COVID-19 sequelae on transthoracic echocardiography findings in pediatric patients with septal congenital heart disease: A retrospective comparative analysis
ORIGINAL RESEARCH ARTICLE
COVID-19 sequelae on transthoracic echocardiography findings in pediatric patients with septal congenital heart disease: A retrospective comparative analysis
Article Summary
- DOI: 10.24969/hvt.2024.486
- Page(s): 244-251
- CARDIOVASCULAR DISEASES
- Published: 31/05/2024
- Received: 10/04/2024
- Revised: 17/05/2024
- Accepted: 22/05/2024
- Views: 3911
- Downloads: 3052
- Keywords: CHD with left-to-right shunting, atrial septal defect, ventricular septal defect, long-COVID syndrome, post-acute sequelae of COVID-19, atrial remodeling, transthoracic echocardiography
Address for Correspondence: Damirbek Abibillaev, Department of Therapeutic Disciplines and Family Medicine, Faculty of Medicine, Ala-Too International University, Bishkek, Kyrgyzstan
E-mail: damirbek.abibillaev@alatoo.edu.kg
ORCID: Damirbek Abibillaev- 0000-0002-4660-3064 Elmira Tukusheva- 0000-0002-6277-8665
Akmaral Kurmanbekova - 0009-0006-9718-7298 Begaiym Ulugbekova- 0009-0009-5456-3312
Raushan Barakanova- 0009-0002-5863-7920 Taalaibek Kudaiberdiev - 0000-0002-3669-066X
Zhumagul Tashbolot kyzy - 0009-0005-7903-4848 Kubra Abdulbakioglu - 0009-00067173-5428
Aziza Rakhmanova - 0009-0006-0207-5876 Aidina Kazymbek - 0009-0004-0298-6392
Kudaibergen Osmonaliev - 0009-0008-4469-1065
Damirbek Abibillaev1a,2*, Elmira Tukusheva2, Akmaral Kurmanbekova3a, Begaiym Ulugbekova1a, Raushan Barakanova3a, Taalaibek Kudaiberdiev3b, Zhumagul Tashbolot kyzy1a, Kubra Abdulbakioglu1b, Azizakan Rakhmanova1c, Aidina Kazymbek4, Kudaibergen Osmonaliev1c
1aDepartment of Therapeutic Disciplines and Family Medicine, 1bDepartment of Fundamental and Morphological Disciplines and 1cDepartment of Surgical Disciplines and Obstetrics-Gynecology, Faculty of Medicine, Ala-Too International University, Bishkek, Kyrgyzstan
2Consultative and Diagnostic Department, Research Institute of Heart Surgery and Organ Transplantation, Bishkek, Kyrgyzstan
3aDepartment of Basic and Clinical Pharmacology and 3bDepartment of Scientific Research Management, Kyrgyz State Medical Academy named after I.K.Akhunbaev, Bishkek, Kyrgyzstan
4Faculty of General Medicine, Kyrgyz Russian Slavic University, Bishkek, Kyrgyzstan
Abstract
Objective: The COVID-19 pandemic has raised concerns about its impact on patients with congenital heart disease (CHD) with left-to-right shunting. We hypothesized that the pandemic, particularly its long-term sequelae known as Post-Acute Sequelae of SARS-CoV-2 infection (PACS) or long COVID syndrome, may influence cardiac chamber size and functional echocardiographic parameters in this population.
Methods: We conducted a retrospective comparative analysis of echocardiographic reports in pediatric CHD patients with left-to-right shunting lesions before (pre-pandemic) and after (post-pandemic) the COVID-19 pandemic. These lesions included atrial (ASD), ventricular septal defects (VSD), and patent ductus arteriosus (PDA). Patients were assessed by following age categories; under one year (infants) and older than one year (older children).
Results: Most of the ASDs presented by secundum type, whereas the majority of VSDs consist of perimembranous type of defects in both groups of age categories. Comparison of echocardiographic parameters revealed a significant increase in left atrial size in the post-pandemic group compared to the pre-pandemic group in both older age and infant categories ((21.0(5.0) mm vs 18.9 (3.7), p=0.01; 14.6 (2.1) vs 11.8 (2.6), p=0.003, respectively)). Although the frequency of ASDs did not differ between groups, there was a trend towards a higher prevalence of VSDs in the post-pandemic group of older children without statistical significance.
Conclusion: Our study provides preliminary evidence of altered cardiac chamber size in CHD patients with left-to-right shunting lesions during the post-COVID-19 period. These findings underscore the need for continued surveillance and multidisciplinary management of CHD patients in the context of the pandemic, highlighting the potential impact of COVID-19 on cardiovascular outcomes in this vulnerable population.
Key words: CHD with left-to-right shunting, atrial septal defect, ventricular septal defect, long-COVID syndrome, post-acute sequelae of COVID-19, atrial remodeling, transthoracic echocardiography
![]()
Graphical abstract
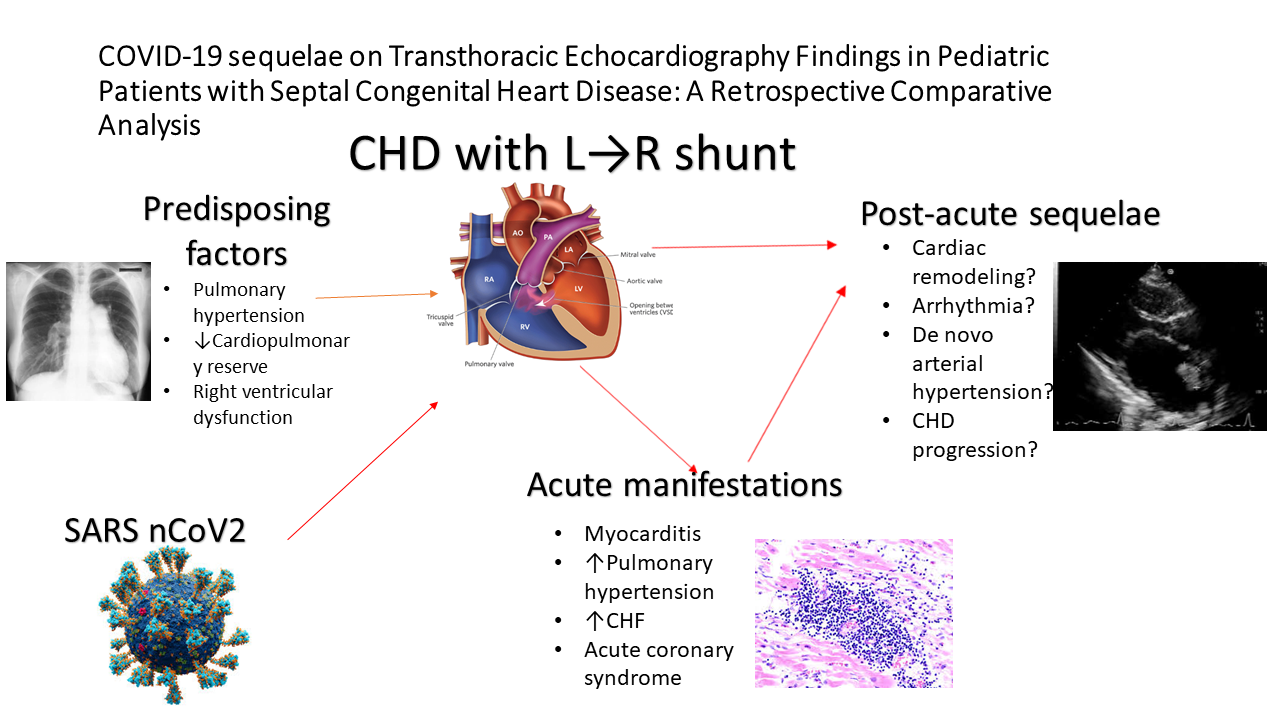
The COVID-19 pandemic caused by the novel coronavirus SARS-CoV-2 has led to significant morbidity and mortality worldwide (1, 2). While primarily known as a respiratory illness, COVID-19 has also been associated with various cardiovascular manifestations, ranging from mild cardiac injury to severe complications such as myocarditis, myocardiodystrophy, and acute coronary syndromes (3, 4). Some individuals experience persistent symptoms following acute COVID-19 infection, a condition referred to as long COVID syndrome or post-acute sequelae of COVID-19 (PASC) (5 ,6). Cardiac manifestations of long COVID may include ongoing fatigue, dyspnea, chest pain, palpitations, postural orthostatic tachycardia, and exercise intolerance, reflecting underlying myocardial and autonomic dysfunction (6).
Congenital heart diseases (CHD) patients, particularly those with significant left-to-right shunting lesions, may have compromised cardiovascular function and reduced cardiopulmonary reserve, placing them at increased risk of severe illness if infected with COVID-19 (7, 8). According to literature findings, pre-existing pulmonary hypertension, pulmonary vascular disease, and right ventricular dysfunction in patients with large defects can exacerbate respiratory compromise and negatively affect the course of COVID-19 (9).
Nonetheless, most of the literature data concerned complex or cyanotic CHDs. Furthermore, long-term cardiovascular sequel remains unelucidated in pediatric cardiology practice. To date, controversy exists regarding the optimal risk stratification and prognostication strategies for these cohorts of patients.
As in other pathologic conditions, COVID-19 pandemics lead to some level of changes in the cardiac structural and functional alterations of patients with congenital septal defects (7, 8). In this study, we aimed to retrospectively analyze and compare the echocardiographic findings of CHD patients with left-to-right shunting examined during the pre-pandemic and post-pandemic periods.
Methods
Study design and population
The study design was retrospective and observational cohort. The echo reports were derived from patients who visited the outpatient department of the Research Institute of Heart Surgery and Organ Transplantation, a leading tertiary care center of CHD during 2018 and 2021 years. Pediatric patients older than one year diagnosed with either isolated or concomitant atrial (ASD), ventricular septal defects (VSD), and patent ductus arteriosus (PDA) were included in the study regardless of being corrected. For pediatric patients younger than one year isolated PDA was not included in the study due to its physiological existence in most of these infants. Due to the high prevalence and relatively benign nature of patent foramen ovale (PFO) in the pediatric population, cases with concomitant PFO were not excluded from the study. Adult patients older than 18 years and individuals without any echocardiographic signs of structural heart disease were excluded. Patients with right-to-left shunting and Eisenmenger syndrome were excluded. As well as cases with the coexistence of septal CHDs with other obstructive lesions were also excluded.
Further, each age group was divided into pre-pandemic (March 2018- December 2019) and post-pandemic (March 2020- December 2021) taking into account the official declaration of the first detection of COVID-19 in the territory of Kyrgyzstan only in March 2020 (12). Due to a lack of accurate information regarding the COVID-19 observations, data from January and February 2020 were not included in the research.
Due to the retrospective nature of the data, no Ethical committee approval was required. Patient’s parents and guardians provided informed consent for all procedures.
Data
Data we obtained retrospectively form patients records and echocardiography records.
Demographic and clinical, perioperative characteristics
Demographic characteristics of the population included age, sex, study period, and operation status. Pediatric patients younger than one year (infants) and older than one year were separately analyzed. Sex percentage was compared in each category and group. Defect type (ASD, VSD, PDA, or combined) and exact anatomical-surgical form of defects were analyzed and compared between groups. Operation status (underwent surgical closure or intervention) was also compared in each group of older patients. Due to a lack of cases of correction, operation status was not analyzed in the infant age category.
Echocardiographic data
TTE data included right and left cardiac chamber sizes, namely, ascending aorta, left atrial anteroposterior size, left ventricular end-diastolic and end-systolic diameters, interventricular septum, left ventricular posterior wall thicknesses, mid-level right ventricular diameter, right ventricular anterior wall diameters. Tricuspid regurgitation severity was evaluated by simple visual assessment through Color Doppler mapping or vena contracta measurement. Left ventricular ejection fraction measured by Tiecholz methods, and mean pulmonary artery pressures derived from right ventricular acceleration time according to the Dabestani or Kitabatake formulas. All measurements were done by existing ASE/EACVI guidelines (10, 11). All investigations were conducted by a single operator experienced in pediatric echocardiography and imaging of CHD.
Statistical analysis
Continuous variables with normal distribution are presented as mean and standard deviation, whereas non-normal distribution is depicted by median and interquartile range. Categorical variables are shown by absolute count and percentage. The normality of distribution was assessed by the Kolmogorov-Smirnov test. Parametric dependent samples t-test or Wilxocon rank test was used for comparison of continuous dependent variables before and after pandemics. Chi-square or Fisher's exact test was applied for categorical variables taking into account sample size and normality of distribution. A statistical significance was adjusted for p-value of less than 0.05 and a confidence interval of 95%. As a statistical software, Stata 16 (Stata Corp, Texas, USA) was used.
Results
Demographic characteristics
A total of 1633 patient’s data for the 2018-2021 periods were analyzed. Out of these numbers, 533 patients were found to have CHD pathology. Due to the presence of other than septal defects (n=235) and the simultaneous presence of obstructive lesions (n=132), a total of 367 cases were excluded from the study. Furthermore, 21 adult patients were excluded from the selected septal CHD cohorts. Finally, 34 infants and 111 older pediatric patients were found to be eligible for the study. Participants were enrolled following existing STROBE guidelines and the chart is described below (Fig. 1) (13).
![]()
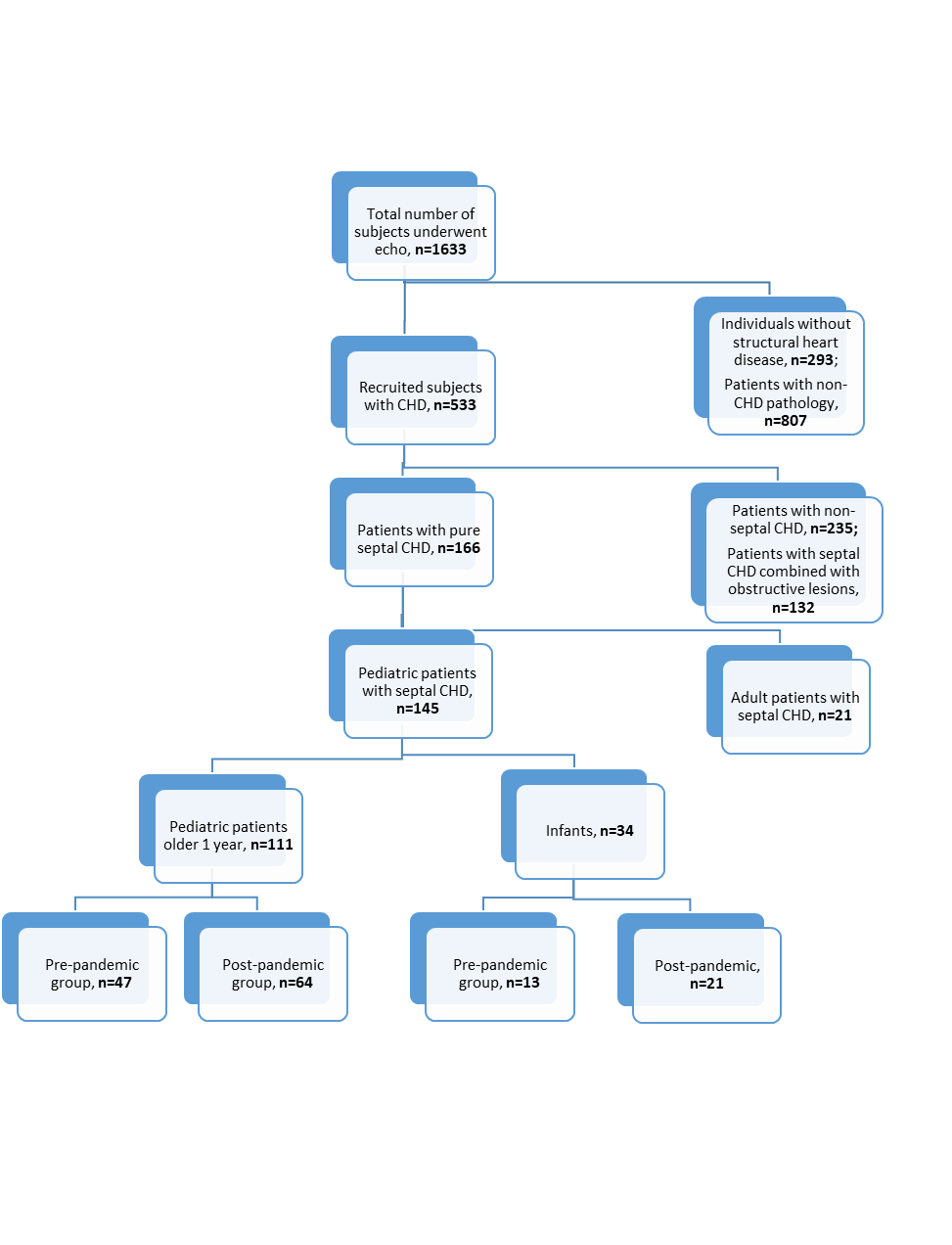
Figure 1. STROBE-chart of participant selection
CHD – congenital heart disease
According to the overall analysis, males constituted 47 % of infants and 53.1% of older children. The mean age in the infants group was found to be 5.6 (3.2) months and 8.45 (0.5) years in the older group years. From infants 13 (37.2%) cases were investigated during pre-pandemic and 21 (62.8%) cases were examined during post-pandemic periods. In the older group, 47 (41.9%) cases were examined during pre-pandemic and 64 (58.1%) cases during post-pandemic periods.
CHD profile of groups before and after pandemics
Both groups in each category were matched by age and gender despite the unequal proportions of CHD cases. The Table 1 highlights the comparative findings of older children group before and after pandemics. In the structure of CHD, VSD prevailed in both periodical groups. Most of the VSDs were presented by a perimembranous type of defect. Nevertheless, no significant difference was noted between groups. In contrast, ASDs were mostly presented by secundum type with occasional cases of primum and venosus types in groups. In 15 (31.9%) cases of pre-pandemic and 28 (43.7%) cases of post-pandemic groups, patients were corrected by surgical or transcatheter closure.
The Table 2 highlighted the CHD details of pediatric patients younger than one year. In this population, ASD was found to be slightly more frequent than VSD. Three cases of pre-pandemic and a single case of post-pandemic combination of ASD with VSD and PDA were noted. All ASDs presented by secundum type, whereas the majority of VSDs were found as pure perimembranous type followed by two cases of perimembranous-muscular and one case of subtricuspid types. None of the infants were operated on.
|
Table 1. Clinical characteristics before and after pandemics in group of pediatric patients older than one year |
||||
|
Variables |
Total (n=111) |
Pre-pandemic (n=47) |
Post-pandemic (n=64) |
p |
|
Sex, male, n(%) |
59 (53.1) |
25 (51.6) |
35 (54.6) |
0.70 |
|
Age, years |
8.45(5.6 |
9.14(5.8 |
7.95(5.39 |
0.27 |
|
Defect type, n(%) ASD VSD PDA |
33 (34.0) 50 (51.5) 14 (14.5) |
16 (39) 18 (43.9) 7 (17) |
17 (30.3) 32 (57.4) 7 (12.5) |
0.43 |
|
ASD type, n(%) secundum primum venosus |
30 (90.9) 1 (3.0) 2 (6.1) |
15 (93.7) 1 (6.3) 0 |
15 (88.2) 0 2 (11.8) |
0.22 |
|
VSD type, n(%) PM ST M PM+ST PM+M |
41 (80.3) 6 (11.7) 1 (1.9) 2 (3.9) 1 (1.9) |
16 (88.9) 1 (5.5) 1 (5.5) 0 0 |
25 (75.8) 5 (15.1) 0 2 (6) 1 (3) |
0.32 |
|
Combined CHD, n(%) Isolated ASD+VSD ASD+PDA VSD+PDA |
97 (87.3) 9 (8.1) 4 (3.6) 1 (0.9) |
41 (87.2) 2 (4.2) 3 (6.3) 1 (2.1) |
56 (87.5) 7 (10.9) 1 (1.6) 0 |
0.20 |
|
Operated, n(%) |
43 (38.7) |
15 (31.9) |
28 (43.7) |
0.20 |
|
ASD - atrial septal defect, CHD –congenital heart disease, M- muscular type, PDA - patent ductus arteriosus, PM – perimembranous type, ST – subtricuspid type, VSD - ventricular septal defect |
||||
|
Table 2. Clinical characteristics before and after pandemics in group of pediatric patients younger than one year |
||||
|
Variables |
Total (n=34) |
Pre-pandemic (n=13) |
Post-pandemic (n=21) |
p |
|
Sex, male n(%) |
16 (47.0) |
6 (41.6) |
10 (47.6) |
0.93 |
|
Age, months |
5.6(3.2 |
5.07(3.09 |
6 (3.31 |
0.41 |
|
Defect type, n(%) ASD VSD combined |
17 (50) 13 (38.2) 4 (11.8) |
5 (38.5) 5 (38.5) 3 (23) |
12 (57.1) 8 (38.1) 1 (4.7) |
0.24 |
|
Combined CHD, n(%) Isolated ASD+VSD ASD+PDA VSD+PDA |
30 (88.2) 2 (5.8) 1 (2.9) 1 (2.9) |
10 (76.9) 1 (7.7) 1 (7.7) 1 (7.7) |
20 (95.2) 1 (4.8) 0 0 |
0.26 |
|
VSD type, n(%) PM PM+M ST |
11 (78.5) 2 (14.4) 1 (7.1) |
5 (100) 0 0 |
6 (66.6) 2 (22.2) 1 (11.1) |
0.67 |
|
ASD - atrial septal defect, CHD –congenital heart disease, M- muscular type, PDA - patent ductus arteriosus, PM – perimembranous type, ST – subtricuspid type |
||||
Comparative analysis of echocardiographic findings
The comparative analysis of chamber quantifications (Tables 3 and 4) did not reveal the echocardiographic difference among groups in both age categories. Only left atrial anteroposterior size in post-pandemic groups was found to be significantly larger in both older children and infants ((21.0 (5.0) mm vs 18.9 (3.7), p=0.01; 14.6 (2.1) vs 11.8 (2.6), p=0.003, respectively)). In older children post-pandemic period, the diameter of the interventricular septum and right ventricular size was presented with slightly higher values in contrast to per-pandemic cases, however, these were not statistically significant.
Among all groups and categories, left ventricular ejection fraction did not differ. As well as comparative analysis of pulmonary arterial pressure in both age categories did not reveal significant findings. None of the cases presented by severe tricuspid regurgitation (TR), instead only two cases of moderate TR were detected in the post-pandemic period without statistical significance. Practically all TR cases were presented as functional and trivial.
Discussion
While the frequency of ASDs did not differ significantly between the pre-pandemic and post-pandemic groups, there was a trend towards a higher prevalence of VSDs in the post-pandemic group of older children, although this difference did not reach statistical significance. The increased incidence of VSDs could be explained just as incidental findings, but at the same time, it raises questions about potential associations between COVID-19 infection and the development of congenital heart diseases. Despite the existence of a strong association between the development of neural tube defects and SARS-CoV2, no robust data is available in the literature related to the development of CHD (14). Furthermore, studies did not reveal the correlation between COVID-19 and adverse outcomes of exact CHD types, instead, they stated the severity of CHD is dependent on the status of surgical repair, their anatomical complexity, and the presence of cyanosis and pulmonary hypertension as adjunct physiological factors (9, 15).
Heart, Vessels and Transplantation 2024; 8: doi: 10.24969/hvt.2024.486
Septal CHD changes on TTE after COVID-19 in children Abibillaev et al.
![]()
Per se, CHD with left-to-right shunting manifests by right heart dilatation and pulmonary hypertension (16). On the other hand, left ventricular remodeling in various forms is observed in congenital aortic valve abnormalities and complex heart diseases in the pediatric population (17). However, our study did not confirm the changes in either right cardiac chamber sizes or pulmonary artery pressure values. Despite the lack of statistical significance, the interventricular septum diameter revealed some extent of thickening in the post-pandemic group of both age categories ((7.02(2.6) mm vs 7.9(2.3) mm, p=0.07)) in older children and ((2.2(1.8) vs 3.3(1.7), p=0.09 in infants)). According to the literature body, long COVID-19 resulted in myocarditis, cardiomyopathies, and heart failure (6, 18). In the light of above-mentioned literature data, we can assume that the mild thickening of the left ventricular myocardium might be explained by some degree of cardiac remodeling in patients with septal CHD.
The chronic volume overload imposed on the left atrium due to the shunting of blood from the systemic-to- pulmonary circulation leads to atrial enlargement as a compensatory response to increased preload and altered hemodynamics. According to these pathophysiological mechanisms, atrial dilatation was known as a marker of pulmonary hypertension. Furthermore, enlarged atria are associated with rhythm disturbances (3, 19). Strikingly, our results revealed a significant increase in left atrial size in the post-pandemic group compared to the pre-pandemic group. This finding in the post-pandemic group can be interpreted as a variant of multi-spectral PASC manifestations. (6, 20). To date, there are no exact studies concerning the association of specific left atrial dilatation and PASC, however arterial hypertension, development of arrhythmias, and exacerbation of heart failure are thought to be manifested by left atrial dilatation (21). We assume that the dilated left atrium along with mildly thickened left ventricular myocardium in these small cohorts increases the likelihood of arrhythmogenicity and remodeling effects of PASC.
![]()
Study limitations
It is essential to interpret our findings in the context of study limitations, including the relatively small sample size and retrospective study design. Due to inconclusive data, most of the echocardiographic parameters, including functional indices have not been analyzed. Furthermore, the lack of laboratory data and other investigations indicative of both CHD and COVID-19 bears a considerable challenge to the interpretation of the abovementioned echocardiographic findings. As well as the absence of longitudinal follow-up data precludes definitive conclusions regarding causality or the temporal relationship between COVID-19 infection and changes in cardiac parameters. Further larger studies need to be addressed.
Conclusion
In conclusion, our study provides preliminary evidence of altered cardiac chamber size in CHD patients with left-to-right shunting lesions during the post-COVID-19 period. These findings underscore the importance of ongoing surveillance, risk stratification, and multidisciplinary management of CHD patients in the context of the long COVID syndrome and highlight the need for further research to elucidate the links between COVID-19 infection and cardiovascular outcomes in this vulnerable population.
Ethics: Patients parent or guardians provided informed consent for the children for all procedures. As per retrospective design of the study there was no need for approval of Ethics committee
Peer-review: External and internal
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
Authorship: D.A., E.T., A.K., B.U., R.B., T. K., Zh. Tk., K.A., A.R., A.K., and K.O. equally contributed to the study and fulfilled authorship criteria
Acknowledgements and funding: None to declare
References
| 1.CDC. Technical notes: provisional death counts for coronavirus disease (COVID-19). Hyattsville, MD: US Department of Health and Human Services, CDC, National Center for Health Statistics; 2023. Availabel at: URL: https://www.cdc.gov/nchs/nvss/vsrr/covid19/tech_notes.htm | ||||
| 2.Zhang JJ, Dong X, Liu GH, Gao YD. Risk and protective factors for COVID-19 morbidity, severity, and mortality. Clin Rev Allergy Immunol 2023; 64: 90-107. doi: 10.1007/s12016-022-08921-5 https://doi.org/10.1007/s12016-022-08921-5 PMid:35044620 PMCid:PMC8767775 |
||||
| 3.Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol 2020; 17: 543-58. doi: 10.1038/s41569-020-0413-9 https://doi.org/10.1038/s41569-020-0413-9 PMid:32690910 PMCid:PMC7370876 |
||||
| 4.Meizinger C, Klugherz B. Focal ST-segment elevation without coronary occlusion: myocardial infarction with no obstructive coronary atherosclerosis associated with COVID-19 case report. Eur Heart J Case Rep 2021; 5: ytaa532. doi: 10.1093/ehjcr/ytaa532 https://doi.org/10.1093/ehjcr/ytaa532 PMid:33768195 PMCid:PMC7982125 |
||||
| 5.Pérez-González A, Araújo-Ameijeiras A, Fernández-Villar A, Crespo M, Poveda E; Cohort COVID-19 of the Galicia Sur Health Research Institute. Long COVID in hospitalized and non-hospitalized patients in a large cohort in Northwest Spain, a prospective cohort study. Scientific reports 2022: 12: 3369. https://doi.org/10.1038/s41598-022-07414-x https://doi.org/10.1038/s41598-022-07414-x PMid:35233035 PMCid:PMC8888560 |
||||
| 6.Raman B, Bluemke DA, Lüscher TF, Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J2022; 43: 1157-72. https://doi.org/10.1093/eurheartj/ehac031 https://doi.org/10.1093/eurheartj/ehac031 PMid:35176758 PMCid:PMC8903393 |
||||
| 7.Alsaied T, Aboulhosn JA, Cotts TB, Daniels CJ, Etheridge SP, Feltes TF, et al. Coronavirus Disease 2019 (COVID-19) pandemic implications in pediatric and adult congenital heart disease. J Am Heart Assoc 2020; 9: e017224. https://doi.org/10.1161/JAHA.120.017224 https://doi.org/10.1161/JAHA.120.017224 PMid:32441586 PMCid:PMC7429046 |
||||
| 8.Ali MI, Alkhuzaie HF, Alhashim SA, Hassan HA, Tawhari AH, Abdulqader AK, et al. Effect of COVID-19 on congenital heart disease children: a literature review. Int J Community Med Public Health 2021; 8: 900-4. https://doi.org/10.18203/2394-6040.ijcmph20210032 |
||||
| 9.Haiduc AA, Ogunjimi M, Shammus R. COVID-19 and congenital heart disease: an insight of pathophysiology and associated risks. Cardiol Young 2021; 31: 233-40. doi:10.1017/S1047951120003741 https://doi.org/10.1017/S1047951120003741 PMid:33172515 |
||||
| 10.Writing Group: Campbell RM, Douglas PS, Eidem BW, Lai WW, Lopez L, Sachdeva R; et al. Echocardiography in outpatient pediatric cardiology; J Am Soc Echocardiogr 2014; 27: 1247-66. doi: 10.1016/j.echo.2014.10.002. 11.Galderisi M, Cosyns B, Edvardsen T, Cardim N, Delgado V, Di Salvo G, et al; 2016-2018 EACVI Scientific Documents Committee; 2016-2018 EACVI Scientific Documents Committee. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2017; 18: 1301-10. doi: 10.1093/ehjci/jex244. https://doi.org/10.1093/ehjci/jex244 PMid:29045589 |
||||
| 12.Available at: URL: https://kabar.kg/news/v-kyrgyzstane-zaregistrirovan-pervye-3-sluchaia-koronavirusa/ | ||||
| 13.Cuschieri S. The STROBE guidelines. Saudi J Anaesth 2019; 13(Suppl 1): S31-4. doi: 10.4103/sja.SJA_543_18. https://doi.org/10.4103/sja.SJA_543_18 PMid:30930717 PMCid:PMC6398292 |
||||
| 14.Khan I, Nabeka H, Akbar SM, Al Mahtab M, Shimokawa T, Islam F et al. Risk of congenital birth defects during COVID-19 pandemic: draw attention to the physicians and policymakers. J Glob Health 2020: 10: 020378 https://doi.org/10.7189/jogh.10.020378 PMid:33274057 PMCid:PMC7690649 |
||||
| 15.Zareef R, Salameh E, Hammoud R, Tannouri T, Bitar F, Arabi, M. COVID-19 in congenital heart disease patients: what did we learn? Frontier Carrdiovasc Med 2023: 10, 1235165. https://doi.org/10.3389/fcvm.2023.1235165 https://doi.org/10.3389/fcvm.2023.1235165 PMid:37719985 PMCid:PMC10501459 |
||||
| 16.Monreal G, Youtz DJ, Phillips AB, Eyman ME, Gorr MW, Velten C, et al. Right ventricular remodeling in the restrictive ventricular septal defect. J Mol Cell Cardiol 2010; 49: 699-706. doi: 10.1016/j.yjmcc.2010.07.005. https://doi.org/10.1016/j.yjmcc.2010.07.005 PMid:20637777 PMCid:PMC4263504 |
||||
| 17.Goo HW, Park SH. Pattern analysis of left ventricular remodeling using cardiac computed tomography in children with congenital heart disease: preliminary results. Korean J Radiol 2020: 21: 717-25. doi: 10.3348/kjr.2019.0689. https://doi.org/10.3348/kjr.2019.0689 PMid:32410410 PMCid:PMC7231616 |
||||
| 18.Mohammad KO, Lin A, Rodriguez JBC. Cardiac manifestations of post-acute COVID-19 Infection. Curr Cardiol Rep 2022: 24: 1775-83. doi: 10.1007/s11886-022-01793-3. https://doi.org/10.1007/s11886-022-01793-3 PMid:36322364 PMCid:PMC9628458 |
||||
| 19.Medi C, Kalman JM, Ling LH, Teh AW, Lee G, Lee G, et al. Atrial electrical and structural remodeling associated with longstanding pulmonary hypertension and right ventricular hypertrophy in humans. J Cardiovasc Electrophysiol 2012; 23: 614-20. https://doi.org/10.1111/j.1540-8167.2011.02255.x PMid:22269035 |
||||
| 20.Varney JA, Dong VS, Tsao T, Sabir MS, Rivera AT, Ghula S, et al. COVID-19 and arrhythmia: An overview. J Cardiol 2022; 79: 468-75. Doi: 10.1016/j.jjcc.2021.11.019 https://doi.org/10.1016/j.jjcc.2021.11.019 PMid:35074257 PMCid:PMC8632592 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

