599270 PMCid:PMC6779564 |
||||
| 4. Chen Y, Yang H, Wei H, Chen Y, Lan M. Stroke-associated pneumonia: A bibliometric analysis of worldwide trends from 2003 to 2020. Medicine (Baltimore) 2021; 100: e27321. doi: 10.1097/MD.0000000000027321. https://doi.org/10.1097/MD.0000000000027321 PMid:34559149 PMCid:PMC8462563 |
||||
| 5.Du M, Mi D, Liu M, Liu J. Global trends and regional differences in disease burden of stroke among children: a trend analysis based on the global burden of disease study 2019. BMC Public Health 2023; 23: 2120. doi: 10.1186/s12889-023-17046-z https://doi.org/10.1186/s12889-023-17046-z PMid:37891500 PMCid:PMC10612321 |
||||
| 6.Turgumbaeva JD, Akynbekov KU, Turgumbaev DD. The structure of the incidence and risk factors of stroke in Bishkek according to the register. Vestnik KazNMU 2015; 3: 92-6. | ||||
| 7.E-health Center at the Ministry of Health of the Kyrgyz Republic. Collection "Public health and the activities of healthcare organizations of the Kyrgyz Republic" for 2019; 382pp. | ||||
| 8.Samudinova TT, Kulov BB, Turgumbaev DD, Abirova AB. Epidemiology of stroke in the city of Bishkek according to the register (2017-2018). Healthcare of Kyrgyzstan 2021; 3: 90-103. Doi: 10.51350/zdravkg2021931290 https://doi.org/10.51350/zdravkg2021931290 |
||||
| 9.Tadi P, Lui F. Acute stroke. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. | ||||
| 10.Sattar SBA, Sharma S. Bacterial pneumonia. [Updated 2022 Aug 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. Available from: URL: https://www.ncbi.nlm.nih.gov/books/NBK513321/ | ||||
| 11.Sanivarapu RR, Gibson J. Aspiration pneumonia. [Updated 2023 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. Available from: URL: https://www.ncbi.nlm.nih.gov/books/NBK470459/ | ||||
| 12.Grossmann I, Rodriguez K, Soni M, Joshi PK, Patel SC, Shreya D, Z et al. Stroke and pneumonia: mechanisms, risk factors, management, and prevention. Cureus 2021; 13: e19912. doi: 10.7759/cureus.19912. https://doi.org/10.7759/cureus.19912 |
||||
| 13.Kulnik ST, Birring SS, Hodsoll J, Moxham J, Rafferty GF, Kalra L. Higher cough flow is associated with lower risk of pneumonia in acute stroke. Thorax 2016; 71: 474-5. doi: 10.1136/thoraxjnl-2015-207810. https://doi.org/10.1136/thoraxjnl-2015-207810 PMid:26834183 |
||||
| 14.Dylla L, Herson PS, Poisson SN, Rice JD, Ginde AA. Association between chronic inflammatory diseases and stroke-associated pneumonia - an epidemiological study. J Stroke Cerebrovasc Dis 2021; 30: 105605. doi: 10.1016/j.jstrokecerebrovasdis.2021.105605 https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.105605 PMid:33482567 PMCid:PMC7946738 |
||||
| 15.Lidetu T, Muluneh EK, Wassie GT. Incidence and predictors of aspiration pneumonia among stroke patients in Western Amhara Region, North-West Ethiopia: A retrospective follow up study. Int J Gen Med 2023; 16: 1303-15. doi: 10.2147/IJGM.S400420 https://doi.org/10.2147/IJGM.S400420 PMid:37089139 PMCid:PMC10115200 |
||||
| 16.Faura J, Bustamante A, Miró-Mur F, Montaner J. Stroke-induced immunosuppression: implications for the prevention and prediction of post-stroke infections. J Neuroinflammation 2021; 18: 127. doi: 10.1186/s12974-021-02177-0 https://doi.org/10.1186/s12974-021-02177-0 PMid:34092245 PMCid:PMC8183083 |
||||
| 17.Ghelani DP, Kim HA, Zhang SR, Drummond GR, Sobey CG, De Silva TM. Ischemic stroke and infection: A brief update on mechanisms and potential therapies. Biochem Pharmacol 2021; 193:114768. doi: 10.1016/j.bcp.2021.114768 https://doi.org/10.1016/j.bcp.2021.114768 PMid:34543657 |
||||
| 18.Zhang SR, Phan TG, Sobey CG. Targeting the immune system for ischemic stroke. Trends Pharmacol Sci 2021; 42: 96-105. doi: 10.1016/j.tips.2020.11.010 https://doi.org/10.1016/j.tips.2020.11.010 PMid:33341247 |
||||
| 19. Kishore AK, Vail A, Jeans AR, Chamorro A, Di Napoli M, Kalra L et al. Pneumonia in Stroke Consensus (PISCES) Group. microbiological etiologies of pneumonia complicating stroke: a systematic review. Stroke 2018l; 49: 1602-9. doi: 10.1161/STROKEAHA.117.020250 https://doi.org/10.1161/STROKEAHA.117.020250 PMid:29915122 |
||||
| 20.Martin-Loeches I, Torres A, Nagavci B, Aliberti S, Antonelli M, Bassetti et al. ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Intensive Care Med 2023; 49: 615-32. doi: 10.1007/s00134-023-07033-8 https://doi.org/10.1007/s00134-023-07033-8 PMid:37012484 PMCid:PMC10069946 |
||||
| 21.Shebl E, Gulick PG. Nosocomial Pneumonia. [Updated 2023 May 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: URL: https://www.ncbi.nlm.nih.gov/books/NBK535441/ | ||||
| 22.Safavi A, Molavynejad S, Rashidi M, Asadizaker M, Maraghi E. The effect of an infection control guideline on the incidence of ventilator-associated pneumonia in patients admitted to the intensive care units. BMC Infect Dis 2023; 23: 198. doi: 10.1186/s12879-023-08151-w https://doi.org/10.1186/s12879-023-08151-w PMid:37003964 PMCid:PMC10067205 |
||||
| 23.Smith CJ, Kishore AK, Vail A, Chamorro A, Garau J, Hopkins SJ, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in stroke consensus group. Stroke 2015; 46: 2335-40. doi: 10.1161/STROKEAHA.115.009617 https://doi.org/10.1161/STROKEAHA.115.009617 PMid:26111886 |
||||
| 24.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988; 16: 128-40. doi: 10.1016/0196-6553(88)90053-3 https://doi.org/10.1016/0196-6553(88)90053-3 PMid:2841893 |
||||
| 25.Zawiah M, Hayat Khan A, Abu Farha R, Usman A, Sha'aban A, Abu Hammour K, et al. Diagnosis and treatment of stroke associated pneumonia: Qualitative exploration of clinicians' practice. Electron J Gen Med 2023;20: em454. https://doi.org/10.29333/ejgm/12849 |
||||
| 26.Liu ZY, Wei L, Ye RC, Chen J, Nie D, Zhang G, Zhang XP. Reducing the incidence of stroke-associated pneumonia: an evidence-based practice. BMC Neurol 2022; 22: 297. doi: 10.1186/s12883-022-02826-8 https://doi.org/10.1186/s12883-022-02826-8 PMid:35953801 PMCid:PMC9367053 |
||||
| 27.Zaragoza R, Vidal-Cortés P, Aguilar G, Borges M, Diaz E, Ferrer R, et al. Update of the treatment of nosocomial pneumonia in the ICU. Crit Care 2020; 24: 383. doi: 10.1186/s13054-020-03091-2 https://doi.org/10.1186/s13054-020-03091-2 PMid:32600375 PMCid:PMC7322703 |
||||
| 28.Wagner C, Marchina S, Deveau JA, Frayne C, Sulmonte K, Kumar S. Risk of stroke-associated pneumonia and oral hygiene. Cerebrovasc Dis 2016; 41: 35-9. doi: 10.1159/000440733 https://doi.org/10.1159/000440733 PMid:26584429 |
||||
| 29.Alghadir AH, Zafar H, Al-Eisa ES, Iqbal ZA. Effect of posture on swallowing. Afr Health Sci 2017; 17:133-7. doi: 10.4314/ahs.v17i1.17 https://doi.org/10.4314/ahs.v17i1.17 PMid:29026386 PMCid:PMC5636236 |
||||
| 30.Kalra L, Irshad S, Hodsoll J, Simpson M, Gulliford M, Smithard D, et al.; STROKE-INF Investigators. Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): a prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet 2015; 386 :1835-44. doi: 10.1016/S0140-6736(15)00126-9 https://doi.org/10.1016/S0140-6736(15)00126-9 PMid:26343840 |
||||
| 31.Sluis WM, Westendorp WF, van de Beek D, Nederkoorn PJ, van der Worp HB. Preventive ceftriaxone in patients at high risk of stroke-associated pneumonia. A post-hoc analysis of the PASS trial. PLoS One 2022; 17: e0279700. doi: 10.1371/journal.pone.0279700 https://doi.org/10.1371/journal.pone.0279700 PMid:36584124 PMCid:PMC9803205 |
||||
| 32.Guillot P, Delamaire F, Gacouin A, Painvin B, Piau C, Reizine F et al. Early discontinuation of combination antibiotic therapy in severe community-acquired pneumonia: a retrospective cohort study. BMC Infect Dis 2023; 23: 611. doi: 10.1186/s12879-023-08493-5 https://doi.org/10.1186/s12879-023-08493-5 PMid:37723456 PMCid:PMC10506273 |
||||
| 33.Kishore AK, Jeans AR, Garau J, Bustamante A, Kalra L, Langhorne P, et al. Antibiotic treatment for pneumonia complicating stroke: Recommendations from the pneumonia in stroke consensus (PISCES) group. Eur Stroke J 2019 Dec; 4: 318-8. doi: 10.1177/2396987319851335 https://doi.org/10.1177/2396987319851335 PMid:31903430 PMCid:PMC6921946 |
||||
| 34.Campanella E, Marino A, Stracquadanio S, Restivo R, Micali C, Nunnari G, et al. Management of ventilator associated pneumonia due to <em>Stenotrophomonas maltophilia</em> infection: A case report and literature review. World Acad Sci J 5: 16, 2023 https://doi.org/10.3892/wasj.2023.193 |
||||
| 35.Buaniam K. Effects of a Nursing Program development in ischemic stroke patients with dysphagia on aspiration pneumonia prevention at stroke unit, Phatthalung Hospital. Health Sci J Thai 2021; 3: 74-87. Available from: URL: https://he02.tci-thaijo.org/index.php/HSJT/article/view/249023 | ||||
| 36. Vermeij JD, Westendorp WF, Dippel DW, van de Beek D, Nederkoorn PJ. Antibiotic therapy for preventing infections in people with acute stroke. Cochrane Database Syst Rev 2018; 1 :CD008530. doi: 10.1002/14651858.CD008530.pub3 https://doi.org/10.1002/14651858.CD008530.pub3 PMid:29355906 PMCid:PMC6491314 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

