Cardiotoxicity of checkpoint inhibitors: focus on immune side effects
SYSTEMATIC REVIEW
Cardiotoxicity of checkpoint inhibitors: focus on immune side effects
Article Summary
- DOI: 10.24969/hvt.2024.480
- Page(s): 302-312
- CARDIOVASCULAR DISEASES
- Published: 21/04/2024
- Received: 08/02/2024
- Revised: 13/03/2024
- Accepted: 14/04/2024
- Views: 5338
- Downloads: 3358
- Keywords: cardiotoxicity, immunotherapy, checkpoint inhibitors, outcomes
Address for Correspondence: Firdavsjon R. Akiljonov, Clinical Diagnostic Department of the Federal State Budgetary Institution National Medical Research Center for Cardiovascular Surgery named after. A.N. Bakulev"
Email: firdavs96_tths@mail.ru Mobile +79994504642;
ORICD: Firdavsjon R. Akiljonov- 0000-0002-1675-4216, Yuri I. Buziashvili - 0000-001-7016-7541,
Elmira U. Asymbekova -0000-0002-5422-2069, Elvina F. Tugeeva - 0000-0003-1751-4924,
Elena V. Artamonova - 0000-0002-8936-3590
Yuri I. Buziashvili1, Elmira U. Asymbekova1, Elvina F. Tugeeva1, Elena V. Artamonova2, Firdavsjon R. Akiljonov1
1Clinical Diagnostic Department, Federal State Budgetary Institution National Medical Research Center for Cardiovascular Surgery named after. A.N. Bakulev" of the Ministry of Health of Russia, Moscow, Russia
2Department for the Study of New Antitumor Drugs Federal State Budgetary Institution “National Medical Research Center of Oncology named after. N.N. Blokhin" of the Ministry of Health of Russia, Moscow, Russia
Abstract
Objective: The use of checkpoint inhibitors in cancer will continue to expand in the coming years, which is a promising area for future clinical research. However, diagnosis of immune-related side effects is challenging due to its heterogeneous clinical manifestations, which range from subclinical to fulminant manifestations with high in-hospital mortality. A potential mechanism may be proliferation and clonal expansion of antigens by T lymphocytes in tumor cells and affected self-tissues. The exact pathophysiological mechanism remains unclear and the risk profile of patients experiencing cardiotoxicity is unknown. The aim of our work is to focus on the immune side effects of immunotherapy and their mechanisms.
Methods: We used PubMed (Medline) database, Google Scholar for evidence search. The search depth was up to 10 years. The search phrases used were: “immune side effects”, “cardiotoxicity of immunotherapy”, “checkpoint inhibitors”. Search result by keywords in the PubMed (Medline) database, Google Scholar found a total of 205 publications. Number publications after removing duplicates amounted to 165. After analyzing the titles and their abstracts, 40 publications met the goal, 17 publications passed the full-text screening. Thus, our review finally included 13 studies.
Results: Immune checkpoint inhibitors are the most notable breakthrough in the treatment of cancer, but the wide range of adverse events associated with the immune system should not be ignored. According to the analysis of the studies cardiac adverse are reported in 13.3% of patients receiving such therapy, myocarditis – 2.1% and cardiac arrhythmias – 9%, other cardiovascular pathology is rare. The exact pathophysiological mechanism remains unclear and the risk profile of patients experiencing cardiotoxicity is unknown.
Conclusion: Continued intensive efforts by the research communities and interdisciplinary collaborations in oncology and cardiology will help address these challenges and thereby allow immunotherapy to achieve its maximum potential benefit in the treatment of cancer.
Key words: cardiotoxicity, immunotherapy, checkpoint inhibitors, outcomes
![]()
Introduction
Over the past decade, expanding therapeutic options have transformed the treatment of virtually all forms of cancer. The global burden of disease is shifting from infectious to noncommunicable diseases, creating increasing challenges for cardiologists and oncologists. During tumorigenesis, tumor cells inhibit T cell activation and effector processing by hijacking immune checkpoint molecules (ICMs), thus evade immunosurveillance and immune system attack. Therefore, regulation of T cell activity is an important target in antitumor therapy. Microenvironmental factors of the malignant process also modify the antitumor immune response involving infiltration of T cells and expression of checkpoint proteins (1).
Graphical abstract

Immune checkpoint inhibitors (ICIs) have been developed to enhance the activity of the body's own immune cells against neoplastic cells. Each group of ICIs acts on different co-stimulatory signaling molecules on T lymphocytes and antigen presenting cells (APCs), such as cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death protein (PD1) and its ligand (PD-L1) and thereby inhibit immune tolerance of T cells to tumor cells (2).
Immunotherapy plays an important role in prolonging disease-free and overall survival in patients with various types of cancer, such as melanoma, renal cell carcinoma, non-small cell lung cancer (NSCLC) and Hodgkin lymphoma. Immune responses are controlled through the modulation and adjustment of a complex network of cell surface receptors, which is necessary to protect the body from potential threats, as well as to develop immunity to self-antigens. Immune checkpoints regulate T cells, controlling their activation and modulating their effector functions, to prevent autoimmune reactions. One of the advantages of the development of ICI-based therapy over the previous decade is its molecular impact on the regression of the malignant process. The emergence and development of ICIs improved existing approaches to antitumor therapy and created the prerequisites for remission of the malignant process by stimulating the immune system against tumor cells. Despite proven effectiveness in clinical studies, the use of ICIs has led to the development of immune-related side effects (ISE) affecting various body systems. ISE are observed in around 60-80% of patients receiving immunotherapy among which 13-23% are severe side-effects, as classified by the Common Terminology Criteria. Also, the severity varies based on the chemotherapeutic agent and is reported to be higher among combination therapies (3–4). Frequently encountered adverse events include myocarditis, endocrinopathies, hepatitis, colitis and pneumonitis.
The aim of our work is to focus on the mechanisms of immune side effects of immunotherapy.
Methods
Algorithm information retrieval was developed in accordance with reporting requirements and provisions for systematic reviews and meta-analyses (PRISMA) in PubMed (Medline) database, and Google Scholar. The search depth was up to 10 years. The search phrases used were: “immune side effects”, “cardiotoxicity of immunotherapy”, “checkpoint inhibitors”. Articles were selected that addressed the topic of cardiotoxicity of immunotherapy, particularly the mechanism of immune side effects. Uninformative articles were rejected.
Inclusion criteria: studies with access to full texts; all participants were adults (18 years and older); research with adequate but the presented source data, in particular about the incidence of endpoints. To select suitable studies to be included in this systematic review, two authors independently studied abstracts and full-text reports to meet inclusion criteria. Search result by key words in the PubMed (Medline) database, and Google Scholar found a total of 205 publications. Number publications after removing duplicates amounted to 165. After analyzing the titles and their abstracts, 40 publications met the goal. 17 publications passed the full-text screening. Thus, our review finally included 13 studies (Fig.1).
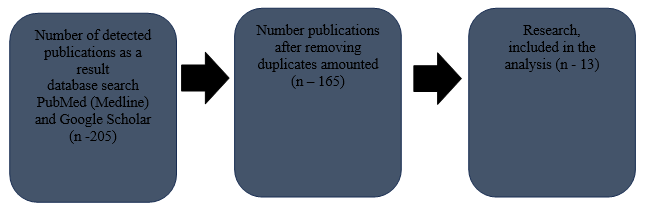
Figure1. Flowchart for selection of studies included in the review
Immune therapy-associated cardiotoxicity (IAC)
Immune therapy-associated cardiotoxicity (IAC) is rare but often fatal. Existing studies report a wide range in the incidence of myocarditis: from 0.06% for nivolumab monotherapy and 0.27% for the combination of nivolumab and ipilimumab (5). This study was conducted while actively studying the epidemiological results of cardiovascular toxicity from immunotherapy. Subsequently, pharmacovigilance studies have increased awareness of ISE, which has led to an increase in the number of reported cases in recent years. However, the diagnosis of myocarditis is challenging due to its heterogeneous clinical manifestations, which range from subclinical to fulminant manifestations (fulminate myocarditis) with high in-hospital mortality. In contrast to traditional myocarditis, immunotherapy-associated myocarditis (IAM) has a fulminant course: the reported mortality rate is 40 – 50% (6). In addition to cardiotoxicity, studies from international registries have reported other potential ISE, which include cardiomyopathies, vasculitis, cardiac arrhythmias, and pericarditis (7). However, large population-based epidemiological studies are limited. Moreover, endomyocardial biopsy remains the gold standard to confirm the diagnosis, which is invasive procedures and is rarely performed in routine clinical practice, thereby remaining unvalidated. On the other hand, immunodiagnostic and immunoassay methods have been developed to facilitate the verification and monitoring of complications of immunotherapy (8). These approaches can provide a tool for accurately identifying and predicting cardiotoxicity in cancer patients. Thus, the incidence of ISE is likely underestimated. Cardiovascular adverse events associated with immunotherapy are increasingly being reported in the literature (9). In a recently published paper on pharmacovigilance, researchers expressed concern that cardiotoxicity may be underrepresented in the current literature (10).
Among all ICIs, cytotoxic anti-CTLA-4, anti-PD-1 and its ligand anti-PD-L1 have been approved for clinical use. Anti-CTLA-4 competes with the co - stimulatory receptor CD28, thereby inhibiting T cell activation. In peripheral tissues, anti-CTLA-4 also inhibits effector T cells, promoting the immunosuppressive function of T lymphocytes. Unlike anti-CTLA-4, PD-1 inhibits T cell activation through intrinsic signaling and moderation of immunosuppression by regulatory T cells (11). To date, more than 20 ICIs have been studied in more than 800 international clinical studies. Anti-CTLA-4 antibodies (ipilimumab), PD-1 antibodies (nivolumab, pembrolizumab, cemiplimab, etc.) and PD-L1 antibodies (atezolizumab, durvalumab, avelumab, etc.) are widely used (12). The first of these drugs was ipilimumab, a CTLA-4 inhibitor, which was approved in 2011 (13). Pembrolizumab (PD-1) and nivolumab studies (PD-L1) followed shortly after clinical testing of ipilimumab. Indications for use and ICIs rapidly is expanding into first-line metastatic and adjuvant settings, with many studies being conducted in combination with standard cytotoxic targeted therapy and radiation therapy. Sustained remission of the malignant process and improved overall survival have been shown in numerous randomized clinical trials in patients treated with ICIs (14). In 2018 alone, an estimated 3.6% of cancer patients in the United States had an indication for cancer immunotherapy. Since then, the Food and Drug Administration (FDA) expanded indications for the use of ICIs (15). However, not all patients respond to immunotherapy, and overall response rates depend on tumor type and chemotherapy agent, ranging from 10.9% for ipilimumab monotherapy to 69% for pembrolizumab. We also emphasize that according to Allied Market Research estimates for 2018, the global market for ICI drugs in 2020 was estimated at 29.8 billion US dollars, and, according to forecasts, by 2030 it will reach 140.9 billion US dollars (16).
Over the past decade, perspectives in anticancer therapy have changed, leading to significant improvements in survival for patients with advanced cancer. For example, targeting PD-1 with pembrolizumab and nivolumab has been shown to restore the ability of the immune system to recognize and destroy tumor cells with improved long-term prognosis in cancer patients (17). Results of a systematic review showed that higher PD-L1 expression in patients with NSCLC leads to higher responsiveness to anti-PD-1/PD-L1 therapy (18). Compared to standard therapeutic regimens, both ICIs are administered either as monotherapy or in combination with radiation or chemotherapy. Recent studies have demonstrated the effectiveness of immunotherapy in the treatment of advanced hepatocellular carcinoma (19). Also in the study, it was shown that the use of ICIs leads to long-term remission in patients with melanoma of various locations (20). The primary mechanism of immunotherapy aims to create antitumor activity in the body by disrupting molecular patterns that allow tumor cells to evade the immune system. Compared with conventional chemotherapy, ICIs are associated with higher levels of quality of life and longer duration of subclinical cardiotoxicity. It is expected that the indications for ICIs in cancer will continue to expand in the coming years, which is a promising area for future clinical research. However, the expansion of indications is accompanied by immune-related adverse events in patients.
Role of immune checkpoints in the immune system and pathogenetic aspect of cardiotoxicity
Immune checkpoints refer to inhibitory pathways of the immune system that are critical for maintaining self-tolerance and gene homeostasis in the body. To minimize damage to self-tissue, immune checkpoints regulate the activation threshold and severity of the physiological immune response in peripheral tissues. Tolerance is defined as homeostasis, which regulates the expansion of immune cells and responses against a particular antigen, in which tumor cells, which are mutated self-peptides, are recognized as self-cells. Tolerance is established by several components, including so-called “immune checkpoint” patterns, which suppress the functions of the immune system by regulating them and producing immunosuppressive cytokines and chemokines. Activated T-cells secrete a number of cytokines that are capable of activating ICI and therefore avoiding tissue damage from inflammation as negative feedback by suppressing T-cells, acting as inhibitors of the immune system (21). From this point of view, ICI molecules play an indispensable role in the regulation of cellular immunity and the mechanisms of its resistance; however, their role in other aspects of cancer remains unclear. Recent studies have shown that ICI molecules control the microenvironment, immune and energetic responses in the tumor niche, leading to remodeling of metabolic patterns of both immune and malignant cell involvement (22).
Tumor cells disrupt T-cell activation by activating certain ICI proteins, which ultimately leads to the development of immune resistance, facilitating its spread malignant process. PD-1 and its ligands (PD-L1 and PD-L2), CTLA-4, as well as inhibitory T-cell receptors, are recognized as the main regulators of the antitumor immune response. In addition, understanding the regulatory mechanisms, including the above-mentioned receptors and their ligands, has opened up the possibility of their application for therapeutic purposes and the creation of a synergistic combination in anticancer therapy.
The mechanism of cardiotoxicity associated with ICI is not fully understood. Activation and proliferation of T-lymphocytes due to antigen exposure involve various complex mechanisms and usually require >1 stimulatory signal. The interaction between T-cell receptors and the major histocompatibility complex (MHC) of antigen presenting cell (APC) causes T-cell activation. The interactions of immune checkpoints in the tumor microenvironment are depicted in Figure 1 (23).
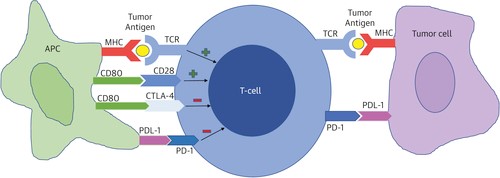
Figure 1. Activation and proliferation of T cells in response to tumor antigens presented by antigen presenting cells (APCs) depend on the net result of active stimulatory and inhibitory signals. The interaction between the major histocompatibility complex and the T -cell receptor forms the main stimulatory signal. One of the most important co - stimulatory signals arises from the interaction of CD80 on the APC and CD28 on the T cell. Inhibitory signals arise from interactions between PD-1/PDL-1 and CD80/CTLA-4. Increased expression of PDL-1 by tumor cells inhibits T cell proliferation. APC = antigen presenting cell; CD = cluster of differentiation; CTLA-4 = cytotoxic T-lymphocyte antigen 4; MHC = major histocompatibility complex; PD-1 = programmed cell death 1; PD-L1 = programmed cell death ligand 1; TCR = T cell receptor. (adapted modified from Alberts B, Johnson A, Luis J, Raff M, Roberts K, Walter P. Helper T Cells and Lymphocyte activation. In: Molecular Biology of the Cell. 4th edition, New York: Garland Science 2002.Copyright© 2002 )
T-lymphocytes are subject to positive and negative selection in the thymus to ensure self-tolerance and with specific immunological recognition of pathogenic organisms. Tumor antigens are taken up by APC, which process and present the molecular complexes of MHC-I and MHC-II to the receptors of CD8+ and CD4+ T-cells, and then identify malignant cells. The combination of these receptors on the surface of APC and CD28 on the surface of T-cells represents synergistic activation signals, leading to cytoskeletal remodeling, cytokine secretion, and lymphocyte differentiation. Activated CD4+ T cells secrete cytokines that stimulate the proliferation of CD8+ T-cells in the lymph nodes. Activated CD8+ T-cells can reach the tumor through the bloodstream, recognize MHC -I and lyse tumor cells. Activated CTLA-4 and PD-1 protect autoantigens from abnormally activated T-cells. CTLA-4 can induce transendocytosis of ligands to reduce the co-stimulatory signal to protect its own cells. PD-1, in combination with PD-L1, negatively mediates T-cell proliferation and activation. CTLA-4 not only competes with CD28, but also causes the death of regulatory T-cells, resulting in an imbalance between regulatory and cytotoxic immune cells. Monoclonal antibody CTLA-4 effectively eliminates regulatory cells from tumors through antibody-dependent cell-mediated cytotoxicity, thereby reducing the immunosuppressive effect to achieve an antitumor response. However, regulatory cells play an important role in peripheral tolerance and the maintenance of immune homeostasis. A decrease in the number of peripheral natural cells causes the immune system to attack the body, leading to the development of ISE. PD-1 plays an important role in T-cell homeostasis and inhibition of inflammation in peripheral tissues (24).
Lymphocyte activation gene 3 (LAG3) protein is a negative immunomodulator that regulates T-cell and dendritic cell function by binding to MHC-II. LAG-3 has an intracellular domain that inhibits effector CD4 + T cells and an extracellular domain similar to CD4, but with higher affinity for MHC-II, which is expressed on the surface of malignant cells. When LAG-3 binds to the surface of T cells, the immune system mistakes tumor cells for its own, facilitating camouflage through the immune system. After " immunoediting " malignant cells can also express ICI to reactivate antitumor immune response mechanisms. Consequently, inhibitors of CTLA-4, PD-1, PD-L1 and LAG3 have been approved by the FDA for the purpose of antitumor therapy for cancer (25).
Several reports have been published on inflammatory cytokines in IAC. Several cytokines have been identified, including interferon γ, interleukin 1β (IL-1β), and chemokine ligand 10. The release of cytokines and chemokines has been attributed to the recruitment of neutrophils and macrophages in cardiomyocytes. In heart failure, elevated levels of these cytokines lead to local recruitment of T lymphocytes and other immune cells for further lysis of cardiomyocytes. Cytokines such as IL -6 may directly increase the risk of cardiac arrhythmias. In addition, some cytokines can directly change automaticity of the heart. IL-1β increases the risk of arrhythmias associated with oxidation and phosphorylation of Ca+ channels in cardiomyocytes (26). However, large randomized studies are needed to determine potential and prognostic role of cytokines in immunotherapy cardiovasculotoxicity.
Risk factors for immunotherapy-associated cardiotoxicity
It is important to identify patients at increased risk of cardiotoxicity before undergoing antineoplastic therapy, and several scores have been proposed for baseline assessment, although none of these prognostic scores have been validated in prospective randomized trials clinical studies. A multimodal approach with sequential clinical assessment based on a detailed history and physical examination, combined with analysis of cardiac-specific biomarkers and cardiovascular imaging techniques, can help in risk stratification and prognosis of cardiovascular events.
In patients receiving combination therapy with ICIs, the incidence of myocarditis was much higher compared to with monotherapy. However, in a systematic review, no significant differences were found for ISE (27). Also registered increased incidence of adverse events in patients receiving anti-PD-1 compared to anti-CTLA-4 (28). In this study, the frequency of complications did not vary depending on the cancer and in patients with an existing cardiac history. Similarly, using univariate regression analysis, Minotti with co-authors (29) found that a previous history of cardiovascular diseases (CVD) is not associated with an increased risk of mortality. On the contrary, Patel et al (30) reported that patients with myocarditis had a higher prevalence of cardiovascular risk factors. However, the results are inconclusive regarding whether patients with pre-existing CVD are at increased risk of cardiotoxicity or not. In turn, it was found that older age, male gender, African race and history of CVD were associated with an increased risk of developing cardiotoxicity within a year after antitumor immunotherapy. Also in the general population, the risk increases with age and is more common among Africans compared to other races. Cardiovascular toxicity was lower in women than in men, although women have a higher mortality rate and poor long-term prognosis. When analyzing the FDA Adverse Event Reporting System database, Baik et al. (31) found 13,096 cases of IAC, and the risk of myocarditis was significantly higher in female patients (p = 0.004) and age over 75 years (p < 0.001). Also, in meta-analysis by Rodgers J. et al. (32) identified direct and indirect correlation risk factors such as age, gender, race and excess body weight with cardiotoxicity. Taken together, there is a lack of large international registries and cohorts to evaluate reliable risk factors for cardiotoxicity.
Adverse cardiovascular events with immune checkpoint inhibitors
Antitumor immunotherapy has the potential to affect every organ system, especially the cardiovascular, gastrointestinal, endocrine, respiratory and nervous systems. According to epidemiological data a higher incidence of cardiotoxicity was found than previously reported in clinical studies (33). Cardiotoxicity developed in 12.5% by 12 months after completion of immunotherapy courses. The most common one was about arrhythmias occurred in 9.3%, and myocarditis developed in 2.1%. Dong H.et al (2022) analyzed 22 clinical trials using PD1 and PD-L1 inhibitor monotherapy in their study. By the 9th month, 9% developed cardiac arrhythmias, 3 % had acute myocardial infarction, 1 % had myocarditis, 1.7 % had pericarditis (34).
|
Table 1. Adverse cardiac effects associated with immune checkpoint inhibitors |
|||||
|
Authors |
Year |
Type of the study |
Therapy |
Follow-up |
Cardiotoxicity |
|
Waheed et al. (33) |
2021 |
Observational |
Immunotherapy |
12 months |
12.5% Arrhythmias 9.3% Myocarditis 2.1% |
|
Dong et al. (34) |
2022 |
Meta-analysis of 22 trials |
PD1 and PD-L1 inhibitors monotherapy |
9 months |
Arrhythmias 9% Acute myocardial infarction – 3% Myocarditis – 1% Pericarditis 1.7% |
|
Ito et al. (35) |
2023 |
Registry |
PD-1 inhibitor CTLA 4 inhibitor |
1 year |
PD – 1 inhibitor – 9.7% CTLA -4 inhibitor – 7.5% |
|
Chitturi et al. (36) |
2019 |
Observational |
Antitumor immunotherapy |
6 months |
13.3% |
|
Cathcart-Rake et al. (38) |
2020 |
Observational |
PD1 and PD-L1 inhibitors |
9 months 1 year |
Myocarditis 9 months - 0.89% 1 year – 2.1% |
Also, a nationwide Danish study examined the cumulative outcomes of cardiotoxicity. The absolute risk within a year after initiation of immunotherapy was 6.6% in patients receiving PD-1 inhibitors; 7.5% – CTLA-4 inhibitors; 9.7% – PD-1 inhibitors (35). Chitturi K. with co-authors (2019) studied 252 patients who received antitumor immunotherapy. In this study, adverse cardiovascular events occurred in 13.3% of patients during a median follow-up period of 6 months (36, 37).
Data on IAC focus primarily on the development of myocarditis. Although clinical studies have reported a low incidence (0.09%), real data indicate a higher prevalence rate. Cathcart-Rake with co-authors (2020) evaluated the incidence of ISE in patients with NSCLC treated with PD-1 or PD-L1 inhibitors and found that the incidence of myocarditis was 0.89% at 9 months and 2.1% at one year after immunotherapy (38). There was also a higher incidence of ISE (0.27% vs 0.06%) in patients receiving the combination of nivolumab and ipilimumab. In some cases, late onset myocarditis occurred more than a year after the start of immunotherapy. Threshold occupancy of PD-1 and PD-L1 receptors may persist long after cessation of infusion, which may partly explain the wide range of time to clinical manifestation and the wide range of immunotherapy toxicities. Infiltration of CD4+/CD8+ T cells and CD68+ macrophages with subsequent lysis of cardiomyocytes is accompanied by IAM. Clonal cytotoxic CD8+ cells are enriched in the blood of patients with cardiotoxicity and are characterized by unique transcriptional changes, including activation of circulating chemokines. Direct cytotoxic destruction of cardiomyocytes by T-cells and overexpression of proinflammatory cytokines contribute to the development of myocarditis. Thavendiranathan with co-authors (39) demonstrated that CTLA4, in the context of complete genetic absence of PD-1, leads to sudden cardiac death, manifested by myocardial infiltration of T-cells and macrophages, and severe life-threatening cardiac arrhythmias. Also, PD-1 deficiency led to the development of fulminate myocarditis with infiltration of T-cells and macrophages into cardiomyocytes. It was also found that different B cell subtypes in the peripheral blood of cancer patients are associated with responsiveness to anticancer therapy (40). In addition to cardio- specific antibodies, autoantibodies induced by ICI-therapy, such as anti-acetylcholine receptors, anti-striated muscle antibodies and anti-mitochondrial antibodies, have been identified. For example, antibodies against striated muscle can react with both cardiomyocyte and skeletal muscle antigens, causing antibody-dependent cellular cytotoxicity. In addition, autoantibodies can induce activation of complement-dependent cytotoxicity, which leads to lysis of self-cells. These studies show that both cellular and humoral immunity contribute to the development of IAC (41). The mechanisms by which immune cells are recruited to the myocardium are not fully understood. ICIs components are constitutively expressed in endothelial cells, cardiomyocytes, etc., providing potential targets for antitumor immunotherapy. Clonal T-cells targeting identical antigens in skeletal muscle and lung tissue may explain the concomitant presentation of pneumonitis and myositis.
At this stage, other theories of pathogenesis are being actively studied, including increased autoantibodies targeting cardiomyocyte autoantigens and inflammatory infiltration.
Takotsubo syndrome manifests itself in the period from 15 weeks to 8 months after the start of ICI courses. Epidemiological data are lacking and the literature remains limited to single case reports. Takotsubo syndrome is an acute and transient syndrome of regional left ventricular failure.
The pathogenetic aspect of this complication when using ICI remains uncertain. The world literature presents isolated clinical cases of the development of this syndrome in patients with melanoma after combination immunotherapy (42).
Similarly, epidemiological data on dilated cardiomyopathy caused by ICIs are limited due to its low incidence. Nishimura and co-authors (43) found that patients with a PD-1 defect are more likely to develop severe dilated cardiomyopathy. Potential pathogenetic mechanisms may include the immune response of activated T cells in the vessels and myocardium (43). Taken together, there is a lack of large randomized studies to provide a more detailed pathophysiological and prognostic assessment of this complication.
Vasculitis is associated with autoimmune processes and can occur in vessels of various sizes and diameters. The frequency of vasculitis is less than 1%, and large epidemiological data are not presented. Currently, according to world literature large vessel vasculitis, such as giant cell arteritis, has been reported in the study by Miyabe et al (44). The likely mechanism is and activation of T cells and NK cells, as well as the secretion of proinflammatory cytokines, which leads to inflammation of the vascular wall with subsequent thrombosis. Computed tomography allows the diagnosis of vasculitis, which is characterized by diffuse peripheral thickening of the vessel wall and thrombosis. Therefore, antitumor immunotherapy offers promising opportunities for the treatment of cancer, but attention needs to be paid to the verification and methods of preventing ISE.
Diagnosis of cardiotoxicity associated with immunotherapy
The European Society of Cardiology guidelines on cardiovascular toxicity associated with anticancer therapy recommend that the frequency of monitoring after an initial assessment be based on the patient's baseline cardiovascular risk and chemotherapy/immunotherapy protocol (45). As part of a multimodal approach, the first step is that patients before and during courses of immunotherapy are recommended to regularly monitor the electrocardiogram (ECG) to assess rhythm disturbances and cardiac conduction. Approximately 40% of patients had ECG abnormalities such as a prolonged PR interval, decreased R wave amplitude, prolonged QT interval, Q waves, and ST-T segment changes. However, a study of 35 patients undergoing immunotherapy reported an 89% incidence of ECG abnormalities (46). Although cardiac rhythm and conduction disturbances are nonspecific, their verification is important, which requires more highly sensitive studies. Cardiac-specific biomarkers, including troponin I (TrI), brain natriuretic peptide (BNP), and N-terminal fragment of brain natriuretic peptide (NT-proBNP), are effective markers for assessing and predicting cardiovascular toxicity. Troponin-I is currently considered the most significant biomarker for the diagnosis of cardiotoxicity associated injury and monitoring is recommended during the first 6 weeks after initiation of immunotherapy courses (47). Large randomized trials have shown that an increase in troponin-I occurs in 94% of patients, and an increase in NT-proBNP may be present in 65% of cases of myocarditis (48). As with ECG, circulating biomarker levels are insufficient to rule out myocarditis. Echocardiography is an important tool for assessing structural and functional changes in the heart. Changes in systolic and diastolic function of the left ventricular myocardium, local contractility index and pericardial effusion compared to baseline values may indicate a diagnosis of IAM. Patients also experience a decrease in the most sensitive echocardiographic parameter, global longitudinal strain (GLS), which directly correlates with the incidence of cardiovascular events (49).
Magnetic resonance imaging (MRI) is currently considered the “gold standard” in the verification of myocarditis. Various MRI modalities, such as diffusion-weighted imaging (T1/2) and late gadolinium enhancement (LGE), can demonstrate myocardial edema and damage. In a retrospective cohort study, myocardial T1 and T2 values were significantly elevated in 78% and 43% of patients, respectively, and T1 values directly correlated with histopathological changes and were independent prognostic parameters of poor long-term prognosis (50). Some patients may show no signs of LGE, in part due to the early phase of the disease.
Therefore, if myocarditis is suspected, repeat cardiac MRI should be considered after 2 to 3 days. Limited availability and high cost of the study may limit the use of cardiac MRI in routine clinical practice.
Endomyocardial biopsy (EMB) is the gold standard for diagnosing myocarditis. However, due to its invasive nature and potential complications (overall incidence 6%), it is rarely used as a first-line test. Data on the use of EMB in myocarditis are limited. Based on data from international studies, the histopathological picture was mainly lymphocytic infiltration, and 56% had myocardial fibrosis (51).
By integrating all clinical and diagnostic characteristics, unified diagnostic criteria for IAM were proposed in 2019. A diagnosis of definite myocarditis can only be made if any of the following criteria are present: (1) EMB is suggestive of myocarditis; (2) myocarditis confirmed by MRI, together with elevated cardiac-specific biomarkers; and (3) diffuse hypokinesis with a high index of local contractility impairment in combination with the clinical picture of myocarditis, elevated biomarkers and the absence of coronary lesions (52).
Once diagnosis is made, treatment should be initiated immediately to avoid high in-hospital mortality. There are currently no randomized studies evaluating the effectiveness of treatment for cardiotoxicity associated injury. At this stage, treatment focuses on immunosuppressive therapy. As a rule, severe manifestations of immune cardiotoxicity require the use of high doses of corticosteroids (prednisolone 1–2 mg/kg/day or methylprednisolone 1–2 mg/kg/day), and their use should be continued for 4–6 weeks. A higher initial dose and earlier initiation of treatment have been found to be associated with a good long-term prognosis (53). Compared with low-dose corticosteroids (<60 mg/day), high doses (501–1000 mg/day) were associated with a 73% reduction in the risk of adverse cardiovascular events. The dose of the drug should be gradually reduced over 4–6 weeks after normalization of clinical and diagnostic parameters. For patient’s refractory to corticosteroid therapy within 24 hours, immunosuppressive treatments should be added, including plasmapheresis, intravenous immunoglobulins, antithymocyte globulin, mycophenolate mofetil, tacrolimus, and infliximab. A recently published report found that abatacept (a CTLA-4 agonist) is effective for the treatment of corticosteroid-resistant myocarditis, but attention should be paid to the potential risk of opportunistic infections (54). Second-line therapy may also be considered, including infliximab, mycophenolate mofetil, and plasmapheresis. There are several reports of the use of infliximab as second-line therapy; however, it should be used with caution. The optimal duration of this therapy is unknown, but it is advisable to continue treatment until symptoms resolve and troponin levels and left ventricular ejection fraction normalize. During therapy with high doses of corticosteroids, preventive strategies are recommended, such as the prevention of Pneumocystis pneumonia, the use of a proton pump inhibitor and nystatin for oral candidiasis. Immunosuppressive therapies such as immunoglobulin, tacrolimus, infliximab, mycophenolate mofetil, and methotrexate may also serve as potential treatments for IAM (55). However, the effectiveness of second-line therapy has not been tested in large observational or prospective studies. Taken together, individualized treatment strategies designed for patients require the active collaboration of a multidisciplinary team consisting of oncologists, cardiologists, and immunologists. Cardiotoxicity of antitumor immunotherapy remains a diagnostic and therapeutic challenge due to the lack of convincing evidence to support the above treatment strategies. One of the major advantages of the development of immunotherapy over the previous decade is its profound impact on anticancer therapy. The advent and development of immune checkpoint inhibitors` therapies have improved current approaches to cancer treatment by stimulating the immune system against tumor cells. Cardiovascular toxicity from immunotherapy is a relatively new clinical entity that continues to expand as more sophisticated immunotherapy regimens are rapidly developed.
Study limitations
First, our systematic review included a small number of studies. Second, inclusion and exclusion criteria varied across studies in most cases. In addition, registries, as an option for observational studies, are also susceptible to the influence of selection bias.
Conclusion
Immune checkpoint inhibitors are the most notable breakthrough in the treatment of cancer, but the wide range of adverse events associated with the immune system should not be ignored. Although cardiotoxicity associated injury is rare, it is potentially malignant and is attracting increasing attention from researchers. The exact pathophysiological mechanism remains unclear and the risk profile of patients experiencing cardiotoxicity is unknown. Future large multicenter prospective studies are of paramount importance and should help improve understanding of the extent of cardiovascular events, as well as provide insight into optimal management of this patient population.
Immunotherapy have revolutionized anticancer therapy, and their clinical use is rapidly expanding, leading to increased life expectancy. Prevention of cardiotoxicity is critical to determining the cardiovascular prognosis of patients undergoing immunotherapy. Continued intensive efforts by the research communities and interdisciplinary collaborations in the fields of oncology and cardiology will help address these challenges and thereby allow immunotherapy to achieve its maximum potential benefit in the treatment of cancer.
Peer-review: External and Internal
Conflict of interest: None to declare
Authorship: Y.I.B., E.U.A., E.F.T., E.V.A. and F.R.A. equally contributed to the study, preparation of manuscript and fulfilled authorship criteria
Acknowledgement and funding: None to declare
References
| 1.Xiang Y, Gong M, Deng Y, Wang H, Ye D. T cell effects and mechanisms in immunotherapy of head and neck tumors. Cell Commun Signal 2023; 21: 49. doi:10.1186/s12964-023-01070-y https://doi.org/10.1186/s12964-023-01070-y PMid:36872320 PMCid:PMC9985928 |
||||
| 2.Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016; 39: 98-106. doi:10.1097/COC.0000000000000239 https://doi.org/10.1097/COC.0000000000000239 PMid:26558876 PMCid:PMC4892769 |
||||
| 3.L'Orphelin JM, Varey E, Khammari A, Dreno B, Dompmartin A. Severe late-onset grade III-IV adverse events under immunotherapy: a retrospective study of 79 cases. Cancers (Basel) 2021; 13: 4928. doi:10.3390/cancers13194928 https://doi.org/10.3390/cancers13194928 PMid:34638410 PMCid:PMC8508100 |
||||
| 4.L'Orphelin JM, Dollalille C, Akroun J, Alexandre J, Dompmartin A. Cardiovascular immunotoxicity associated with immune checkpoint inhibitors in metastatic melanoma. Cancers (Basel) 2023; 15: 2170. doi:10.3390/cancers15072170 https://doi.org/10.3390/cancers15072170 PMid:37046831 PMCid:PMC10093552 |
||||
| 5.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016; 375: 1749-55. doi:10.1056/NEJMoa1609214 https://doi.org/10.1056/NEJMoa1609214 PMid:27806233 PMCid:PMC5247797 |
||||
| 6.Frascaro F, Bianchi N, Sanguettoli F, Marchini F, Meossi S, Zanarelli L. Immune checkpoint inhibitors-associated myocarditis: diagnosis, treatment and current status on rechallenge. J Clin Med 2023; 12: 7737. doi:10.3390/jcm12247737 https://doi.org/10.3390/jcm12247737 PMid:38137806 PMCid:PMC10744238 |
||||
| 7.Bi H, Ren D, Wang Q, Ding X, Wang H. Immune checkpoint inhibitor-induced myocarditis in lung cancer patients: a case report of sintilimab -induced myocarditis and a review of the literature. Ann Palliat Med 2021; 10: 793-802. doi:10.21037/apm-20-2449 https://doi.org/10.21037/apm-20-2449 PMid:33545801 |
||||
| 8.Wong CK, Lam TH, Liao SY, Lau YM, Tse HF, So BYF. Immunopathogenesis of immune checkpoint inhibitor induced myocarditis: insights from experimental models and treatment implications. Biomedicines 2023; 11: 107. doi:10.3390/biomedicines11010107 https://doi.org/10.3390/biomedicines11010107 PMid:36672615 PMCid:PMC9855410 |
||||
| 9.Chiang CH, Chiang CH, Ma KS, Hsia YP, Lee YW, Wu HR, et al. The incidence and risk of cardiovascular events associated with immune checkpoint inhibitors in Asian populations. Jpn J Clin Oncol 2022; 52: 1389-98. doi:10.1093/ jjco /hyac150 https://doi.org/10.1093/jjco/hyac150 PMid:36208180 PMCid:PMC9721460 |
||||
| 10.Ball S, Ghosh RK, Wongsaengsak S, Bandyopadhyay D, Ghosh GC, Aronow WS, et al. Cardiovascular toxicities of immune checkpoint inhibitors: JACC Review Topic of the Week. J Am Coll Cardiol 2019; 74: 1714-27. doi:10.1016/ j.jacc.2019.07.079 https://doi.org/10.1016/j.jacc.2019.07.079 PMid:31558256 |
||||
| 11.Hayashi H, Nakagawa K. Combination therapy with PD-1 or PD-L1 inhibitors for cancer. Int J Clin Oncol 2020; 25: 818-30. doi: 10.1007/s10147-019-01548-1 https://doi.org/10.1007/s10147-019-01548-1 PMid:31549270 |
||||
| 12.Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H et al. Immune checkpoint inhibitors in cancer therapy. Curr Oncol 2022; 29: 3044-60. doi:10.3390/curroncol29050247 https://doi.org/10.3390/curroncol29050247 PMid:35621637 PMCid:PMC9139602 |
||||
| 13.Savoia P, Astrua C, Fava P. Ipilimumab (Anti-Ctla-4 Mab) in the treatment of metastatic melanoma: Effectiveness and toxicity management. Hum Vaccin Immunother 2016; 12: 1092-101. doi:10.1080/21645515.2015.1129478 https://doi.org/10.1080/21645515.2015.1129478 PMid:26889818 PMCid:PMC4963052 |
||||
| 14.Ventola CL. Cancer Immunotherapy, Part 3: challenges and future trends. P.T. 2017; 42: 514-21. | ||||
| 15.Eno J. Immunotherapy through the years. J Adv Pract Oncol 2017; 8: 747-53. https://doi.org/10.6004/jadpro.2017.8.7.8 |
||||
| 16.Pan C, Liu H, Robins E, Song W, Liu D, Li Z, et al. Next-generation immuno-oncology agents: current momentum shifts in cancer immunotherapy. J Hematol Oncol 2020; 13: 29. doi:10.1186/s13045-020-00862-w https://doi.org/10.1186/s13045-020-00862-w PMid:32245497 PMCid:PMC7119170 |
||||
| 17.Parvez A, Choudhary F, Mudgal P, Khan R, Qureshi KA, Farooqi H, et al. PD-1 and PD-L1: architects of immune symphony and immunotherapy breakthroughs in cancer treatment. Front Immunol 2023; 14: 1296341. doi:10.3389/fimmu.2023.1296341 https://doi.org/10.3389/fimmu.2023.1296341 PMid:38106415 PMCid:PMC10722272 |
||||
| 18.Xu Y, Wan B, Chen X, Zhan P, Zhao Y, Zhang T et al. The association of PD-L1 expression with the efficacy of anti-PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: a meta-analysis of randomized controlled trials. Transl Lung Cancer Res 2019; 8: 413-28. doi:10.21037/tlcr.2019.08.09 https://doi.org/10.21037/tlcr.2019.08.09 PMid:31555516 PMCid:PMC6749123 |
||||
| 19.Mandlik DS, Mandlik SK, Choudhary HB. Immunotherapy for hepatocellular carcinoma: Current status and future perspectives. World J Gastroenterol 2023; 29: 1054-75. doi:10.3748/ wjg.v29.i 6.1054 https://doi.org/10.3748/wjg.v29.i6.1054 PMid:36844141 PMCid:PMC9950866 |
||||
| 20.Knight A, Karapetyan L, Kirkwood JM. Immunotherapy in melanoma: recent advances and future directions. Cancers (Basel) 2023; 15: 1106. doi:10.3390/cancers15041106 https://doi.org/10.3390/cancers15041106 PMid:36831449 PMCid:PMC9954703 |
||||
| 21.Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol 2020; 17: 807-21. doi:10.1038/s41423-020-0488-6 https://doi.org/10.1038/s41423-020-0488-6 PMid:32612154 PMCid:PMC7395159 |
||||
| 22.Eskandari-Malayeri F, Rezaei M. Immune checkpoint inhibitors as mediators for immunosuppression by cancer-associated fibroblasts: A comprehensive review. Front Immunol 2022; 13: 996145. doi:10.3389/fimmu.2022.996145 https://doi.org/10.3389/fimmu.2022.996145 PMid:36275750 PMCid:PMC9581325 |
||||
| 23.Huang Q, Zheng Y, Gao Z, Yuan L, Sun Y, Chen H. Comparative efficacy and safety of PD-1/PD-L1 inhibitors for patients with solid tumors: a systematic review and Bayesian network meta-analysis. J Cancer 2021; 12: 1133-43. doi:10.7150/jca.49325 https://doi.org/10.7150/jca.49325 PMid:33442411 PMCid:PMC7797652 |
||||
| 24.Boussiotis VA, Chatterjee P, Li L. Biochemical signaling of PD-1 on T cells and its functional implications. Cancer J 2014; 20: 265-71. doi:10.1097/PPO.0000000000000059 https://doi.org/10.1097/PPO.0000000000000059 PMid:25098287 PMCid:PMC4151049 |
||||
| 25.Paucek RD, Baltimore D, Li G. The cellular immunotherapy revolution: arming the immune system for precision therapy. Trends Immunol 2019; 40: 292-309. doi:10.1016/j.it.2019.02.002 https://doi.org/10.1016/j.it.2019.02.002 PMid:30871979 |
||||
| 26.Liu H, Zhao Y, Xie A, Kim TY, Terentyeva R, Liu M, et al. Interleukin-1 β , oxidative stress, and abnormal calcium handling mediate diabetic arrhythmic risk. JACC Basic Transl Sci 2021; 6: 42-52. doi:10.1016/ j.jacbts.2020.11.002 https://doi.org/10.1016/j.jacbts.2020.11.002 PMid:33532665 PMCid:PMC7838050 |
||||
| 27.Cone EB, Haeuser L, Reese SW, Marchese M, Nguyen DD, Nabi J, et al. Immune checkpoint inhibitor monotherapy is associated with less cardiac toxicity than combination therapy. PLoS One 2022; 17: e 0272022. doi:10.1371/ journal.pone.0272022 https://doi.org/10.1371/journal.pone.0272022 PMid:36318537 PMCid:PMC9624428 |
||||
| 28.Shalata W, Abu-Salman A, Steckbeck R, Mathew Jacob B, Massalha I, Yakobson A. Cardiac toxicity associated with immune checkpoint inhibitors: a systematic review. Cancers (Basel) 2021; 13: 5218. doi:10.3390/cancers13205218 https://doi.org/10.3390/cancers13205218 PMid:34680365 PMCid:PMC8534225 |
||||
| 29.Minotti G, Menna P, Camilli M, Salvatorelli E, Levi R. Beyond hypertension: Diastolic dysfunction associated with cancer treatment in the era of cardio-oncology. Adv Pharmacol 2022; 94: 365-409. doi:10.1016/bs.apha.2022.02.002 https://doi.org/10.1016/bs.apha.2022.02.002 PMid:35659376 |
||||
| 30.Dal'bo N, Patel R, Parikh R, Shah SP, Guha A, Dani SS, Ganatra S. Cardiotoxicity of contemporary anticancer immunotherapy. Curr Treat Options Cardiovasc Med 2020; 22: 62. doi:10.1007/s11936-020-00867-1 https://doi.org/10.1007/s11936-020-00867-1 PMid:33162729 PMCid:PMC7605901 |
||||
| 31.Baik AH, Oluwole OO, Johnson DB, Shah N, Salem JE, Tsai K, et al. Mechanisms of cardiovascular toxicities associated with immunotherapies. Circ Res 2021; 128: 1780-801. doi:10.1161/CIRCRESAHA.120.315894 https://doi.org/10.1161/CIRCRESAHA.120.315894 PMid:33934609 PMCid:PMC8159878 |
||||
| 32.Rodgers JL, Jones J, Bolleddu SI, Vanthenapalli S, Rodgers LE, Shah K, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis 2019; 6: 19. doi:10.3390/jcdd6020019 https://doi.org/10.3390/jcdd6020019 PMid:31035613 PMCid:PMC6616540 |
||||
| 33.Waheed N, Fradley MG, DeRemer DL, Mahmoud A, Shah CP, Langaee T, et al. Newly diagnosed cardiovascular disease in patients treated with immune checkpoint inhibitors: a retrospective analysis of patients at an academic tertiary care center. Cardiooncology 2021; 7: 10. doi:10.1186/s40959-021-00097-9 https://doi.org/10.1186/s40959-021-00097-9 PMid:33736707 PMCid:PMC7977591 |
||||
| 34.Dong H, Qi Y, Kong X, Wang Z, Fang Y, Wang J. PD-1/PD-L1 Inhibitor-associated myocarditis: epidemiology, characteristics, diagnosis, treatment, and potential mechanism. Front Pharmacol 2022; 13: 835510. doi:10.3389/fphar.2022.835510 https://doi.org/10.3389/fphar.2022.835510 PMid:35517794 PMCid:PMC9062035 |
||||
| 35.Ito T, Kaku-Ito Y, Ohno F, Nakahara T. A real-world study on the safety profile of extended-interval dosing of immune checkpoint inhibitors for melanoma: a single-center analysis in Japan. Front Med (Lausanne) 2023; 10: 1293397. doi:10.3389/fmed.2023.1293397 https://doi.org/10.3389/fmed.2023.1293397 PMid:38143437 PMCid:PMC10740208 |
||||
| 36.Chitturi KR, Xu J, Araujo-Gutierrez R, Bhimaraj A, Guha A, Hussain I, et al. Immune checkpoint inhibitor-related adverse cardiovascular events in patients with lung cancer. JACC CardioOncol 2019; 1: 182-92. doi:10.1016/ j.jaccao.2019.11.013 https://doi.org/10.1016/j.jaccao.2019.11.013 PMid:34396181 PMCid:PMC8352266 |
||||
| 37.Zhao F, Shen D, Shang M, Yu H, Zuo X, Chen L, et al. Immunotherapy: A new target for cancer cure (Review). Oncol Rep 2023; 49: 100. doi:10.3892/or.2023.8537 https://doi.org/10.3892/or.2023.8537 PMid:36999633 PMCid:PMC10091078 |
||||
| 38.Cathcart -Rake EJ, Sangaralingham LR, Henk HJ, Shah ND, Riaz IB, Mansfield AS. A population-based study of immunotherapy-related toxicities in lung cancer. Clin Lung Cancer 2020; 21: 421-7.e2. doi:10.1016/ j.cllc.2020.04.003 https://doi.org/10.1016/j.cllc.2020.04.003 PMid:32446852 PMCid:PMC7486993 |
||||
| 39.Thavendiranathan P, Zhang L, Zafar A. Myocardial T1 and T2 mapping by magnetic resonance in patients with immune checkpoint inhibitor-associated myocarditis. J Am Coll Cardiol 2021; 77: 1503-16. doi:10.1016/ j.jacc.2021.01.050 https://doi.org/10.1016/S0735-1097(21)04410-7 |
||||
| 40.Zotova L. Immune checkpoint inhibitors-related myocarditis: a review of reported clinical cases. Diagnostics (Basel) 2023; 13: 1243. doi:10.3390/diagnostics13071243 https://doi.org/10.3390/diagnostics13071243 PMid:37046461 PMCid:PMC10093455 |
||||
| 41.Bockstahler M, Fischer A, Goetzke C. Heart-specific immune responses in an animal model of autoimmune-related myocarditis mitigated by an immunoproteasome inhibitor and genetic ablation. Circulation 2020; 141: 1885-902. doi:10.1161/CIRCULATIONAHA.119.043171 https://doi.org/10.1161/CIRCULATIONAHA.119.043171 PMid:32160764 |
||||
| 42.Trontzas IP, Vathiotis IA, Kyriakoulis KG, Sofianidi A, Spyropoulou Z, Charpidou A, et al. Takotsubo cardiomyopathy in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-summary of included cases. Cancers (Basel) 2023; 15: 2637. doi:10.3390/cancers15092637 https://doi.org/10.3390/cancers15092637 PMid:37174104 PMCid:PMC10177389 |
||||
| 43.Verbeek JS, Hirose S, Nishimura H. The complex association of Fc γ RIIb with autoimmune susceptibility. Front Immunol 2019 https://doi.org/10.3389/fimmu.2019.02061 PMid:31681256 PMCid:PMC6803437 |
||||
| 10: 2061. doi:10.3389/fimmu.2019.02061 https://doi.org/10.3389/fimmu.2019.02061 PMid:31681256 PMCid:PMC6803437 |
||||
| 44.Miyabe C, Dong Y, Ikeda T, Takahashi K, Miyabe Y, Kawakami T. Immune checkpoint molecule expression is altered in the skin and peripheral blood in vasculitis. Sci Rep 2021; 11: 20019. doi:10.1038/s41598-021-99558-5 https://doi.org/10.1038/s41598-021-99558-5 PMid:34625602 PMCid:PMC8501116 |
||||
| 45.Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022; 43: 4229-361. doi:10.1093/ eurheartj /ehac244 | ||||
| 46.Teymouri N, Mesbah S, Navabian SMH, Shekouh D, Najafabadi MM, Norouzkhani N, et al. ECG frequency changes in potassium disorders: a narrative review. Am J Cardiovasc Dis 2022; 12: 112-24. | ||||
| 47.Ganesh S, Zhong P, Zhou X. Cardiotoxicity induced by immune checkpoint inhibitor: The complete insight into mechanisms, monitoring, diagnosis, and treatment. Front Cardiovascular Med 2022; 9: 997660. doi:10.3389/fcvm.2022.997660 https://doi.org/10.3389/fcvm.2022.997660 PMid:36204564 PMCid:PMC9530557 |
||||
| 48.Zhao Y, Lyu N, Zhang W, Tan H, Jin Q, Dang A. Prognosis Implication of N-terminal Pro-B-type natriuretic peptide in adult patients with acute myocarditis. Front Cardiovascular Med 2022; 9: 839763. doi:10.3389/fcvm.2022.839763 https://doi.org/10.3389/fcvm.2022.839763 PMid:35433855 PMCid:PMC9009355 |
||||
| 49.Laufer-Perl M, Gilon D, Kapusta L, Iakobishvili Z. The role of speckle strain echocardiography in the diagnosis of early subclinical cardiac injury in cancer patients-is there more than just left ventricle global longitudinal strain?. J Clin Med 2021; 10: 154. doi:10.3390/jcm10010154 https://doi.org/10.3390/jcm10010154 PMid:33466260 PMCid:PMC7795612 |
||||
| 50.Cadour F, Cautela J, Rapacchi S, Varoquaux A, Habert P, et al. Cardiac MRI features and prognostic value in immune checkpoint inhibitor-induced myocarditis. Radiology 2022; 303: 512-21. doi:10.1148/radiol.211765 https://doi.org/10.1148/radiol.211765 PMid:35230185 |
||||
| 51.Ammirati E, Buono A, Moroni F, Gigli L, Power JR, Ciabatti M. et al. State-of-the-Art of endomyocardial biopsy on acute myocarditis and chronic inflammatory cardiomyopathy. Curr Cardiol Rep 2022; 24: 597-609. doi:10.1007/s11886-022-01680-x https://doi.org/10.1007/s11886-022-01680-x PMid:35201561 PMCid:PMC8866555 |
||||
| 52.Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation 2019; 140: 80-91. doi:10.1161/CIRCULATIONAHA.118.034497 https://doi.org/10.1161/CIRCULATIONAHA.118.034497 PMid:31390169 PMCid:PMC6779326 |
||||
| 53.Bai X, Hu J, Betof Warner A. Early use of high-dose glucocorticoid for the management of irae is associated with poorer survival in patients with advanced melanoma treated with anti-PD-1 monotherapy. Clin Cancer Res 2021; 27: 5993-6000. doi:10.1158/1078-0432.CCR -21-1283 https://doi.org/10.1158/1078-0432.CCR-21-1283 PMid:34376536 PMCid:PMC9401488 |
||||
| 54.Nguyen LS, Bretagne M, Arrondeau J. Reversal of immune-checkpoint inhibitor fulminant myocarditis using personalized-dose-adjusted abatacept and ruxolitinib: proof of concept. J Immunother Cancer 2022; 10: e 004699. doi:10.1136/jitc-2022-004699 https://doi.org/10.1136/jitc-2022-004699 PMid:35383117 PMCid:PMC8984056 |
||||
| 55.Wang A, Xu Y, Fei Y, Wang M. The role of immunosuppressive agents in the management of severe and refractory immune-related adverse events. Asia Pac J Clin Oncol 2020; 16: 201-10. doi:10.1111/ajco.13332 https://doi.org/10.1111/ajco.13332 PMid:32212243 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

