Clinical and neuroimaging predictors of early hospital mortality in patients with hemorrhagic stroke
ORIGINAL RESEARCH ARTICLE
Clinical and neuroimaging predictors of early hospital mortality in patients with hemorrhagic stroke
Article Summary
- DOI: 10.24969/hvt.2024.488
- Page(s): 263-276
- RELEVANT DISCIPLINES
- Published: 11/06/2024
- Received: 28/01/2024
- Revised: 23/04/2024
- Accepted: 24/04/2024
- Views: 4610
- Downloads: 3110
- Keywords: hemorrhagic stroke, retrospective study, predictors, fatal outcome, hospital stage, risk stratification
Address for Correspondence: Elmira Mamytova, Department of Neurology and Clinical Genetics, Kyrgyz State Medical Academy named after I.K. Akhunbayev, Bishkek, Kyrgyzstan E-mail: elmiramamytova@yahoo.com
ORCID: Darikha I. Bakaeva - 0009-0006-6789-3271 Khalida Sh. Musaeva - 0000-0003-0334-1700 Elmira M. Mamytova - 0000-0002-4322-5555 Mitalip M. Mamytova - 0000-0002-0586-9480 Damirbek A. Abibillaev - 0000-0002-4660-3064 Nurbek K. Monolov 0000-0001-7589-582012
Citation: Bakaeva DI, Mamytova EM, Mamytov MM, Musaeva KS, Abibillaev DA, Monolov NK. Clinical and neuroimaging predictors of early hospital mortality in patients with hemorrhagic stroke. Heart Vessels Transplant 2024; 8: doi: 10.24969/hvt.2024.488
Darikha I. Bakaeva1, Elmira M.Mamytova1,2* , Mitalip M.Mamytov³, Khalida Sh. Musaeva¹, Damirbek A. Abibillaev4, Nurbek K. Monolov²
¹ Department of Neurology and Clinical Genetics, KGMA named after I.K. Akhunbayev, Bishkek, Kyrgyzstan
² Department of Clinical Disciplines, Salymbekov University, Bishkek, Kyrgyzstan
3Department of Neurosurgery of undergraduate and postgraduate study, KGMA named after I.K. Akhunbayev, Bishkek, Kyrgyzstan
4Ala-Too International University, Bishkek, Kyrgyzstan
Abstract
Objective: To date the complex interplay of predictors of stroke-associated mortality have been stated. Despite the abundancy of literature data on stroke-associated mortality, little is known about the predictors of early post-stroke mortality in developing countries. In this study, we aimed to analyze predictors of in-hospital stroke-associated mortality in Kyrgyz patients.
Methods: Research design – retrospective, cross-sectional. Retrospective findings from medical records of 64 patients with hemorrhagic stroke (HS) were used for study. Collected data included clinical, laboratory, neuroimaging parameters, as well as autopsy protocols, which could be associated with a stroke-associated mortality in patients hospitalized in the tertiary care center with distinct stroke department. All patients were divided into 2 groups: deceased and survived ones. Initially comparison tests were applied for groups. Then multiple regression analysis was conducted to determine predictors of stroke associated mortality.
Results: The study included patients with the following clinical subtypes: 12 patients were with subarachnoid hemorrhage (SAH), 42 patients with intracerebral hemorrhage (ICH) and 10 patients with combined (SAH+ICH) HS.
Our study showed that the severe disorder of consciousness (coma) (66%-5.7%, p<0.001), impaired functioning of vital organs (hypoxemia 62%-11.4% - p<0.001; tachycardia – 51%-14.3% - p<0.006), the need for ventilator support and catheterization of the bladder (100% - 0% - p<0.001), neuroimaging picture of threatening cerebral edema with signs of its insertion (50%-20% - p<0.006) and expansion of hematoma into the ventricular system (60%-20% - p<0.006), hyperglycemia (65%-16.7% - p<0.001) and hyperthermia (55%-2.9% - p<0.001), as well as impaired kidney function (65%-16.7% - p<0.001) were significantly more common in mortality groups than in survived patients. Multiple regression analysis demonstrated that only systolic blood pressure level was associated with better survival (OR – 0.04, 95%CI 0.93-0.99, p=0.01).
Conclusions: Thus, as our research shows, that severe unconsciousness, the expansion of hematoma into the ventricular system, progressive cerebral edema with dislocation of medial structures and impairment of the brain, multi-organ insufficiency, tachypnoe/bradypnoe, tachycardia/bradycardia, diabetes mellitus and increased level of cholesterol were more common in HS patients with in-hospital mortality as compared to survivors. The only significant predictor of in-hospital stroke associated mortality among clinical and laboratory variables was systolic blood pressure at onset, low oxygen saturation had borderline significance.
Key words: hemorrhagic stroke, retrospective study, predictors, fatal outcome, hospital stage, risk stratification
![]()
Graphical abstract
Introduction
Inevitably, stroke remains one of the deadliest conditions globally with higher mortality and post-stroke sequelae. According to Bishkek Stroke Registry (BSR) the stroke incidence is 2.6-2.67 cases per 1000 population, with a total mortality rate of 1.17, whereas in-hospital mortality (IHM) is of 14.3%, and extra-hospital mortality (EHM) rate during prehospital stage of management is 51.3%. The incidence of ischemic stroke (IS) is 1.87 per 1000 population (men - 1.64, women - 2.06), while for acute hemorrhagic stroke (AHS), it is 0.54 per 1000 population. Despite the lower incidence rates of AHS in contrast to AIS (1.9 vs 0.5 per 1000, respectively), former is associated with higher mortality rates (54.4% vs 3.8% respectively) (3).
As a rule, almost all patients necessitate hospitalization into specialized intensive care units, which is usually termed as stroke units. According to a recent study, 35% of patients with a hemorrhagic stroke die within 7 days of a stroke, and about 50% of patients - within 30 days. Early prediction of mortality and identification of associated factors is essential for in-time decision making in comprehensive management with targeted interventions (9).
Early prediction of mortality and identification of factors related to the mortality of patients with hemorrhagic stroke in the intensive care unit can potentially reduce the mortality rate through targeted interventions.
As stated by epidemiological analysis of stroke in Kyrgyz Republic (KR), not everything is known about of predictors of AHS-associated IHM.
Therefore, in this article we aimed to perform comprehensive analysis of clinical, laboratory and imaging predictors of in-hospital mortality in AHS patients.
Methods
Study design and population
Research design – retrospective, cross-sectional.
The medical records of patients hospitalized in stroke units of National Hospital of Ministry of Healthcare of KR (NHMHKR) were analyzed. Patients data from January to June of 2021 was included into the study. Patients were subcategorized into deceased and survived groups. Inclusion criteria consisted of any adult patient from all over the regions of KR; with emergent hospitalization into stroke department via ambulance line or transferred from other healthcare institutions within 24 hours after establishment of AHS diagnosis; diagnosed in accordance with World Health Organization (WHO) guidelines and documented by neuroimaging; any clinical forms of AHS, i.e. primary or recurrent, and regardless of complications during admission. ![]()
Patients younger than 18 years; whose death was documented within 24 hours and/or due to other comorbid causes before the neuroimaging confirmation; patients with hemorrhagic transformation of ischemic stroke (IS), neoplasia-associated or trauma-related bleeding were excluded from study.
Informed consent was obtained from patients for all procedures. As to retrospective design of the study, the Ethic committee approval was not sought.
Operational definitions
In-hospital mortality was defined as all in-hospital stroke mortality from all types of stroke occurred in the follow-up period (10).
Hypertension: Any hypertensive condition regardless of type (symptomatic vs essential), stage, severity (urgency vs emergency), onset (new-onset vs long-standing), control (controlled vs uncontrolled) when blood pressure (BP) exceed 140/90 mmHg was defined as hypertension. Stage II arterial hypertension defined from AHA 2017 guidelines (11).
Obesity: Both obesity and overweight cases included when BMI exceeded 25 kg/m2 according to Quotelet formula (12).
Coronary artery disease: Any form of coronary artery disease detected during first medical contact, regardless of anamnestic and documental considerations (13).
Pneumonia: Lung infection diagnosed through clinical examination (crackles, ronchi) and documented by imaging regardless of cause and severity (14).
Diabetes mellitus: Diabetic blood glucose level consistent with lab definitions of current ADA guidelines, regardless of treatment and HbA1c status (15).
Chronic kidney disease was defined according to current KDIGO guidelines, regardless of therapy (16).
Cerebral symptoms are common nonspecific symptoms, such as diffuse headache, syncope etc.
Focal symptoms: Any form of focal neurological deficits;
Tachypnea was accepted when respiratory rate exceeded 20 beats per minute (17).
Hypoxemia was accepted when non-invasive oxygen saturation was measured less than 95% during daytime and less than 92% during night-time (18).
Tachycardia was accepted when resting heart rate exceeded 100 beats per minute
GCS: it measures the level of consciousness categorized as normal/mild impairment GCS (13–15), moderate impairment GCS (9–12), and severe impairment GCS (3–8) (19).
Demographic and clinical data
The standard demographic data including age, gender, ethnicity, place of residence were identified. Baseline vital signs including blood pressure, heart rate, pulse oxymetry recordings obtained. A detailed history including stroke severity, risk factors and comorbidities was noted. Glasgow coma scale and other tests were implied for patients presented with debilitated or comatose states.
Neuroimaging data
Non-contrast computed tomography (CT) is the gold standard of imaging for the diagnosis of intracerebral hemorrhage, but magnetic resonance tomography (MRI) was performed in most patients with fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted imaging (DWI) regimens. Interventional CT angiography was performed only for those patients who were potential candidates for surgical examination after consultation with a neurosurgeon. However, these patients were not included in the study, as the causes of death could be related to surgery and its complications. Therefore, mostly patients underwent contrast-free MR angiography.
The following variables were assessed as hematoma, arterial aneurysm, brain herniation, white matter gliotic changes, dislocation syndrome, bleeding, and arterio-venous malformation.
Statistical analysis
For statistical analysis SPSS 23 (IBM, New-York, USA) and Stata 16, (StataCorp, Texas, USA) tools were used. The independent samples t-test and Chi-square test were used initially for comparative study of deceased and survived patients. Then multiple logistic regression was conducted for predictor analysis. The categorical variables denoted by absolute count and percentage, whereas metric variables depicted via mean, standard deviation, median, minimal and maximal range values. Statistical significance was adjusted for p-value of less than 0.05, confidence interval by 95%.
Results
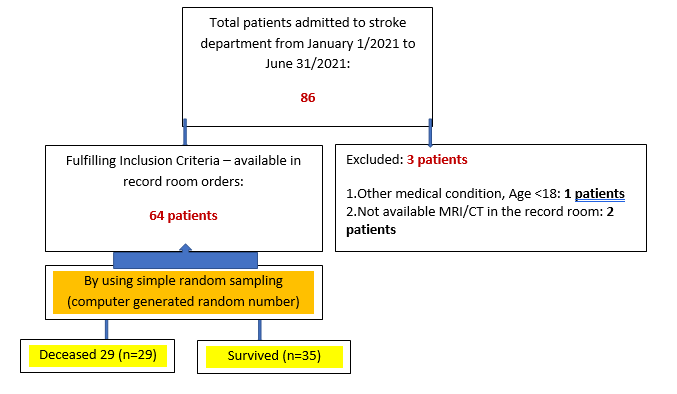
A total of seventy-one patients were included into the study. Due to lack of inclusion criteria, three patients were excluded from the study. The patients further were classified into two groups based on IHM during hospital period: survived (n=35) and non-survived (n=29) (Fig. 1). Each clinical, laboratory, imaging findings were separately analyzed.

Figure 1. Flowchart of study according to STROBE guidelines.
Baseline demographics, clinical signs and comorbid conditions
Clinical-demographic characteristics of survived and non-survived patients during first admission are comparatively highlighted in Table 1.
A classical atherosclerosis-associated disease, including hypertension, coronary artery disease and obesity frequently observed in both groups, despite the lack of statistical difference. Only diabetes showed significant difference among groups (27.6% vs 5.7%, p=0.01).
Admission physical findings revealed the significant differences in diastolic blood pressure in survived and deceased populations (p <0.001). Similarly, the average systolic blood pressure was higher in survived group compared to deceased one, however statistical significance was not noted (p=0.61). Furthermore, most of the survived patients were diagnosed by stage II arterial hypertension according to AHA 2017 guidelines (32 vs 24 cases, respectively; p<0.001). The fever was observed in sixteen cases of non-survived patients, whereas only single patient presented with fever in survived group (p<0.001). All vital signs upon admission differed significantly between groups despite the limited cases, tachypnea was noted in 7, hypoxemia in 18 and symptomatic tachycardia in 15 cases of non-survived patients.
Rest of the demographic data, including gender, age, ethnicity, accommodation did not reveal statistical significance. Strikingly, almost all patients in both groups were non-compliant with their antihypertensive treatment with various forms, such as untreated, unrecognized or incompliant.
Stroke-specific clinical, neuroimaging variables and acute complications
Clinical and neuroimaging parameters including acute period complications of AHS among groups are cshown in Table 2.
Most of the patients could not complain to altered consciousness or speech problems, therefore ideal anamnestic information could not be gained. From non-survived patients only four of them presented by headache of various degree. Whereas, in the structure of complaints of survived group, 11 had headache, 6 had limb weakness; in addition to headache, 5 of them had speech problems including aphasia. Nausea and vomiting were noted in only two cases.
![]()
|
Table 1. Baseline demographics, clinical signs and comorbid conditions |
|||
|
Variables |
Deceased (n=29) |
Survived (n=35) |
p |
|
Sex, male |
13 (44.8) |
14 (40) |
0.69 |
|
Age, years |
59.8(15.5) 64 (31-86) |
58.7(12.9) 58 (34-85) |
0.76 |
|
WHO age gradation, n(%) Young (18-44) Middle age (45-59) Old (60-74) Senile (75-90) |
7 (24.1) 6 (20.7) 12 (41.4) 4 (13.8) |
6 (17.1) 12 (34.3) 13 (37.1) 4 (11.4) |
0.66 |
|
Turcic-speaking ethnicity, n(%) |
22 (75.8) |
31 (88.5) |
0.18 |
|
Urban residents, n(%) |
14(48.2) |
9(25.7) |
0.06 |
|
DBP, mmHg |
85.8 (15.6) |
98 (17.2) |
<0.001 |
|
SBP, mmHg |
145.5 (13.5) |
180.5 (34.0) |
<0.001 |
|
Fever, n(%) |
16(55.1) |
1(2.8) |
<0.001 |
|
Abnormal RR, n(%) |
8(27.5) |
2(5.7) |
0.01 |
|
Abnormal HR, n(%) |
11(37.9) |
28(80) |
<0.001 |
|
Abnormal SPO2, n(%) |
11(37.9) |
31(88.5) |
<0.001 |
|
HTN, n(%) |
25 (86.2) |
33 (94.2) |
0.27 |
|
Stage II HTN, n(%) |
11 (37.9) |
31 (88.5) |
<0.001 |
|
Treatment compliance, n(%) Untreated Incompliant Unexamined |
13 (44.8) 8 (27.6) 8 (27.6) |
24 (68.6) 5 (14.3) 6 (17.1) |
0.15 |
|
Obesity, n(%) |
11 (37.9) |
15 (42.8) |
0.69 |
|
CAD, n(%) |
25 (86.2) |
32 (91.4) |
0.50 |
|
Pneumonia, n(%) |
4 (13.8) |
5 (14.3) |
0.95 |
|
DM, n(%) |
33 (94.2) |
21 (72.4) |
0.01 |
|
CKD, n(%) |
7 (24.1) |
14 (40) |
0.17 |
|
COVID-19, n(%) |
4 (13.7) |
2 (5.7) |
0.27 |
|
Combination of comorbidities, n(%) Single Double Triple Quadruple |
3(10.3) 14(48.2) 7 (24.1) 5(17.2) |
4(11.4) 12(34.2) 12(34.2) 7(20) |
0.7 |
|
Data are presented as mean (SD) and n(%) CAD – coronary artery disease, CKD – chronic kidney disease, DBP – diastolic blood pressure, DM – diabetes mellitus, HR – heart rate, HTN – arterial hypertension, RR- respiratory rate, SBP – systolic blood pressure |
|||
The neurological examination revealed existence of all four classical symptom complexes: cerebral, focal, meningeal and symptoms of secondary brain stem involvement. Cross-tab analysis revealed statistically significant differences of occurrences of these symptoms among groups (p<0.001). In seventeen cases of non-survived patients, severe clinical symptoms were developed including combination of cerebral, focal, meningeal and medullary signs whereas only 6 cases had the similar pattern in survived group. Furthermore, survived patients mostly presented by combined form of either cerebral and focal (n=11) or cerebral and meningeal (n=12) symptoms. Isolated symptoms were seen rarely in both groups. In the structure of cerebral symptoms, loss of consciousness was found significantly different among groups (p<0.001). Most of the non-survived patients developed deep coma states in contrast to survived ones. For instance, coma stage 1 was observed in 9, coma stage 2 in 8 and sopor in 6 cases of non-survived group. Whereas, most of survived patients were conscious (n=10) and presented by mild alteration of consciousness (n=19).
Analysis of cerebral and extra-cerebral complications of stroke revealed statistically significant differences. In non-survived group, the most prevalent sequel was intraventricular bleeding with 12 cases then followed by 9 cases of cerebral edema and combinations of extra-cerebral complications in 8 cases. Among the survived group of patients 17 cases did not show any kind of complications, 10 of them presented by intraventricular bleeding and some isolated complications.
Magnetic resonance imaging has the same accuracy for the diagnosis of acute intracerebral hemorrhage as routine CT, and surpasses CT in the difference between intracerebral hemorrhage and hemorrhagic transformation after acute ischemic stroke. Gradient echo sequences of MRI can identify microbleeding in the parenchyma of the brain, as well as blood products that represent chronic lesions and cavernomas – i.e., such findings that, as a rule, cannot be diagnosed by non-contrast tomography (20). An acute hematoma is noted on the head tomogram in T1 regimen as an area of increased density inside the parenchyma with surrounding hypodensity, which indicates perivascular edema (21).
According to neurovizualization imaging volume of cerebral hematoma varied as follows: small-sized (less than 30 mm3), medium-sized (30-50 mm3) and large (more than 50 mm3) (Fig. 2). Despite the statistically non-significant findings (p=0.42), the non-survived group revealed predominantly large-sized (n=11) and medium-sized (n=10) hematomas. Strikingly, arterial aneurysms frequently seen in survived ones. Furthermore, arterio-venous malformation was detected in single case of survived group. White matter gliotic changes, as the universal response to stroke injury of central nervous system were noted in 32 cases of survived and 23 cases of non-survived patients without significant difference. The dislocation syndrome, a very devastating pattern of brain damage mostly visualized in non-survived group in 13 cases of compressed and 3 cases of non-compressed type. Only 7 patients developed brain herniation with compression in survived group.
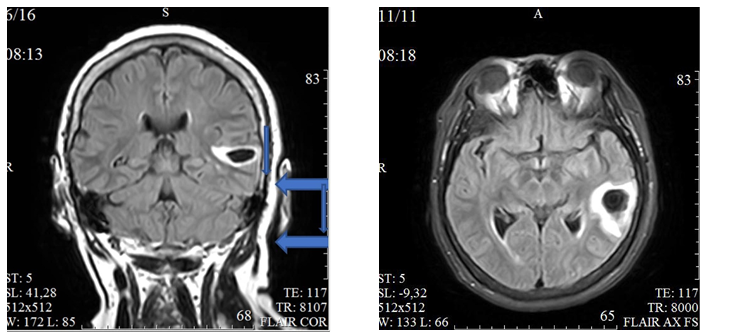
Figure 2. In the superior temporal gyrus on the left an intracerebral hematoma is shown (wide arrow) measuring up to 28*20*9 mm, with a hypointensive magnetic resonance signal in the center and an enhanced rim on T2 DWI and FLAIR, isointensive in the center and a moderately hypointensive rim on T1 DWI, moderate perifocal edema (narrow arrow) ( left – coronal plane, right – axial plane, FLAIR)
Therapeutic interventions in hemorrhagic stroke are aimed at treating complications of the acute/subacute phase, such as mass effect due to hemorrhage and edema, secondary ischemia, increased intracranial pressure (ICP) and increased hematoma volume, as well as eliminating the long-term effects of stroke, such as deep vein thrombosis, infection or recurrence of bleeding.
|
Table 2. Stroke-specific clinical, neuroimaging variables and acute complications |
|||
|
Variables |
Deceased (n=29) |
Survived (n=35) |
p |
|
Hospitalization time, n (%) Within 4-5 hours Within 24hours Within 48hours
|
21(72.4) 3(10.3) 5(17.2) |
14(40) 4(11.4) 17(48.5) |
0.02 |
|
Disease-onset, daytime, n (%) Morning Day Evening Night |
7 (24.1) 5 (17.2) 11 (37.9) 4 (13.8) |
4 (11.4) 7 (20.0) 6 (17.1) 4 (11.4) |
0.02 |
|
Disease onset, acute, n (%) |
25(86.2) |
35(100) |
0.02 |
|
Disease onset, background, n (%) sleepy state awake time physical exertion emotional exertion alcohol intake unknown |
3 (10.3) 5 (17.2) 1 (3.4) 0 (0.0) 3(10.3) 17(58.6) |
5 (14.3) 13 (37.1) 3 (8.6) 3 (8.6) 2(5.7) 9(25.7) |
0.06 |
|
Disease onset, hypertensive, n (%) |
28(96.5) |
33(94.2) |
0.65 |
|
Neurological status on admission, n (%) 1) Focal symptoms 2) Cerebral + Focal 3) Focal + Cerebral + Meningeal 4) General + Focal + Stem |
7 (15.6) 25 (55.6) 12 (26.7) 1 (2.2%) |
28 (62.2) 17 (37.8) 0 0 |
<0.001 |
|
Disease onset, symptoms, n (%) Cerebral Focal Mixed |
17 (58.6) 0 8 (27.6) |
17 (48.6) 5 (14.3) 13 (37.1) |
0.02 |
|
Complaints upon presentation, n (%) Impossible to complaint1 Headache Kinetic problems2 Speech problems Speech and kinetic problems Speech, kinetic and swallowing |
31 (68.9) 0 2 (4.4) 4 (8.9) 5 (11.1) 3 (6.7) |
5 (11.1) 2 (2.2) 9 (20) 5 (11.1) 22 (48.9) 2 (4.4) |
<0.001 |
|
Dislocation syndrome, n (%) |
14 (48.2%) |
7 (20%) |
<0.05 |
|
Focal deficit, n (%) |
29(100) |
26(76.4) |
0.005 |
|
Focal symptoms, n (%) 1) Hemiplegia 2) Speech disorder 3) Hemiplegia + Oculomotor 4) Hemiplegia + Aphasia 5) Alternating Syndrome 6) Cerebellar symptoms 7) Others 8) Tetraparesis + Bulbar syndrome 9) Tetraparesis |
21 (46.7) 2 (4.4) 2 (4.4) 11 (24.4) 2 (4.4) 1 (2.2) 2 (4.4) 2 (4.4) 2 (4.4) |
25 (55.6) 2 (4.4) 0 16 (35.6) 2 (4.4) 0 0 0 0 |
0.25 |
|
Focal neurological symptoms, n (%) |
20(71,4) |
23(65.7) |
0.62 |
|
Meningeal symptoms, n (%) |
19(65.5) |
19(54.2) |
0.36 |
|
Cerebral symptoms, n (%) |
29(100) |
31(88.5) |
0.06 |
|
SAH, n(%) |
8(27.5) |
14(40) |
0.29 |
|
Table 2. Stroke-specific clinical, neuroimaging variables and acute complications (continued from page ???) |
|||
|
Variables |
Deceased (n=29) |
Survived (n=35) |
p |
|
ICB, n (%) |
24(82.7) |
26(74.2) |
0.41 |
|
IVH, n (%) |
23(79.3) |
16(45.7) |
0.006 |
|
Hematoma size, mm3 |
34.51 (12.40) |
28.31 (43.93) |
0.18 |
|
Large hematoma , n (%) |
12 (41.3) |
9 (25.7) |
0.18 |
|
White matter degeneration (gliosis) , n (%) |
23(79.3) |
32(91.4) |
0.16 |
|
Primary stroke, n (%) |
27(93.1) |
28(80) |
0.13 |
|
Recurrent HS, n (%) |
5(17.2) |
6(17.1) |
0.99 |
|
Complications, n (%) |
29(100) |
18(51.4) |
<0.001 |
|
Combined complications, n (%) |
17(58.6) |
2(5.7) |
<0.001 |
|
Non-neurological , n (%) |
16(55.1) |
2(5.7) |
<0.001 |
|
Brain edema, n (%) |
25(86.2) |
4(11.4) |
<0.001 |
|
ACS, n (%) |
5(17.2) |
0 |
0.01 |
|
AKI, n (%) |
3(10.3) |
0 |
0.05 |
|
Opportunistic infections, n (%) |
4(13.7) |
0 |
0.02 |
|
Length of hospital stay, n (%) Up to 1 day Up to 3 days Up to 1 week Up to 2 weeks Over 2 weeks |
3 (10.3) 5 (17.2) 13 (44.8) 4 (13.8) 4 (13.8) |
3 (8.6) 0 6 (17.1) 22 (62.9) 4 (11.4) |
<0.001 |
|
GCS, points |
7.6 (2.5) |
11.1 (2.5) |
<0.001 |
|
UC insertion9, n (%) |
29 (100) |
11 (31.4) |
<0.001 |
|
MV need, n (%) |
22 (75.8) |
0 |
<0.001 |
|
Data are presented as mean (SD) and n(%) ACS – acute coronary syndrome, AKI – acute kidney injury, GCS – Glasgow Coma Scale, HS – hemorrhagic stroke, ICB – intracerebral (parenchymatous) bleeding, IVH – intraventricular hemorrhage, SAH – subarachnoid hemorrhage, MV – mechanical ventilation, UC – urinary catheter 1Impossible to collect anamnesis due to motor or sensory aphasia; 2Any form of focal deficits manifested as kinetic problems, hemiplegia, hyperkinesia etc.; 3Abrupt onset of disease which necessitated immediate call of ambulance and prompt medical intervention; 4Cerebral symptoms are common nonspecific symptoms, such as diffuse headache, syncope etc.; 5Any form of focal neurological deficits; 6Tachypnea was accepted when respiratory rate exceeded 20 beats per minute; 7Hypoxemia was accepted when non-invasive oxygen saturation measured less than 95% during daytime and less than 92% during night-time; 8Tachycardia was accepted when resting heart rate exceeded 100 beats per minute 9Urinary catheter inserted upon arrival to stroke unit due to autonomic pelvic dysregulations |
|||
Laboratory and clinical investigations
The laboratory and other imaging findings, such as chest radiography, electrocardiographic and transthoracic echocardiographic findings are presented in Tables 3. When studying the main components of the general blood test, there was also no statistically significant difference between the study groups (p>0.05). It should be noted that the average values of the main blood components do not differ significantly from the reference norm.
Laboratory findings revealed different results in terms of blood glucose level and total cholesterol. Blood glucose levels were significantly higher in deceased population in contrast to surviving ones (p=0.01). The structure of glycemic profile revealed unequivocal findings, both mild and severe hyperglycemic cases registered frequently in non-survived group. ![]()
|
Table 3. Laboratory, CXR, TTE and ECG findings |
|||
|
Variables |
Deceased (n=29) |
Survived (n=35) |
p |
|
Hemoglobin, g/L |
130.4(19.8) 132 (88-175) |
139.1(19.7) 142 (87-182) |
0.09 |
|
Erythrocytes, 1012 |
4.8(0.9) 4.8 (2.8-6.7) |
4.6(0.6) 4.6 (2.9-6.2) |
0.28 |
|
Leukocytes, 109 |
9.2(3.8) 7 (4-16) |
8.2(3.1) 8 (3.7-21) |
0.29 |
|
Segments, % |
67.9(10.9) 67 (51-88) |
70.8(9.2) 72 (54-92) |
0.26 |
|
Lymphocytes, % |
30.7(7.9) 34 (10-39) |
26.6(11.1) 25 (6-48) |
0,12 |
|
Thrombocytes, 103 |
241.9(38) 239 (185-320) |
254.3(72.9) 250 (83-443) |
0,44 |
|
ESR, mm/hour |
11(10.2) 7 (2-46) |
14.3(11.5) 10 (2-45) |
0.23 |
|
Urine protein, |
0.13(0.26) |
0.11(0.24 |
0.77 |
|
Leukocytes (urine), |
7.2(9.9) 2 (2-30) |
7.0(7.9) 3 (1-30) |
0.92 |
|
Erythrocytes (urine) |
4.0(8.7) 0 (0-30) |
2.6(6.0) 0 (0-25) |
0.44 |
|
Bacteria (urine), |
0.5(0.9 0 (0-3) |
0.1(0.5) 0 (0-3) |
0.07 |
|
Glucose, mmol/L |
7.9(3.08) 7.8 (4-15) |
5.6(1.5) 5.5 (3.8-15) |
<0.001 |
|
Total cholesterol, mmol/L |
5.3(1.3) 4.9 (3.2-7.5) |
4.3(0.9) 4.3 (1.9-6.8) |
<0.001 |
|
AST, mmol/L |
23.8(12.5) 18 (12-56) |
22.3(14.8) 18.9 (10-81) |
0.68 |
|
ALT, mmol/L |
26.3(14.1 20 (13-62) |
23.2(19.8) 17 (10-92) |
0,47 |
|
Creatinine, mcmol/L |
180.6(101.0) 178 (54-430) |
98.2(37.8) 88 (59-210) |
<0.001 |
|
Fibrinogen, mmol/L |
3680.6(945.2) 3667 (1888-5665) |
3617.7(772.7) 3554 (2004-5772) |
0.77 |
|
PTI, % |
99.2(17.3) 98 (68-120) |
91.2(11.9) 90 (69-121) |
0.03 |
|
TTE, n(%) Normal Abnormal No data |
4(13.7) 4(13.7) 21(72.4) |
10(28.5) 3(8.5) 22(62.8) |
0.33 |
|
CXR, n(%) Normal Abnormal |
15(51.7) 14(48.2) |
27(77.1) 8(22.8) |
0.03 |
|
ECG, n(%) Normal Abnormal |
4(13.7) 25(86.2) |
23(65.7) 12(34.2) |
<0.001 |
|
ALT – alanine aminotransferase, AST – aspartate aminotransferase, CXR – chest X-ray, ECG –electrocardiogram, ESR – erythrocyte sedimentation rate, TTE – transthoracic echocardiography, PTI – prothrombin index |
|||
Kidney function tests revealed substantial changes in both patient`s groups. Creatinine level was presented by significant difference among groups ((180.6 (101.0) vs 98.2 (37.8) mcmol/l, p<0.001)). Furthermore, increased blood urea nitrogen and urea were mostly observed in deceased group (19 vs 4 cases, p<0.001).
Fat metabolism was assessed by the level of total cholesterol, the level of which was slightly increased when compared with the reference norm and its values were significantly higher in deceased individuals and amounted to 5.3 (1.3) mmol/l, while in living individuals its values remained within normal limits and amounted to 4.3 (0.9_ mmol/L (P<0.001). Hypercholesterolemia occurred in 1/3 of the group of deceased (n=10-34.5%), whereas in living individuals it occurred less frequently (4 - 11.4%).
The hemostasiogram and the nature of hemostasis disorders were also analyzed according to fibrinogen, prothrombin index (PTI) and other indicators. Thus, when compared with the group of deceased, the level of fibrinogen did not differ significantly from the level of the group of living persons and did not exceed the reference values, although there was a statistically significant difference between the groups for PTI (p=0.03). Attention is drawn to the analysis of deviations in the hemostasiogram by the fact that in deceased persons, disorders in hemostasis shifted towards hypercoagulation and were 3 times more common (n=12-41.4%) than among living persons (n=4-11.4%), hypocoagulation, on the contrary, was significantly found in deceased patients less frequently (n=8 - 22.9%) than in surviving patients (n= 4 - 13.9%), signs of disseminated intravascular coagulation syndrome were found only in 3 deceased (10.3%) patients.
The analysis of hemodynamics by echocardiography did not reveal a statistically significant difference between the study groups. Echocardiograms most often showed signs of atherosclerotic damage to the valves and aorta, signs of left ventricular failure or diastolic dysfunction of the left ventricle. In isolated cases, rhythm disturbances in the form of atrial fibrillation in combination with atherosclerosis and signs of acute coronary syndrome (left ventricular myocardial infarction) were detected.
A similar pattern was observed with respect to the chest X-ray when comparing the main and control groups. There was no statistically significant difference between the groups. The most common was a normal picture of the chest organs, which was more typical for survivors (n=27 – 77.1%), a normal picture of chest organs in the deceased occurred in half of the group of deceased (n=15 – 51.7%). Signs of the following pathological conditions were observed significantly less frequently in both groups with different frequency: unilateral lobular pneumonia, bilateral pneumonia, residual effects of tuberculosis (both pulmonary and intrathoracic node), pneumofibrosis, and active pulmonary tuberculosis.
Clinical, laboratory and imaging predictors of stroke-associated in-hospital mortality of patients with AHS
Multivariate logistic regression analysis of risk factors for death in the early post-stroke period in patients with hemorrhagic stroke: death was defined as dependent variables and increased systolic blood pressure,tachypnoe/bradypnoe, tachycardia/ bradycardia, decreased saturation in the onset of stroke, associated diabetes mellitus, huge size of hematoma, total cholesterol, prothrombin index were defined as independent variables (Table 4).
|
Table 4. Assignment of independent variables |
|||
|
Independent variable |
Assignment |
Independent variable |
Assignment |
|
Onset SBP |
Yes =1, no =0 |
GCS scores |
Continuous variable |
|
Onset RR |
Yes =1, no =0 |
Cholesterol |
Continuous variable |
|
Onset HR |
Yes =1, no =0 |
PTI |
Continuous variable |
|
Onset SPO2 |
Yes =1, no =0 |
|
|
|
Diabetes Mellitus |
Yes =1, no =0 |
|
|
|
Hematoma Size |
Yes =1, no =0 |
|
|
|
SBP – systolic blood pressure, RR – respiratory rate, HR – heart rate, SPO2 – saturation pressure O2, GCS – Glasgow coma scale, PTI – prothrombin index |
|||
|
Table 5. Multiple regression analysis of clinical and laboratory data as predictors of in-hospital death |
|||||
|
Variables |
OR |
Standard error |
z/χ2 value |
p |
95% CI |
|
Onset SBP |
0.94 |
0.02 |
-2.35 |
0.01 |
0.90 – 0.99 |
|
Onset RR |
2.68 |
4.28 |
0.62 |
0.53 |
0.11 – 61.18 |
|
Onset HR |
3.27 |
4.62 |
0.84 |
0.39 |
0.20 – 51.9 |
|
Onset SPO2 |
0.07 |
0.10 |
-1,80 |
0.07 |
0.004 – 1.26 |
|
Diabetes mellitus |
25.4 |
52.04 |
1.59 |
0.11 |
0.46 – 1395 |
|
Hematoma |
0.81 |
0.86 |
-0.20 |
0.84 |
0.09 – 6.60 |
|
GCS scores |
0.83 |
0.17 |
-0.85 |
0.39 |
0.54 – 1.26 |
|
CHOL |
2.23 |
1.36 |
1.32 |
0.18 |
0.67 – 7.42 |
|
PTI |
0.99 |
0.02 |
-0.15 |
0.87 |
0.94 – 1.05 |
|
CHOL – cholesterol, CI – confidence interval, GCS – Glasgow coma scale, HR – heart rate, OR – odds ratio, PTI – prothrombin index, RR – respiratory rate, SBP – systolic blood pressure, SPO2 – saturation pressure O2 |
|||||
As can be seen from Table 5 among all variables entered in the model, only high systolic blood pressure at onset of stroke was significant predictor of survival (OR 0.94, 95%CI 0.90-0.99, p=0.01) and low oxygen saturation had borderline predictive value in prediction of death (p=0.07.) All other variables as SPO2 at onset of stroke, hematoma size, Glasgow coma score, heart rate and respiratory rate, coagulation parameter despite being significantly impaired in univariate analysis (Tables 1-3) did not sustain predictive value in multiple analysis model.
Discussion
Hemorrhagic stroke is one of the main causes of death and the main cause of disability worldwide and including Kyrgyz Republic (3, 8). According to a recent study, 35% of patients with a hemorrhagic stroke die within 7 days of a stroke, and about 50% of patients within 30 days. Practically all patients necessitate hospitalization into intensive care units. Early prediction of mortality and identification of associated factors have the potential to improve survival through targeted interventions (9).
In our study, both deceased and surviving patients in 4/5 had moderate to large-sized hematomas, which caused acute neurological syndromes (general cerebral, focal, meningeal, stem).
Despite the same proportion of patients with medium to large-sized hematomas, the symptoms differed in the comparison groups. For example, deceased patients are reliably more likely to have severe cognitive impairment in the form of a sopor or coma (79.3% of cases), while survivors are reliably more likely (82.9% of cases) to have clear consciousness or a low level of unconsciousness (stunning). Other authors (22), who observed adverse outcomes in patients with large-scale hematomas, also point to the association of impairment with adverse clinical outcomes. Cognitive impairment is a consequence of increased intracranial pressure with compression or deformation of the reticular activating system, thalamus and brain stem (23).
Another feature of the deceased group is that they are reliably more likely to show signs of impaired functioning of vital organs and metabolism, resulting in conditions such as tachypnoe, hypoxia, tachycardia, hypertension and hyperthermia with hyperglycemia. All these conditions were observed in the main group patients. The appearance of this kind of disorders is explained by involvement in the pathological process of the centers of vegetative control as a consequence of either direct topical «neighbourhood» of the hematoma itself with the structures of the limbico-reticular complex, or progressive cerebral edema and impairment of vital centres located in the brain stem (24). Rapid clinical evolution of symptoms can occur within a few hours with the development of acute reactive intracranial hypertension, which, according to the authors, is particularly noticeable with medium- to high-magnitude (25).
Severe hypoxemia and severe unconsciousness also caused 80% of deceased patients to have access to ventilators at different times after admission. The link between the need to use a ventilator and high mortality is also indicated by other authors. Thus, Japanese scientists conducted a retrospective study to study the effect of ventilation on hospital mortality in coma patients with inoperable intracerebral hemorrhage (26). The mortality rate of comatose patients connected to ventilators according to Japanese researchers was 80% (27), while other researchers indicate a high frequency variation - 57 - 90% (28 ).
Respiratory pathological patterns are well known in comatose patients with intracerebral hemorrhage when, as in our study, respiratory distress syndrome develops either on or later in the early post-onset (29).
The authors point out that death in such patients is often associated with both respiratory distress syndrome (and subsequent ventilation), and with factors such as: intraventricular hemorrhage, brain stem compression and pupillary abnormality (30) .
It is also worth noting that individual authors are distinguished such a condition as ``sudden death syndrome in brain stroke``, the pathogenesis of which is not yet fully studied, but which is also based on neurogenic causes such as progressive cerebral edema with the development of rostro-caudal damage, acute occlusive hydrocephalus after subarachnoid hemorrhage, hemorrhagic transformation of the heart zone and secondary stem syndrome. As the authors point out, all these causes increase the phenomena of brain edema and cause compression of vital centers (31).
The most common cerebral complication, as we noted earlier in deceased patients, was the expansion of the hematoma into the intraventricular space, followed by progressive cerebral edema with medial structure dislocation and brain compression.
The most common extracerebral complications was a combination of septic and cardiovascular disorders + kidney damage according to laboratory and instrumental methods of examination. The association of high hospital mortality with septic, cardiovascular complications and acute kidney damage was reflected in the work of a number of researchers (32-34). The authors note that the brain-kidney relationship is mediated by the autonomic nervous system, in particular the sympathetic nervous system, and - the neuroendocrine system with the participation of inflammatory and immune response (35). However, the effect of hemorrhagic stroke on kidney function remains relatively unstudied. Acute kidney damage is related to hyperosmolarity of the blood especially linked to administration of mannitol to reduce intracranial pressure, older age associated with hypertension. Almost 30% of patients with acute hemorrhagic stroke have chronic kidney disease, mainly elderly patients, women with related diseases such as diabetes mellitus. What is remarkable is that in most cases, renal dysfunction after intracerebral hemorrhage is usually a transient phenomenon and rarely requires hemodialysis (36).
It is particularly noteworthy that the above complications may occur within the so-called multi-organ deficiency syndrome, which is the leading cause of death in intensive care patients, which is defined as progressive dysfunction of 2 or more organs with systemic homeostasis disorder. It occurs in 14% of ICU admissions and is responsible» for 80% of their deaths. During the first weeks in patients with severe stroke develops an inadequate generalized inflammatory response that creates favorable conditions for joining medical complications such as brain edema, pneumonia, urinary infection, heart failure, stressful ulcers, vein thrombosis (37, 38).
On multiple regression analysis only 2 variables had predictive value the high blood pressure at onset of stroke had protective value against death and low oxygen saturation had borderline value. These should be confirmed further on larger number of patients.
Study limitations
Due to single-center study and low number of patients, the data partially highlight the real clinical scenarios of stroke patients in KR. It is well-known that hospital mortality is also closely related to the level of medical care - the availability of high-precision therapeutic and diagnostic equipment, medications with proven efficacy, and the appropriate training of medical personnel in the management of stroke patients. At least patients were able to take tertiary care in Republican center. The conditions in rural areas cannot be estimated in accordance with these findings. Furthermore, study design was limited to retrospective manner with limited patient data. Due to scarcity of patient records the sample size also suboptimal. Further research is needed to investigate potential mechanisms underlying these associations and to develop targeted interventions to improve patient outcomes. Larger patient cohort is needed to confirm predictors of in=hospital mortality in hemorrhagic stroke patients.
Conclusion
Thus, as our research shows, that severe unconsciousness, the expansion of hematoma into the ventricular system, progressive cerebral edema with dislocation of medial structures and impairment of the brain, multi-organ insufficiency, tachypnoe/bradypnoe, tachycardia/bradycardia, diabetes mellitus and increased level of cholesterol were more common in HS patients with in-hospital mortality as compared to survivors. The only significant predictor of in-hospital stroke associated mortality among clinical and laboratory variables was systolic blood pressure at onset, low oxygen saturation had borderline significance.
Ethics: Written informed consent was obtained from patients for all procedures and due to retrospective mature of the study the Ethics Committee approval was not sought.
Peer-review: External and internal
Conflict of interest: No potential conflict of interest relevant to this article was reported.
Authorship: D.I.B, E.M.M, M.M.M., K.S.M., D.A.A., and N.K.M equally contributed to the study and all authors fulfilled authorship criteria.
Acknowledgements and funding: None to declare
References
| 1.Johnson CO, Nguyen M, Roth GA, Nichols E, Alam T, Abate D, et al. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 439-58. https://doi.org/10.1016/S1474-4422(19)30034-1 PMid:30871944 |
||||
| 2.Feigin VL. Anthology of stroke epidemiology in the 20th and 21st centuries: Assessing the past, the present, and envisioning the future. Int J Stroke 2019; 14: 223-37. https://doi.org/10.1177/1747493019832996 PMid:30794102 |
||||
| 3.Samudinova TT, Kulov BB, Turgumbaev DD, Abirova AB. Epidemiology of stroke in the city of Bishkek according to the register (2017-2018). Healthcare Kyrgyzstan 2021; 3: 90-103. https://doi.org/10.51350/zdravkg2021931290 |
||||
| 4.He Q, Guo H, Bi R, et al. Prediction of Neurological deterioration after intracerebral hemorrhage: The SIGNALS Score. J Am Heart Assoc 2022; 11: e026379. https://doi.org/10.1161/JAHA.122.026379 PMid:35916347 PMCid:PMC9375508 |
||||
| 5.Turgumbaev DD, Artykbaev AS, Kadyrova N, Abdraimova A, Urmanbetova A. Verification analysis diagnosis of "stroke" in persons who died at home. Den-Sooluk 2014; 23. | ||||
| 6.O'Carroll CB, Brown BL, Freeman WD. Intracerebral hemorrhage: a common yet disproportionately deadly stroke subtype. Mayo Clin Proc 2021; 96: 1639-1654. https://doi.org/10.1016/j.mayocp.2020.10.034 PMid:33952393 |
||||
| 7.Norrving B, Barrick J, Davalos A, Dichgans M, Cordonnier C, Guekht A, et al. Action plan for stroke in Europe 2018-2030. Eur Stroke J 2018; 3: 309-36. doi: 10.1177/2396987318808719 https://doi.org/10.1177/2396987318808719 PMid:31236480 PMCid:PMC6571507 |
||||
| 8.Okazaki S, Yamamoto H, Foster LD, Fukuda-Doi M, Koga M, Ihara M, et al. Late neurological deterioration after acute intracerebral hemorrhage:a post hoc analysis of the ATACH-2 trial. Cerebrovasc Dis 2020; 49: 26-31. https://doi.org/10.1159/000506117 PMid:32045911 |
||||
| 9.Radu RA, Terecoasa EO, Tiu C, Ghita C, Purcaru LI, Marinescu AN, et al. Clinical characteristics and outcomes of patients with intracerebral hemorrhage - a feasibility study on romanian patients. J Med Life 2020; 13: 125-31. https://doi.org/10.25122/jml-2020-0042 PMid:32742502 PMCid:PMC7378341 |
||||
| 10.Xia Y, Ma H, Buckeridge DL, Brisson M, Sander B, Chan A, et al. Mortality trends and length of stays among hospitalized patients with COVID-19 in Ontario and Québec (Canada): a population-based cohort study of the first three epidemic waves. Int J Infect Dis 2022; 121: 1-10. doi: 10.1016/j.ijid.2022.04.048 https://doi.org/10.1016/j.ijid.2022.04.048 PMid:35477050 PMCid:PMC9040412 |
||||
| 11.Unger T, Borghi C, Charchar F, et al. International Society of Hypertension global hypertension practice guidelines. J Hypertens 2020; 75: 1334-57. https://doi.org/10.1161/HYPERTENSIONAHA.120.15026 PMid:32370572 |
||||
| 12.Lin X, Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol (Lausanne) 2021; 12: 706978. doi: 10.3389/fendo.2021.706978. https://doi.org/10.3389/fendo.2021.706978 PMid:34552557 PMCid:PMC8450866 |
||||
| 13.Shahjehan RD, Bhutta BS. Coronary Artery Disease. (Updated 2023 Aug 17). In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK564304/ | ||||
| 14.Mackenzie G. The definition and classification of pneumonia. Pneumonia 2016; 8: 1-5. https://doi.org/10.1186/s41479-016-0012-z PMid:28702293 PMCid:PMC5471962 |
||||
| 15.Sacks DB, Arnold M, Bakris GL. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2011; 34: e61-e99. https://doi.org/10.2337/dc11-9998 PMid:21617108 PMCid:PMC3114322 |
||||
| 16.Vaidya SR, Aeddula NR. Chronic Kidney Disease. (Updated 2022 Oct 24). In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535404/ | ||||
| 17.Park SB, Khattar D. Tachypnea. (Updated 2023 Feb 13). In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK541062/ | ||||
| 18.Bhutta BS, Alghoula F, Berim I. Hypoxia. (Updated 2022 Aug 9). In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482316/ | ||||
| 19.Jain S, Iverson LM. Glasgow Coma Scale. (Updated 2023 Jun 12). In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513298/ | ||||
| 20.Sporns PB, Psychogios, MN, Boulouis G, Charidimou A, Li Q, Fainardi E, et al. Neuroimaging of acute intracerebral hemorrhage. J Clin.Med 2021; 10: 1086. doi.org: 10.3390/jcm10051086 https://doi.org/10.3390/jcm10051086 PMid:33807843 PMCid:PMC7962049 |
||||
| 21.Unnithan AKA, M Das J, Mehta P. Hemorrhagic Stroke. (Updated 2023 May 8). In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559173/ | ||||
| 22.Law ZK, Dineen R, England TJ, Cala L, Mistri AK, Appleton JP, et al. Predictors and outcomes of neurological deterioration in intracerebral hemorrhage: results from the TICH‐2 randomized controlled trial. Transl Stroke Res 2021; 12: 275-83. https://doi.org/10.1007/s12975-020-00845-6 PMid:32902808 PMCid:PMC7925446 |
||||
| 23.Lord AS, Gilmore E, Choi HA, Mayer SA, Collaboration V‐I . Time course and predictors of neurological deterioration after intracerebral hemorrhage. Stroke 2015; 46: 647-52. https://doi.org/10.1161/STROKEAHA.114.007704 PMid:25657190 PMCid:PMC4739782 |
||||
| 24.Ovesen C, Christensen AF, Havsteen I, Krarup Hansen C, Rosenbaum S, Kurt E, et al. Prediction and prognostication of neurological deterioration in patients with acute ich: a hospital‐based cohort study. BMJ Open 2015; 5: e008563. https://doi.org/10.1136/bmjopen-2015-008563 PMid:26220872 PMCid:PMC4521514 |
||||
| 25.Brouwers HB, Chang Y, Falcone GJ, Cai X, Ayres AM, Battey TW, et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol 2014; 71: 158-64. https://doi.org/10.1001/jamaneurol.2013.5433 PMid:24366060 PMCid:PMC4131760 |
||||
| 26.Fukuhara T, Aoi M, Namba Y. Mechanical ventilation for comatose patients with inoperative acute intracerebral hemorrhage: possible futility of treatment. PLoS ONE 2014; 9: e103531. https://doi.org/10.1371/journal.pone.0103531 PMid:25062014 PMCid:PMC4111623 |
||||
| 27.O'Carroll CB, Brown BL, Freeman WD. Intracerebral hemorrhage: a common yet disproportionately deadly stroke subtype. Mayo Clin Proc 2021; 96: 1639-54. https://doi.org/10.1016/j.mayocp.2020.10.034 PMid:33952393 |
||||
| 28.You S, Zheng D, Delcourt C, Sato S, Cao Y, Zhang S, et al. Determinants of early versus delayed neurological deterioration in intracerebral hemorrhage. Stroke 2019; 50: 1409-14. https://doi.org/10.1161/STROKEAHA.118.024403 PMid:31136288 |
||||
| 29. Hillal, A., Ullberg, T., Ramgren, B. et al. Computed tomography in acute intracerebral hemorrhage: neuroimaging predictors of hematoma expansion and outcome. Insights Imaging 2022; 13: 180. https://doi.org/10.1186/s13244-022-01309-1 PMid:36417131 PMCid:PMC9684397 |
||||
| 30.Ovesen C, Christensen AF, Havsteen I, Krarup Hansen C, Rosenbaum S, Kurt E. Prediction and prognostication of neurological deterioration in patients with acute ich: a hospital‐based cohort study. BMJ Open 2015; 5: e008563. https://doi.org/10.1136/bmjopen-2015-008563 PMid:26220872 PMCid:PMC4521514 |
||||
| 31.Qi H, Wang D, Deng X, Pang X. Lymphocyte‐to‐monocyte ratio is an independent predictor for neurological deterioration and 90‐day mortality in spontaneous intracerebral hemorrhage. Med Sci Monit 2018; 24: 9282-91. https://doi.org/10.12659/MSM.911645 PMid:30572340 PMCid:PMC6320655 |
||||
| 32.Fan JS, Chen YC, Huang HH, How CK, Yen DH, Huang MS. The association between on‐scene blood pressure and early neurological deterioration in patients with spontaneous intracerebral haemorrhage. Emerg Med J 2015; 32: 239-43. https://doi.org/10.1136/emermed-2013-203114 PMid:24123169 |
||||
| 33.El Husseini N, Fonarow GC, Smith EE. Association of kidney function with 30-day and 1-year poststroke mortality and hospital readmission. Stroke 2018; 49: 2896-903. https://doi.org/10.1161/STROKEAHA.118.022011 PMid:30571413 PMCid:PMC6338440 |
||||
| 34.Bobot M, Suissa L, Hak JF, Burtey S, Guillet B, Hache G. Kidney disease and stroke: epidemiology and potential mechanisms of susceptibility. Nephrol Dial Transplant 2023; 38: 1940-51. https://doi.org/10.1093/ndt/gfad029 PMid:36754366 |
||||
| 35.Zhao Q, Yan T, Chopp M, Venkat P, Chen J. Brain-kidney interaction: Renal dysfunction following ischemic stroke. J Cereb Blood Flow Metab 2020; 40: 246-62. https://doi.org/10.1177/0271678X19890931 PMid:31766979 PMCid:PMC7370616 |
||||
| 36.Du F, Zheng JW, Zhao YB, Yang K, Li HN. Full neurological recovery from severe nonexertional heat stroke with multiple organ dysfunction: A case report. World J Clin Cases 2023; 11: 2355-62. https://doi.org/10.12998/wjcc.v11.i10.2355 PMid:37122509 PMCid:PMC10131031 |
||||
| 37. Robba C, Battaglini D, Samary CS. Ischemic stroke-induced distal organ damage: pathophysiology and new therapeutic strategies. ICMx 2020; 8 (Suppl 1): 23. https://doi.org/10.1186/s40635-020-00305-3 PMid:33336314 PMCid:PMC7746424 |
||||
| 38.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2: 81-4. doi:10.1016/s0140-6736(74)91639-0 https://doi.org/10.1016/S0140-6736(74)91639-0 PMid:4136544 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

