Ischemic stroke from non-bacterial thrombotic endocarditis embolization in Li-Fraumeni syndrome: A case report
CASE REPORT
Ischemic stroke from non-bacterial thrombotic endocarditis embolization in Li-Fraumeni syndrome: A case report
Article Summary
- DOI: 10.24969/hvt.2024.487
- Page(s): 313-318
- CARDIOVASCULAR DISEASES
- Published: 03/06/2024
- Received: 25/05/2024
- Revised: 31/05/2024
- Accepted: 01/06/2024
- Views: 3759
- Downloads: 2934
- Keywords: Li-Fraumeni syndrome, non-bacterial thrombotic endocarditis, ischemic stroke, TP53 mutation, embolization
Address for Correspondence: Stefano Cacciatore, Department of Geriatrics, Orthopedics and Rheumatology, Università Cattolica del Sacro Cuore, L.go F. Vito 1, 00168, Rome, Italy
E-mail: stefano.cacciatore01@icatt.it
ORCID: Silvia Andaloro: 0009-0004-0734-9712 Stefano Cacciatore: 0000-0001-7504-3775;
Maria Anna Nicolazzi: 0000-0002-6900-1963; Federico Biscetti: 0000-0001-7449-657X
Clarissa Modafferi: 0009-0004-6518-1700; Emanuela Lucci-Cordisco: 0000-0002-6279-7604
Antonio Gasbarrini: 0000-0002-7278-4823; Andrea Flex: 0000-0003-2664-4165
Citation: Andaloro S, Cacciatore S, Nicolazza MA, Biscetti F, Modaffer C, Lucci-Cordisco E, et al. Ischemic stroke from non-bacterial thrombotic endocarditis embolization in Li-Fraumeni syndrome: A case report. Heart Vessels Transplant 2024; 8: doi: 10.24969/hvt.2024.487
Silvia Andaloro1a, Stefano Cacciatore1b*, Maria Anna Nicolazzi2a, Federico Biscetti2a, Clarissa Modafferi1c, Emanuela Lucci-Cordisco2a, 2b, Antonio Gasbarrini2c, 2d, Andrea Flex2a
1aDepartment of Translational Medicine and Surgery, 1b*Department of Geriatrics, Orthopedics and Rheumatology, and 1cDepartment of Life Sciences and Public Health, Section of Genomic Medicine, Universita Cattolica del Sacro Cuore, L.go F. Vito 1, 00168, Rome, Italy.
2aCardiovascular Internal Medicine Unit, Department of Cardiovascular Sciences; 2bMedical Genetics Unit; 2cInternal Medicine and Gastroenterology Unit and 2dDepartment of Medical and Surgery Sciences, Fondazione Policlinico Universitario A. Gemelli IRCCS, L.go A. Gemelli 8, 00168 Rome, Italy.
Abstract
Objective: Li-Fraumeni Syndrome (LFS) is a rare autosomal-dominant syndrome caused by a heterozygous germline mutation of the TP53 gene. It is characterized by early-onset malignancies and high penetrance. Non-bacterial thrombotic endocarditis (NBTE) is an uncommon condition for which cancer is a significant risk factor.
Here we present a complex case of LFS unveiled by a NBTE-related ischemic stroke.
Case presentation: A 41-year-old woman was admitted following a syncopal episode, preceded by a history of ischemic stroke. She had a notable family history of cancers. Imaging studies revealed ischemic damage and hemorrhagic infarcts, indicating a possible embolic origin or neoplastic involvement. Subsequent examinations revealed a NBTE on the aortic valve as well as multiple primary malignancies including high-grade invasive ductal carcinoma in the breast and primary lung adenocarcinoma. Genetic testing confirmed the presence of a pathogenic variant in TP53.
Conclusion: This case underscores the intricate interplay between LFS, oncological manifestations, and thrombotic complications leading to ischemic stroke through NBTE embolization.
Key words: Li-Fraumeni syndrome, non-bacterial thrombotic endocarditis, ischemic stroke, TP53 mutation, embolization
Introduction
Li-Fraumeni syndrome (LFS) is a rare autosomal-dominant syndrome caused by a heterozygous germline mutation of the TP53 gene. It is characterized by early-onset tumors, including bone and soft-tissue sarcomas, breast cancer, adrenocortical carcinomas, neoplasms of the central nervous system, among many others, and high penetrance (1). The prognosis of LFS is highly dependent on early detection through family history and genetic testing, as well as on prompt monitoring. According to Villani et al. (2), 5-year survival rate was 88.8% in patients who underwent surveillance and 59.6% in the non-surveillance group. Non-bacterial thrombotic endocarditis (NBTE), formerly known as marantic or terminal endocarditis, is a serious condition for which cancer represents a substantial risk factor. It is recommended that cancer should be ruled out as a probable cause in situations when the etiology of NBTE is unknown (3, 4).
We present a case of NBTE-related embolic stroke unveiling multifocal LFS in a 41-year-old woman.
Case presentation
In August 2022, a 41-year-old woman was admitted to our University Hospital for a syncopal episode. Written informed consent was obtained for all procedures and publication of this case report.
She had been hospitalized one month prior for ischemic stroke presenting as left facio-brachio-crural hemiparesis. She had no additional relevant medical conditions in her history, except for arterial hypertension. The patient had a family history of cancer and p53 protein mutation. One sibling was diagnosed with breast cancer at 58 years old, another sibling passed away from adrenal cancer at 46 years old, a niece was born with congenital adrenal carcinoma, while a brother who was a mutation carrier appeared to be in good health. However, she had never been screened prior to admission.
Upon physical examination, she revealed an extensive hard nodularity in the superior-outer quadrant of the right breast. Furthermore, she displayed deep venous thromboses in both typical (right obstructive thrombosis of the soleal veins, and pre-obstructive thrombosis of the common and left superficial femoral arteries) and atypical (common hepatic artery and splenic artery thrombosis) sites.
A brain computer tomography (CT) scan revealed subacute ischemic distress with a probable embolic etiology in the territories of the right middle cerebral artery; additionally, it revealed three areas suggestive of hemorrhagic infarction in the left parietal and occipital lobes, due either to hemorrhagic angiopathy or a neoplasm of unknown localization (Fig. 1). These findings were later confirmed by brain magnetic resonance imaging (MRI) (Fig. 2).
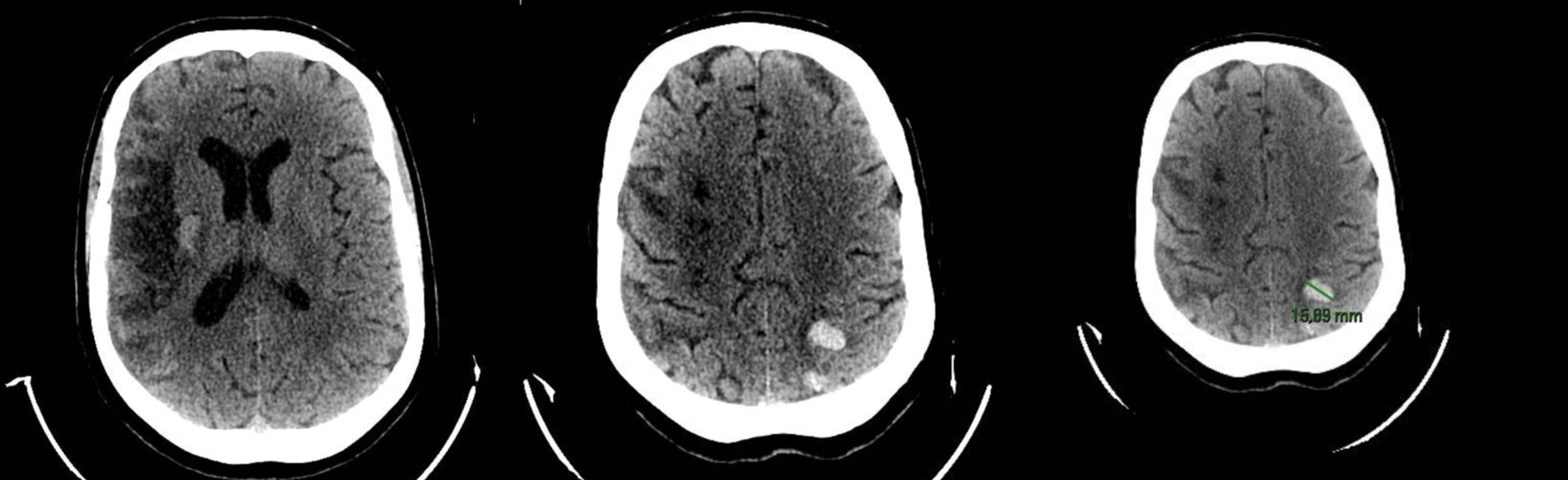
Figure 1. Computer tomography scan revealed multiple areas consistent with ischemic tissue distress in the subacute phase, along with associated areas of hyperdensity indicative of hemorrhagic infarction
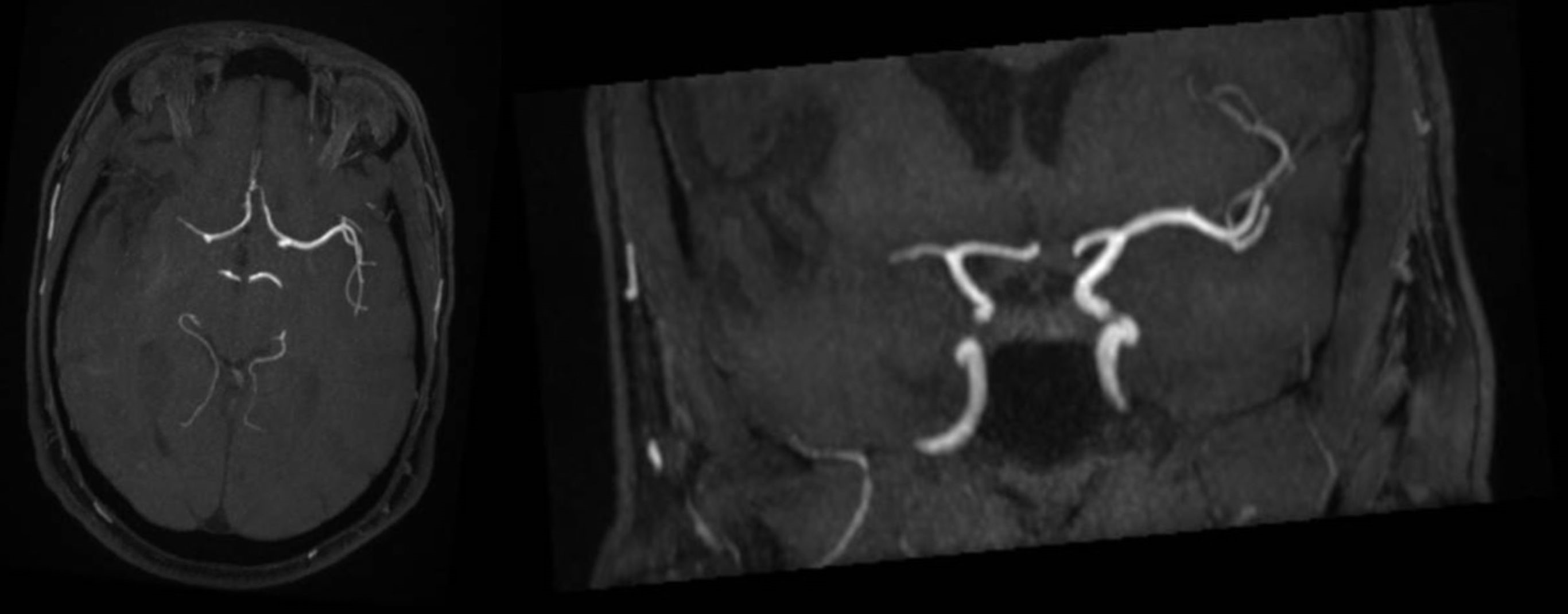
Figure 2. The magnetic resonance imaging scan revealed signs of ischemic distress of embolic origin, with extensive involvement and initial malacic transition into the territory of the right middle cerebral artery and late subacute hemorrhagic conversion of left occipito-parietal cortico-subcortical foci
![]()
Transthoracic and then transesophageal echocardiography revealed a dysmorphic aortic valve with multiple vegetating masses adhered to all aortic valve cusps resulting in a large central coaptation deficit and subsequent severe valvular insufficiency with initial unfavorable left ventricular remodeling (Fig. 3).
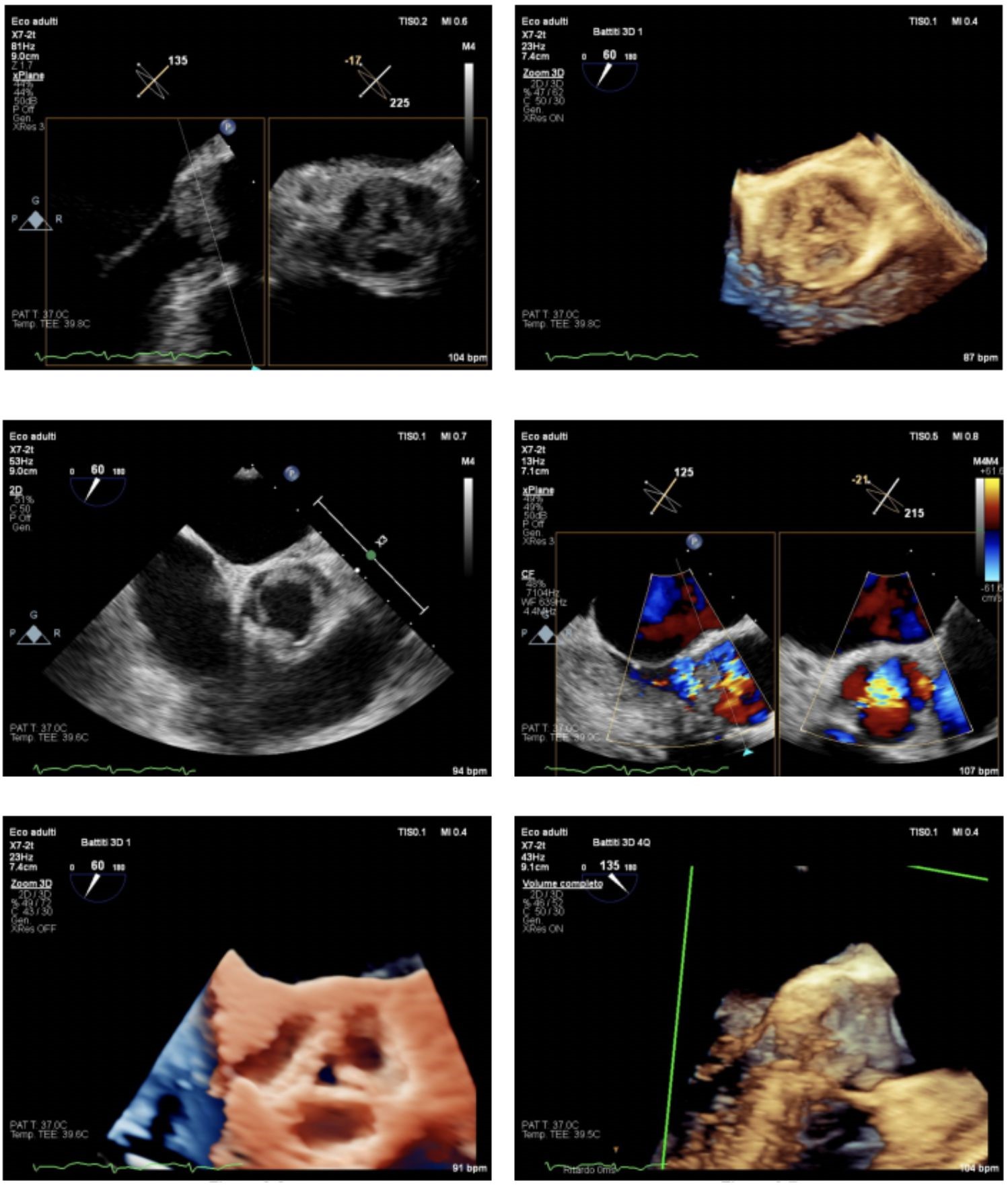
Figure 3. Transesophageal echocardiography showed alterations of the aortic valve with evidence of multiple vegetations adhered to all aortic valve cusps, predominantly at the non-coronary cusp, resulting in a large central coaptation deficit and consequent severe valvular insufficiency with initial unfavorable left ventricular remodeling
Blood culture tests were conducted to rule out infective endocarditis, and empiric antibiotic treatment was initiated. Blood cultures for bacteria including HACEK group (Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, Kingella, Coxiella, Bartonella, and Chlamidia spps.), serologies for mycoplasma, legionella, HIV, HBV, HCV, as well as the Widal-Wright reaction tested all negative. The vegetations did not change in size with antibiotic therapy, raising the possibility of a NBTE.
In the presence of a high clinical suspicion of an underlying tumor, a total-body CT scan was performed, revealing abnormalities consistent with multiple neoplasms, which were subsequently investigated further with targeted diagnostic testing. First, several nodules with irregular margins and associated lymphadenopathy in the superior-outer and superior-inner quadrants of the right breast were found. Histologic examination of the primary lesion revealed high-grade invasive ductal carcinoma (G3) with lobular-like growth features (E-cadherin: 20%), positive estrogen receptor (ER+: 90%), negative progestin receptor (PR: <1%), HER2 positivity, and high proliferation index (Ki-67: 35%). Second, the right lower lung lobe displayed a solid heteroformation with irregular margins and tightly adhered to the pleura (Fig. 4A). Additional nodules and micronodules were found bilaterally. CT-guided biopsy of the major lesion confirmed the presence of a primary lung adenocarcinoma (TT1 and napsin positive, ER- and GATA3-negative). Third, a suspicious nodule was found in the right lobe of the thyroid gland (Fig. 4B), which was confirmed by ultrasonography. This finding was no further investigated. Fourth, several bone metastases were found in pelvic bones, iliac wing, femoral pertrochanter region bilaterally, all sacral metameres, left clavicle, right scapula (Fig. 4C). Fifth, an additional finding was reported in the left adnexal site, however the lesion appeared with features of likely benignity.
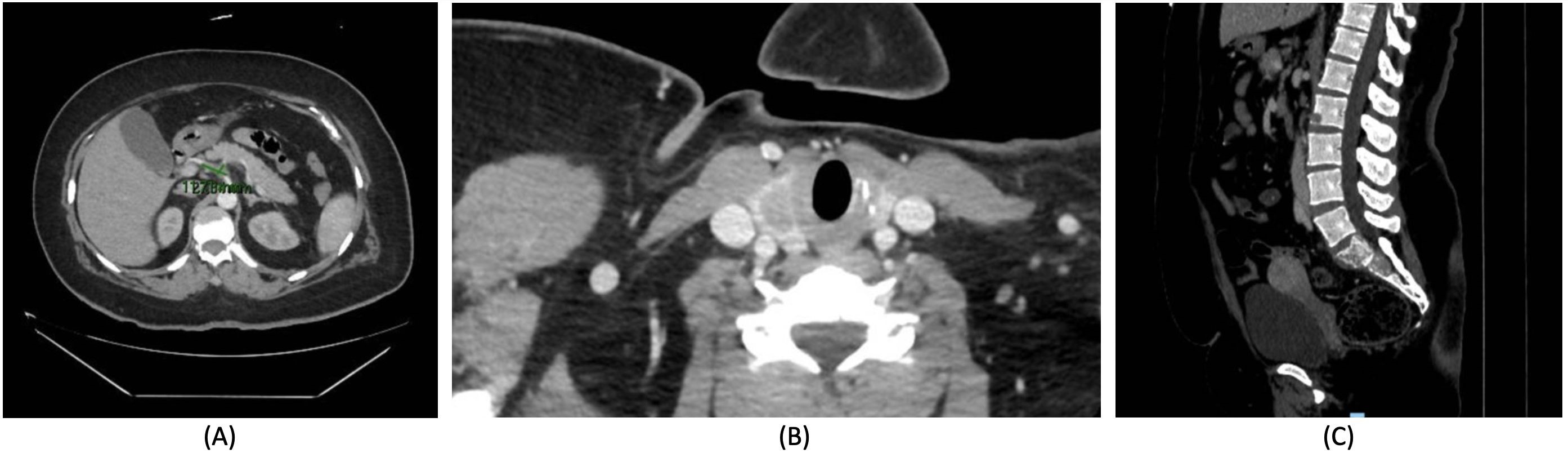
Figure 4. Total-body computerized tomography scan showed A) a pulmonary lesion in the posterior basal segment of the right lower lobe, with the maximum axial dimensions of 40 x 30 mm and craniocaudal extension of 47 mm, B) a nodule in the right thyroid lobe (15 x 10 mm), and C) several osteolytic lesions in pelvic bones, iliac wing, femoral pertrochanter region bilaterally, all sacral metameres
Upon informed consent the genetic analysis revealed the presence of the c.1010 G>A (p.Arg337His) pathogenic variant previously identified in her brother. This variant, reported in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/variation/12379/), has been identified in several Brazilian individuals and families affected by Li-Fraumeni syndrome. Families carrying this variant show an extremely variable cancer risk and several unaffected adult carriers have been described, suggesting an incomplete/age-dependent penetrance (5).
The case underwent collegial discussion. Any surgical interventions for the NBTE, as well as radiation therapy on the involved bone segments or stereotactic therapy on the lung lesion were excluded. The patient was therefore referred for palliative care.
Discussion
In the present case, we address a highly intricate clinical scenario of LFS presenting with NBTE-related ischemic stroke.
Individuals with LFS are lifelong susceptible to developing cancers. Overall lifetime risk is estimated to be over 70% for men and over 90% for women, and cumulative incidence is of 100% by the age of 70 (6, 7). The average age at the diagnosis of first cancers in LFS is 21.9 years, with a tendency for earlier onset in women (8). In particular, breast cancer is by far the most common cancer in women with TP53 mutation accounting for 27-31% of all LFS-related tumors (9). To date, several treatment options to reduce the incidence of cancer in LFS are under investigation on preclinical models (10), however none of them is currently available in humans (11). Therefore, individuals diagnosed with TP53 mutation necessitate a comprehensive and proactive monitoring plan to closely monitor for malignancies in different organ systems, to establish early intervention and improve the rates of survival, complications and quality of life (11). In the present case, as a result of inadequate cancer surveillance LFS manifested itself when several tumors in advanced stages had already developed.
NBTE is one of the consequences of hypercoagulable states induced by advanced malignancy (3). In NBTE, degenerating platelets interwoven with strands of fibrin lead to the formation of sterile vegetation on previously undamaged heart valves (most often aortic and mitral), without bloodstream bacterial infection (3). The most frequently malignancies associated with NBTE include mucin-secreting adenocarcinomas of the lung, stomach, biliary system, pancreas, and ovary, however association with thyroid cancer, head and neck squamous cell carcinoma has been reported in literature (3, 12). The diagnosis of NBTE is considerably more elusive than that of infective endocarditis, as there are no bloodstream markers and vegetations are smaller, easily friable and frequently embolize, leaving only small remnants to be identified through echocardiography. Embolic stroke is the most common manifestation of NBTE (3). Lopez et al. (4) addressed that 5-10 % of the patients with cryptogenic stroke have an underlying malignancy (4). The management of NBTE includes full-dose intravenous unfractionated heparin or subcutaneous low molecular weight heparin, as well as the management of underlying conditions (3). Surgery is rarely indicated since patients with NBTE often present with advanced malignancies and therefore an unfavorable benefit-to-risk ratio (3).
Conclusion
This case highlights the complex relationship between LFS, cancer-related manifestations, and thrombotic complications. It underscores the need for a vigilant, comprehensive approach to care, emphasizing the importance of genetic counseling, personalized surveillance, and an integrated, multidisciplinary management strategy integrating oncology, genetics, cardiology, neurology, and internal medicine. As our knowledge of the genetic basis of cancer progresses, we need to adjust our strategy for handling complex hereditary cancer syndromes such as LFS.
Ethics: Written informed consent was obtained from patient for all procedures and for the publication of this case report.
Peer-review: External and internal
Conflict of interest: No potential conflict of interest relevant to this article was reported.
Authorship: Conceptualization: SA. Investigation: SA, CM, ELC, MAN and FB. Methodology: MAN and FB. Validation: AF and AG. Visualization: SA and SC. Writing – original draft: SA. Writing – review & editing: SC and FB. Supervision: AF and AG. All authors fulfilled authorship criteria.
Acknowledgements and funding: None to declare
References
| 1.Kamihara J, Rana HQ, Garber JE. Germline TP53 mutations and the changing landscape of Li-Fraumeni syndrome. Hum Mutat 2014; 35: 654-62. Doi: 10.1002/humu.22559 https://doi.org/10.1002/humu.22559 PMid:24706533 |
||||
| 2.Villani A, Shore A, Wasserman JD, Stephens D, Kim RH, Druker H, et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 year follow-up of a prospective observational study. Lancet Oncol 2016; 17: 1295-305. Doi: 10.1016/S1470-2045(16)30249-2 https://doi.org/10.1016/S1470-2045(16)30249-2 PMid:27501770 |
||||
| 3.Zmaili M, Alzubi J, Lo Presti Vega S, Ababneh E, Xu B. Non-bacterial thrombotic endocarditis: A state-of-the-art contemporary review. Prog Cardiovasc Dis 2022; 74: 99-110. Doi: 10.1016/j.pcad.2022.10.009 https://doi.org/10.1016/j.pcad.2022.10.009 PMid:36279942 |
||||
| 4.Salazar-Camelo RA, Moreno-Vargas EA, Cardona AF, Bayona-Ortiz HF. Ischemic stroke: A paradoxical manifestation of cancer. Crit Rev Oncol Hematol 2021; 157: 103181. Doi: 10.1016/j.critrevonc.2020.103181 https://doi.org/10.1016/j.critrevonc.2020.103181 PMid:33264715 |
||||
| 5.Achatz MI, Hainau P, Ashton-Prolla P. Highly prevalent TP53 mutation predisposing to many cancers in the Brazilian population: a case for newborn screening? Lancet Oncol 2009; 10; 920-5. Doi: 10.1016/S1470-2045(09)70089-0 https://doi.org/10.1016/S1470-2045(09)70089-0 PMid:19717094 |
||||
| 6.Mai PL, Best AF, Peters JA, DeCastro RM, Khincha PP, Loud JT, et al. Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer 2016; 122: 3673-81. Doi: 10.1002/cncr.30248 https://doi.org/10.1002/cncr.30248 PMid:27496084 PMCid:PMC5115949 |
||||
| 7.Guha T, Malkin D. Inherited TP53 Mutations and the Li-Fraumeni Syndrome. Cold Spring Harb Perspect Med 2017;7. https://doi.org/10.1101/cshperspect.a026187 https://doi.org/10.1101/cshperspect.a026187 PMid:28270529 PMCid:PMC5378014 |
||||
| 8.Gonzalez KD, Noltner KA, Buzin CH, Gu D, Wen-Fong CY, Nguyen VQ, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27: 1250-6. Doi: 10.1200/JCO.2008.16.6959 https://doi.org/10.1200/JCO.2008.16.6959 PMid:19204208 |
||||
| 9.Id Said B, Kim H, Tran J, Novokmet A, Malkin D. Super-transactivation TP53 variant in the Germline of a family with Li-Fraumeni syndrome. Hum Mutat 2016; 37: 889-92. doi: 10.1002/humu.23025 https://doi.org/10.1002/humu.23025 PMid:27297285 |
||||
| 10.Wang PY, Li J, Walcott FL, Kang JG, Starost MF, Talagala SL, et al. Inhibiting mitochondrial respiration prevents cancer in a mouse model of Li-Fraumeni syndrome. J Clin Invest 2017; 127: 132-6. Doi: 10.1172/JCI88668 https://doi.org/10.1172/JCI88668 PMid:27869650 PMCid:PMC5199691 |
||||
| 11.Kumamoto T, Yamazaki F, Nakano Y, Tamura C, Tashiro S, Hattori H, et al. Medical guidelines for Li-Fraumeni syndrome 2019, version 1.1. Int J Clin Oncol 2021; 26: 2161-78. Doi: 10.1007/s10147-021-02011-w https://doi.org/10.1007/s10147-021-02011-w PMid:34633580 PMCid:PMC8595164 |
||||
| 12.Umeojiako WI, Kasouridis I, Sargent R, Ghani S. Atypical marantic endocarditis. BMJ Case Rep 2019; 12. Doi: 10.1136/bcr-2019-232057 https://doi.org/10.1136/bcr-2019-232057 PMid:31712241 PMCid:PMC6855875 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

