Single-chamber permanent pacemaker implantation in patient with persistent left superior vena cava anomaly: a case report
CASE REPORT
Single-chamber permanent pacemaker implantation in patient with persistent left superior vena cava anomaly: a case report
Article Summary
- DOI: 10.24969/hvt.2024.493
- Page(s): 411-416
- CARDIOVASCULAR DISEASES
- Published: 20/06/2024
- Received: 13/05/2024
- Revised: 12/06/2024
- Accepted: 13/06/2024
- Views: 3834
- Downloads: 2991
- Keywords: Persistent left superior vena cava, thoracic venous anomalies, permanent pacemaker implantation
Address for Correspondence: Mykhaylo Sorokivskyy, Lviv National Medical University, Lviv, Ukraine
E-mail: msorokivskyy2@gmail.com
Case report
Single-chamber permanent pacemaker implantation in patient with persistent left superior vena cava anomaly: a case report
Oleh Semeniuk1 , Oleksiy Myshakivskyy1,2 , Mykhaylo Sorokivskyy2, Ulyana Chernyaha-Royko2
1Lviv Regional Hospital, Lviv, Ukraine
2Lviv National Medical University, Lviv, Ukraine
Abstract
Objective: Persistent left superior vena cava (PLSVC) is not uncommon venous return anomaly (0.3-05% of the general population). It is usually asymptomatic but can complicate transvenous cardiac interventions, particularly implantations of cardiac pacemakers. We present a case of need for pacemaker implantation in a patients with PLSVC.
Case presentation: An 84-year-old woman was referred to hospital with frequent syncopal episodes, dizziness, and fatigue. Electrocardiogram showed atrial fibrillation with bradycardia (35-40 bpm). The patient was fully investigated and was qualified for permanent single-chamber pacemaker implantation. The patient had an isolated PLSVC. Additionally, she had right breast cancer; therefore we performed left axillary access for pacemaker implantation. The pacing lead was inserted via left axillary vein through the PLSVC to the coronary sinus. Afterwards, we looped lead in the right atrium, which helped us to put it through the tricuspid valve and implant the lead in apex of right ventricle. All lead measurements at implantation were acceptable. The patient was discharged three days post-implantation without any complications. In a 1-year follow-up we have noticed good lead parameters at interrogation and stable lead position on the X-ray.
Conclusion: Certainly, clinicians must be aware of this anomaly and the challenges it presents during pacemaker implantation in affected patients, as well as potential solutions to address these challenges.
Key words: Persistent left superior vena cava, thoracic venous anomalies, permanent pacemaker implantation
Introduction
Persistent left superior vena cava (PLSVC) is one of the most common congenital vein outflow pathologies. The incidence of PLSVC is present in 0.3-0.5% individuals in the general population (1, 2). PLSVC can be associated with other heart abnormalities, including heart rhythm disturbances. Most often PLSVC drains into right atrium via coronary sinus (2). In the majority of cases, the anatomy of right-sided vein remains unchanged, allowing for the pacemaker implantation from the right side. However, there are patients which do not have right superior vena cava (RSVC) or in which right-sided access is undesirable for other reasons. In these scenarios, the implantation of pacemaker electrodes in such patients can present a significant challenge.
We report a case of successful and uncomplicated pacemaker implantation through the PLSVC in a patient with atrial fibrillation and bradycardia.
Case report
An 84-year-old Caucasian woman was referred to our hospital by ambulance with frequent syncopal episodes, dizziness, and fatigue. She had these symptoms for last 2 weeks, but at the day before hospitalization these episodes became more frequent, and the patient experienced 3 syncope episodes on this day. She had an irregular heart rate of 35-40 beats per minute, her blood pressure was 115/70 mmHg and oxygen saturation was 96% on room air. During the physical examination, signs of congestive heart failure and swelling on the lower limbs were observed. The patient also had a history of extracardiac conditions: she had right breast cancer with regional metastases in the right axillary lymph nodes.
Two years ago, she underwent surgical intervention for this condition, specifically mastectomy with regional axillary lymphadenectomy, and has shown no signs of recurrence during follow-ups. Before the admission, the only medication she received was rivaroxaban 15 mg daily.
Laboratory tests revealed mild anemia (Hb – 10.1 g/dL), elevated renal parameters (creatinine – 123.5 mmol/l, GFR (Cockroft formula) – 31 ml/min/1,73m2). Other laboratory results fell within the normal range. The standard 12-lead electrocardiogram revealed base rhythm atrial fibrillation with a slow ventricular response and heart rate of 35-40 bpm (Fig. 1).
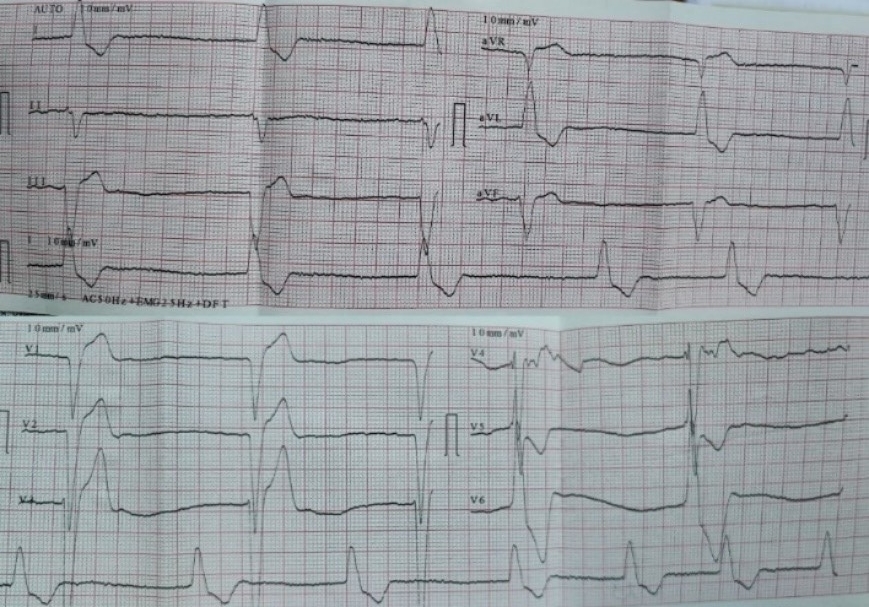
Figure 1. Electrocardiogram at admission – atrial fibrillation with slow ventricular response
Her echocardiogram showed normal left ventricular ejection fraction (60%), absence of asynergy zones, and an enlarged left atrium (size -6.0 cm). Additionally, there were moderate mitral, aortic, and tricuspid regurgitations, mild pulmonary hypertension. We also noted significantly dilated coronary sinus (15x18 mm), which suggested PLSVC.
After further investigations, we excluded any reversable causes of bradycardia, and the patient was qualified to permanent pacemaker implantation.
|
The procedure was planned by a multidisciplinary team comprising a cardiologist, electrophysiologist, interventional cardiologist and echocardiographist. We aimed to avoid right subclavian access because of right breast cancer, so we decided to perform procedure from the left side through the left axillary vein puncture. After puncture, venography was performed (Fig. 2). It revealed that PLSVC was drained into right atrium through dilated coronary sinus. We inserted the pacing electrode (Medtronic 5076 – 58 cm) through PLSVC into the coronary sinus. However, we encountered difficulty placing the electrode into right ventricle (RV) because the coronary sinus ostium was located very close to the tricuspid valve and directed in opposite side from the RV. Besides, due to anomalous vein anatomy we lacked electrode length for comfortable lead placement. The most challenging part of the procedure was crossing the tricuspid valve. Initially, we entered the right atrium and attempted to turn electrode in the opposite side using a pre-shaped J-stylet, but this was unsuccessful. Subsequently, we made a loop in the right atrium and positioned electrode into the RV using this loop. Finally, we advanced straight stylet and placed electrode in RV apex (Fig. 3). It was performed by gentle stylet advancement and slight lead manipulations.
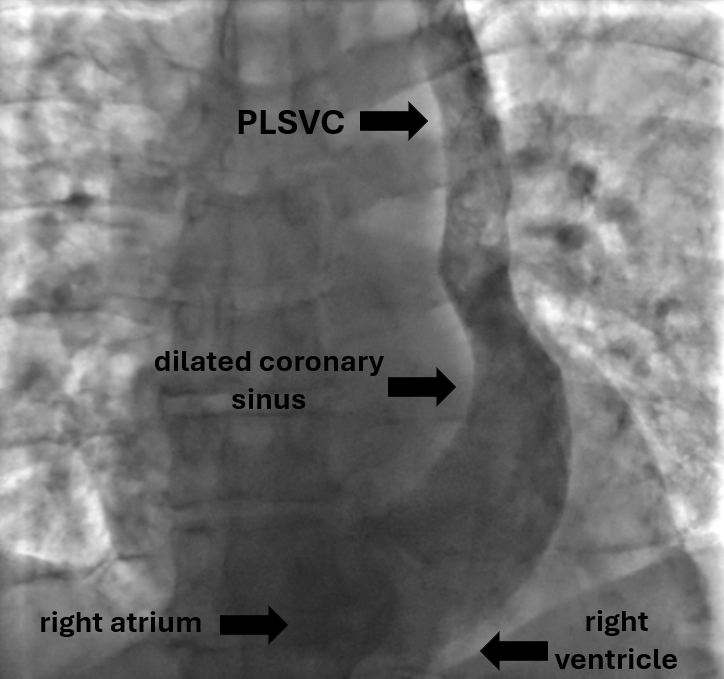
Figure 2. Venography image shows PLSVC draining to the right atrium through the coronary sinus
PLSVC – persistent left superior vena cava
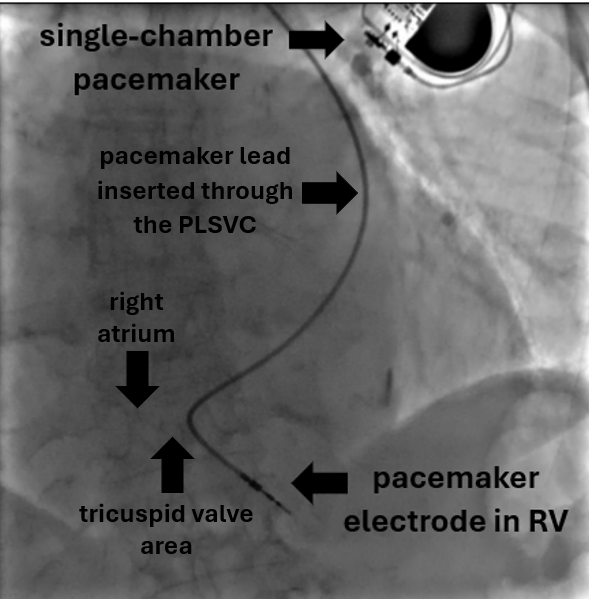
Figure 3. Chest X-ray image showing final position of the ventricular electrode in right ventricular apex
PLSVC – persistent left superior vena cava, RV – right ventricle
Additionally, asking the patient to take deep inspirations and expirations was helpful because as it induced slight changes between different heart structures. The stages of lead advancement are schematically depicted in Figures 4-8.
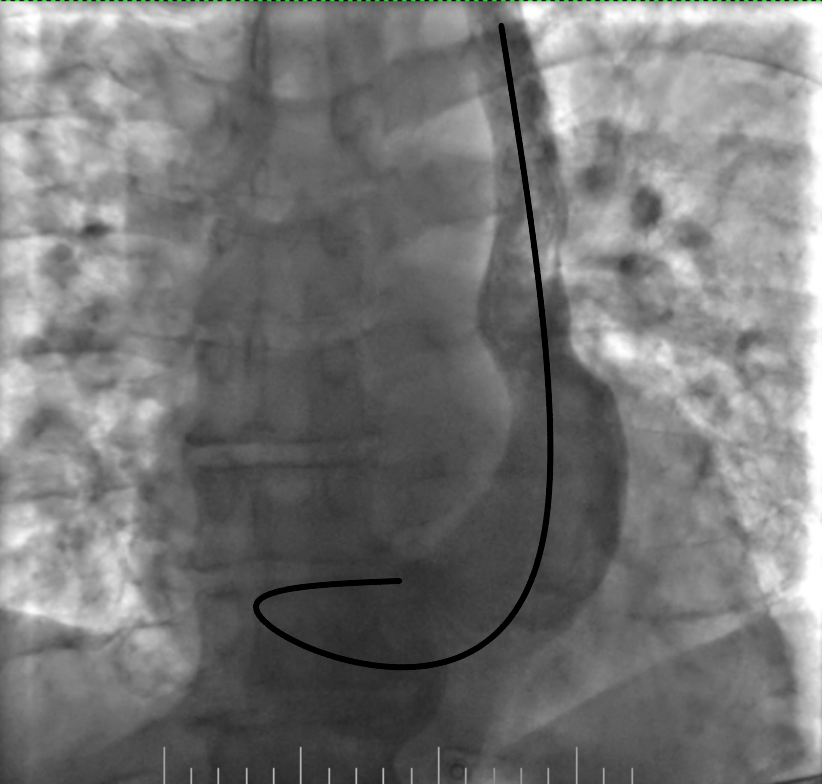
Figure 4. Lead looping in the coronary sinus
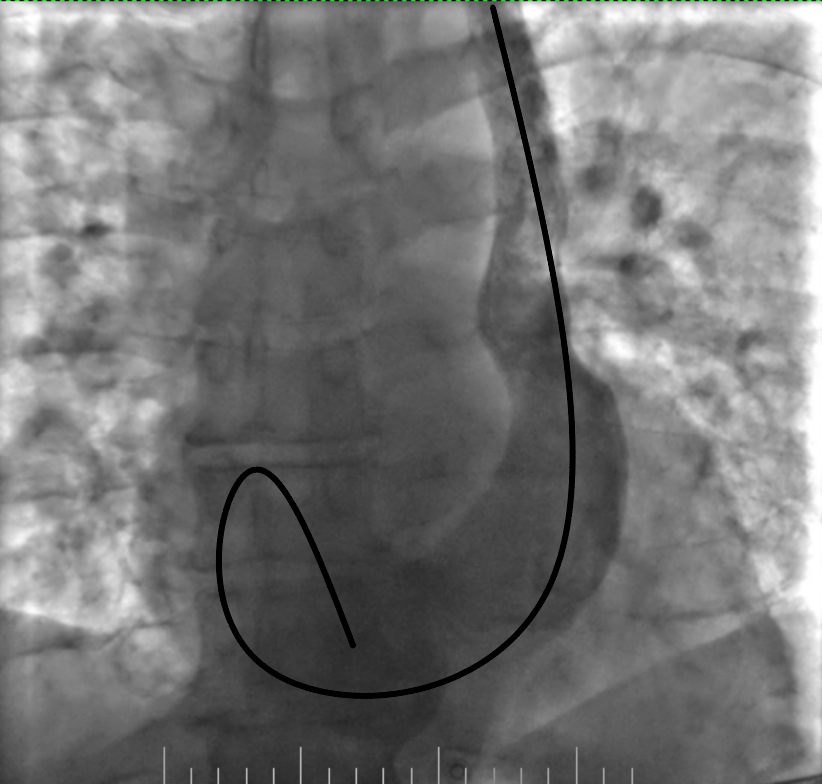
Figure 5. Advancement of the loop into the right atrium
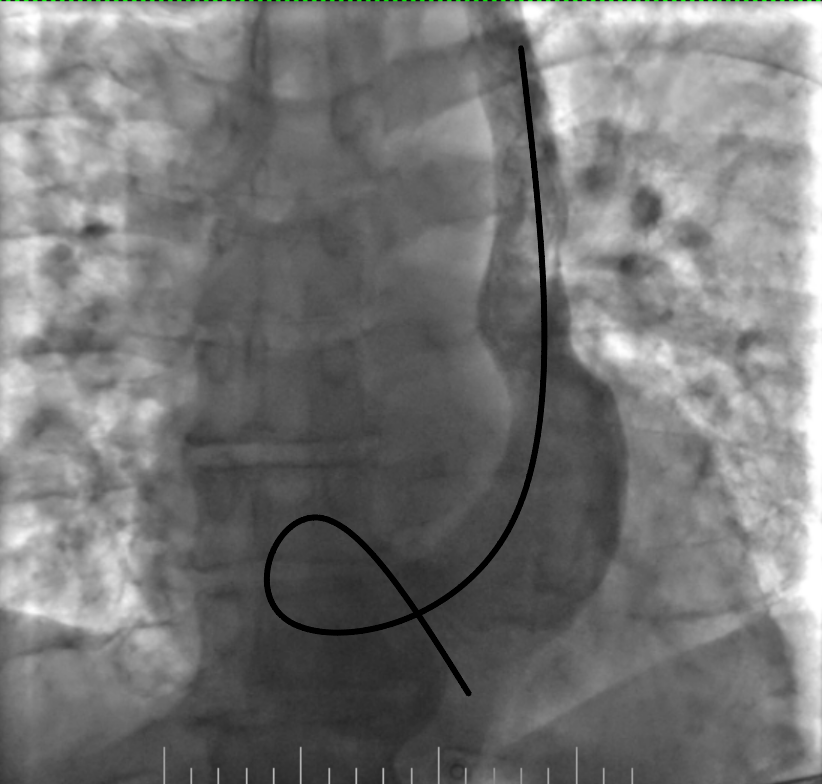
Figure 6. Advancement of the lead into the right ventricle and its fixation by screw
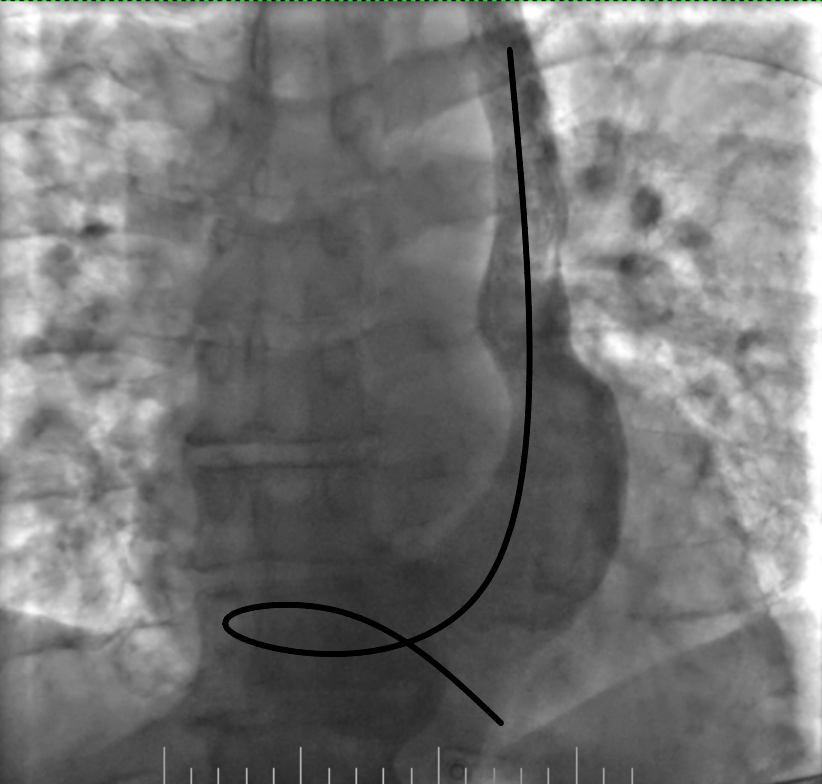
Figure 7. Removing the loop with deep inspiration and expiration and gentle lead manipulation
We obtained good electrode parameters, R-wave detection was 8-11 mV, pacing threshold was 0.7 V at a 0.4-ms pulse width, slew rate 3.0 V/s, a pacing impedance – 465 Ohms. The electrode was connected to a single-chamber pacemaker.
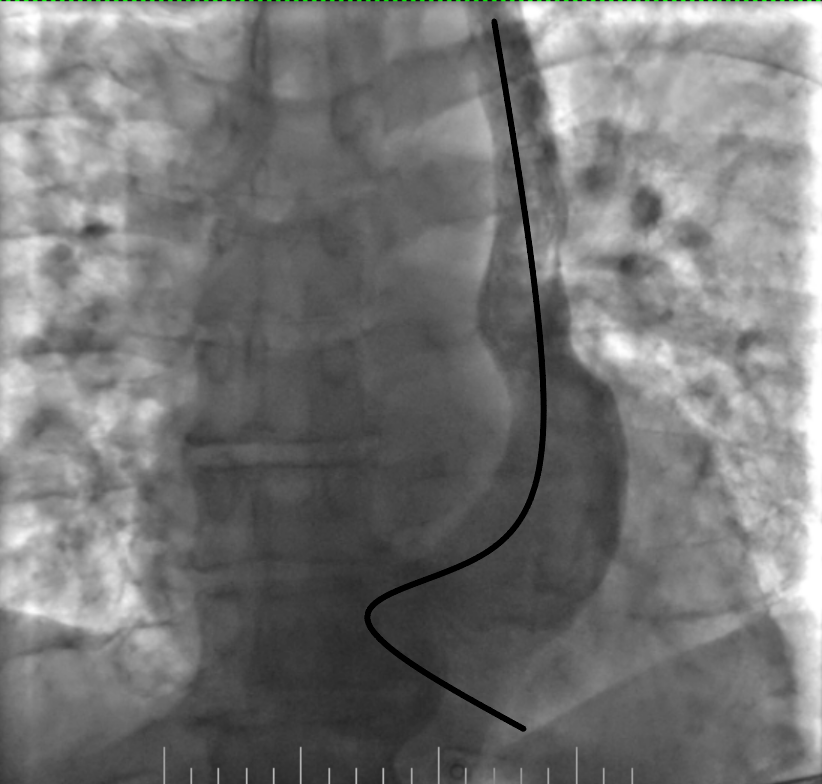
Figure 8. Final position of the lead
The patient was discharged without any complications. We prescribed her rivaroxaban 15 mg daily to prevent thromboembolic events in atrial fibrillation, eplerenone 25 mg daily and torasemide 2.5 mg daily. Next follow-ups showed no major changes in parameters. The chest X-ray confirmed the stable position of the electrode.
Discussion
In this case report, we describe our experience in pacemaker implantation in a patient with PLSVC. The lead implantation via PLSVC is uncommon and we found several publications, which describe this procedure (3-11). A PLSVC is not very rare anomaly and occurs in 0.3-0.5% of population, but in most cases it co-occurs with the right superior vena cava (1, 2). In 10% to 20% of cases, PLSVC drains into the left atrium, which is condition that is more dangerous and consequently causes right-to-left cardiac shunt with hypoxemia and desaturation. Most often PLSVC drains to the unroofed coronary sinus. In such cases, it causes no hemodynamic issues and usually is diagnosed incidentally. The presence of PLSVC affects the heart and vessel anatomy. Especially it causes change of the coronary sinus anatomy which drains about 20% of whole venous return and therefore becomes significantly dilated.
A PLSVC is more often observed in patients with congenital heart diseases and is present in 4.3% of patients with CHD (1). Besides, PLSVC is associated with conduction disturbances – tachyarrhythmias and bradyarrhythmias. Invasive treatment of rhythm disturbances in patients with PLSVC usually can be complicated, particularly pacemaker implantation via PLSVC. To enhance the outcomes of implantation, it is recommended to identify PLSVC before surgery and ensure proper preparation, i.e. additional visualization methods, if necessary. There may be concerns about safety issue of lead implantation via PLSVC, as evidenced by a limited number of reports highlighting increased risks of coronary sinus damage due to its large size and thin wall (6, 10). Additionally, access through PLSVC is technically complex procedure and may have higher risk of unsuccessful implantation and difficulty in obtaining a stable lead position with stable and acceptable lead parameters (3, 7). It is considered that the risk of other complications is not higher than that associated with the standard approach. Alternative in patients with PLSVC is the implantation of a leadless pacemaker via the femoral vein approach, which is comparable to transvenous leads in terms of safety and efficiency. In some patients, such as infants and children with congenital heart disease or patients with complex anatomy, surgical epicardial lead implantation may become a preferable technique. It is important to consider different possibilities and to choose the option, which is most suitable and safe for each individual case.
Conclusion
Venous anomalies, which include PLSVC, can complicate a pacemaker implantation procedure significantly. To enhance outcomes in such cases, early diagnosis before the procedure is crucial. Additional visualization methods may be used, if needed. It is better to involve multidisciplinary team and to discuss the preferrable access and alternative approaches. Familiarity with different techniques and tools which can be used for successful lead implantation is also important for proper operative planning.
Ethics: Written informed consent was obtained from patient for all procedures
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: O.S., O.M., M.S., and U.C-R. equally contributed to the case management and preparation for manuscript, and fulfilled authorship criteria.
Acknowledgements and funding: None to declare
Statement on A.I.-assisted technologies use: We declare that we did not use AI-assisted technologies in preparation of this manuscript
References
| 1. Tak Т, Crouch E, Drake GB. Persistent left superior vena cava: incidence, significance and clinical correlates. Int J Cardiol 2002; 82: 91-3. https://doi.org/10.1016/S0167-5273(01)00586-1 PMid:11786168 |
||||
| 2. Kamil W, Tyrak KW, Hołda MK, Koziej M, Piątek K, Klimek-Piotrowska W. Persistent left superior vena cava. Cardiovasc J Afr 2017; 28: e1-e4. https://doi.org/10.5830/CVJA-2016-084 PMid:28759082 PMCid:PMC5558145 |
||||
| 3. Razi M, Madaan A, Goel A, Sinha SK. Troubleshooting during pacemaker implant in persistent left superior vena cava with absence of right superior vena cava (isolated persistent left superior vena cava). Avicenna J Med 2016; 6: 47-50. https://doi.org/10.4103/2231-0770.179550 PMid:27144141 PMCid:PMC4849188 |
||||
| 4. Mikami T. Permanent pacemaker implantation in a patient with persistent left superior vena cava with an absent right superior vena cava: A case report. J Cardiol Cases 2021; 24: 34-6. https://doi.org/10.1016/j.jccase.2020.11.024 PMid:34257759 PMCid:PMC8258175 |
||||
| 5. Rizkallah J, Burgess J, Kuriachan V. Absent right and persistent left superior vena cava: troubleshooting during a challenging pacemaker implant: a case report. BMC Res Notes 2014; 7: 462. https://doi.org/10.1186/1756-0500-7-462 PMid:25047923 PMCid:PMC4112616 |
||||
| 6. Sundhu M, Syed M, Gul S, Saqi B, Mosteller R. Pacemaker placement in persistent left superior vena cava. Cureus 2017; 9: e1311. https://doi.org/10.7759/cureus.1311 |
||||
| 7. Li T, Xu Q, Liao HT, Asvestas D, Letsas KP, Li Y. Transvenous dual-chamber pacemaker implantation in patients with persistent left superior vena cava. BMC Cardiovasc Disord 2019; 19: 100. https://doi.org/10.1186/s12872-019-1082-7 PMid:31035937 PMCid:PMC6489345 |
||||
| 8. Hassine M, Hamdi S, Chniti G, Boussaada M, Bouchehda N, Mahjoub M, et al. Permanent cardiac pacing in a patient with persistent left superior vena cava and concomitant agenesis of the right-sided superior vena cava. J Arrhyth 2015; 31: 326-7. https://doi.org/10.1016/j.joa.2015.03.008 PMid:26550093 PMCid:PMC4600936 |
||||
| 9. Sirugo P, Marchini F, Bertini M, Malagù M. Dual- chamber pacemaker implantation via both right and persistent left superior vena cava. A case report. Europ Heart J 2022; 6: ytac296. https://doi.org/10.1093/ehjcr/ytac296 PMid:35935396 PMCid:PMC9350431 |
||||
| 10. Girerd N, Gressard A, Berthezene Y, Lantelme P. Persistent left superior vena cava with absent right superior vena cava: a difficult cardiac pacemaker implantation. Int J Cardiol 2009; 132: e117-9. https://doi.org/10.1016/j.ijcard.2007.07.161 PMid:18031849 |
||||
| 11. Kumar S, Moorthy N, Kapoor A, Sinha N. A challenging dual -chamber permanent pacemaker implantation in persistent left superior vena cava with absent right superior vena cava. J Cardiol Cases 2012; 5: e122-4. https://doi.org/10.1016/j.jccase.2011.12.003 PMid:30532919 PMCid:PMC6265434 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

