Review of AHA scientific statement on pharmacological management of cardiac arrhythmias in the fetal and neonatal periods
EDITORIALS
Review of AHA scientific statement on pharmacological management of cardiac arrhythmias in the fetal and neonatal periods
Article Summary
- DOI: 10.24969/hvt.2024.501
- Page(s): 336-343
- CARDIOVASCULAR DISEASES
- Published: 05/08/2024
- Received: 20/07/2024
- Accepted: 22/07/2024
- Views: 6308
- Downloads: 3310
- Keywords: fetal, arrhythmias, management, guidelines
Address for Correspondence: Yuriy Ivaniv, Danylo Halytsky Medical University, Lviv, Ukraine
E-mail: yivaniv@gmail.com ORCID: 0000-0002-2153-9262
Yuriy Ivaniv and Natalia Lozynska, Danylo Halytsky Medical University, Lviv, Ukraine
Graphical abstract
Key words: fetal, arrhythmias, management, guidelines
This scientific statement (1) provides an overview of the main provisions of American Heart Association for management of cardiac arrhythmias in the fetal and neonatal periods. Authors discuss the mechanism of arrhythmias, pharmacological treatment options, and distinct aspects of pharmacokinetics for the fetus and neonate. It is noted that although there has been an improvement in non-invasive diagnosis of fetal and newborns arrhythmias, there are no evidence-based recommendations for the pharmacological management of these disorders, as well as future directions for research are presented.
Fetal arrhythmias
Classification and diagnosis
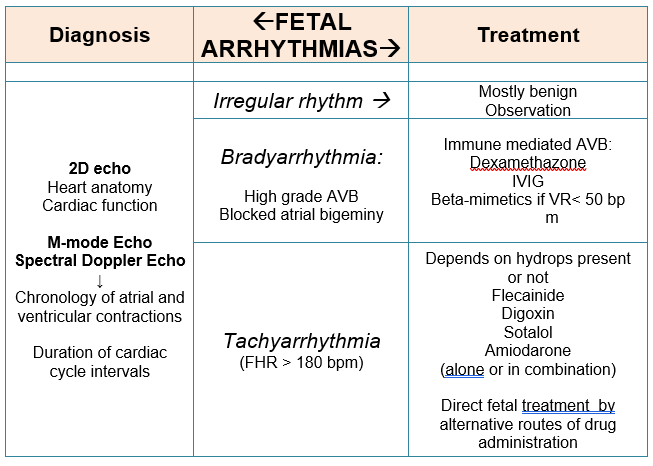
Arrhythmias are the common cardiac pathology and an important cause of morbidity and mortality in fetuses. Approximately 10% of all fetal arrhythmias require in utero treatment and follow up. As a result of the difficulties in the recording of fetal electrocardiogram (ECG), cardiac rhythm determines through registration of the mechanical consequences of the electrical activity of the heart with the help of 2-dimensional, M-mode, and spectral Doppler fetal echocardiography. This method delineates arrhythmia mechanism by determining the rates, chronology of atrial and ventricular contractions and duration of the intervals of cardiac cycle. In addition, fetal echocardiography allows to evaluate fetal heart anatomy to reveal congenital defects, cardiac function, presence and degree of hemodynamic complications. Moreover this method provides continuous surveillance for the fetuses that is a very important task too.
Fetal electrocardiography and fetal magnetocardiography are diagnostic modalities with high ability to diagnose arrhythmias and repolarization abnormalities of the fetal heart, especially long QT syndrome or exposures to antiarrhythmic medications. Although these modalities are not currently widely available but may become the mainstream diagnostic modality in the future.
The publication presents recommendations for differential diagnosis of fetal arrhythmia based on heart rate and atrioventricular ratio (AVR) (1:1, >1:1, <1:1) and distinguishes three main groups of arrhythmias: irregular rhythm, bradyarrhythmia and tachyarrhythmia. The differential diagnosis of fetal arrhythmias is presented in Table 1.
|
Table 1. The differential diagnosis of fetal arrhythmias |
|||
|
Atrioventricular ratio |
Bradycardia: below third percentile for gestational age |
Tachycardia >180 bpm |
Irregular rhythm |
|
1:1 |
Sinus bradycardia Maternal Anti-Ro Maternal viral infection Ectopic atrial rhythm Inherited arrhythmia
Ectopic atrial rhythm |
Sinus tachycardia Maternal TRab Maternal stimulants Maternal Anti-Ro
SVT (AVRT, PJRT) Some VT and JET AET |
PAC
PVC without retrograde ventriculoatrial conduction
Intermittent SVT |
|
>1:1 |
AVB Maternal anti-Ro Kearns-Sayre syndrome Long-QT syndrome
BAB |
Atrial flutter
AET |
Type 1, second-degree AVB
Intermittent type 2, second-degree AVB
Nonconducted PACs
PVC with retrograde ventriculoarterial conduction |
|
<1:1 |
Ventricular bigeminy |
VT JET |
Intermittent VT or JET |
|
AET - atrial ectopic tachycardia, AVB - atrioventricular block, AVRT - atrioventricular reentry tachycardia, BAB - blocked atrial bigeminy, JET - junctional ectopic tachycardia, PAC - premature atrial contractions, PJRT - permanent junctional reciprocating tachycardia, PVC, premature ventricular contractions, SVT - supraventricular tachycardia; Trab - thyroid-stimulating receptor antibody, VT- ventricular tachycardia. |
|||
Irregular rhythm
In addition to atrial and ventricular ectopies, other types of arrhythmias are included to the group of irregular rhythm. Differential diagnosis is based on atrioventricular ratio. The subgroup with a 1:1 AVR includes nonblocked atrial extrasystoles, ventricular extrasystoles without retrograde ventriculoatrial (VA) conduction and intermittent supraventricular tachycardia (SVT).
The subgroup with > 1:1 AVR includes infrequent blocked atrial extrasystoles, ventricular extrasystoles with retrograde ventricular-atrial conduction and second-degree atrioventricular (AV) blockade (type I and intermittent type II). Intermittent ventricular tachycardia (VT) and junctional ectopic tachycardia (JET) are included to subgroup with < 1:1.
These arrhythmias are potentially benign because they do not significantly affect fetal heart rate and do not cause hemodynamic disturbances in the fetuses, but some of them require follow-up. Premature atrial contractions (PAC) are the most common fetal arrhythmia and may present at any time during gestation. This arrhythmia does not require treatment, but monitoring of the fetal heart rhythm is advised because tachyarrhythmia develops in 1-2 % cases. Premature ventricular contractions (PVC) are very rare, can occur as single or complex beats and often combined with structural abnormalities of the fetal heart (cardiomyopathy, cardiac tumors and other). PVC, second-degree AV blockade, VT and JET can be a manifestation of inherited arrhythmic syndromes that is why close prenatal and postnatal follow-up is necessary.
Fetal tachycardia
Tachycardia is defined when fetal heart rate is more than 180 bpm. For differential diagnosis of fetal tachycardia with 1:1 AVR the comparison of the duration of AV and VA intervals of the fetal cardiac cycle is necessary. Short VA interval is a sign of atrioventricular reentry tachycardia (AVRT) – the most common type of fetal tachyarrhythmia. Long VA interval indicates of atrial ectopic tachycardia, permanent junctional reciprocating tachycardia, or sinus tachycardia. Persistent fetal sinus tachycardia can occur as a result of maternal influence (illness, medication), so it is important to conduct laboratory tests to rule out Grave's disease or autoimmune diseases in the mother or to prevent the influence of stimulant drugs. In rare cases, this type of fetal tachyarrhythmia can occur in the presence of JET or VT, which makes differential diagnosis difficult.
Atrial flutter (AFl) is the most common tachycardia with AVR >1:1 (2:1, 3:1, or 4:1). Atrial ectopic tachycardia with variable degrees of AV block also belongs to this subgroup of fetal tachyarrhythmias.
In case of fetal tachycardia with AVR <1:1 VT or JET are possible. Characteristic features of these arrhythmias are AV dissociation, relatively low ventricular rates (<180 bpm) and rapid development of hydrops. In suspected cases, testing for maternal anti-Ro/SSA antibodies is recommended. In cases of VT, it is important to exclude long QT syndrome by questioning the family for a history of this syndrome and obtaining ECGs of the parents and also evaluating fetal heart for structural pathology.
Fetal bradycardia
Fetal bradycardia is defined as a permanent fetal heart rate less than the third percentile for gestational age. Evaluation of the AVR can be helpful in the differential diagnosis of fetal bradycardia, as with tachycardia.
Sustained bradycardia with 1:1 AVR may indicate an ectopic atrial rhythm in fetuses with congenital heart defects, or sinus bradycardia in cases of hereditary syndromes (long QT interval syndrome), or be a consequence of the influence of the pregnant woman conditions (autoimmune diseases, viral infections, or medication). In order to improve the diagnosis, it is recommended to carry out genetic testing, ECG recording of parents and collection of family anamnesis.
Fetal bradycardia with an AVR >1:1 may be a manifestation of high-grade AV block (AVB) or blocked atrial bigeminy. Second-degree AVB is differentiated from blocked atrial bigeminy by measuring the interatrial intervals. In second-degree AVB, the interatrial rate is regular. In blocked atrial bigeminy the interatrial intervals vary and the premature atrial beats not conducted to the ventricles through the refractory AV node. Fetuses with blocked atrial bigeminy can develop SVT, therefore observation of fetal cardiac rhythm is necessary.
AVB can occur as a result of damage to the conduction system of the fetal heart by circulating anti-Ro/SSA antibodies, long QT interval syndrome, or be caused by fetal congenital heart defects, such as left atrial isomerism or corrected transposition of the great vessels. When AVB is combined with congenital heart defects of the fetus, the prognosis is unfavorable.
Antibody–mediated fetal AVB occurs in 2% to 6% of anti-Ro–positive mothers and recurs up to 18% of subsequent pregnancies. Anti-Ro/SSA antibodies can also cause endocardial fibroelastosis and dilated cardiomyopathy, which, even in the absence of AVB, can significantly worsen outcomes of affected fetuses.
Pharmacological treatments of fetal arrhythmias
SVT and atrial flutter
The objectives of antiarrhythmic drug therapy for fetal tachyarrhythmias are to restore a normal fetal heart rate, to prevent or reverse fetal heart failure, and to avoid premature delivery and its consequences. Transplacental antiarrhythmic treatment requires several days to achieve therapeutic fetal drug levels. Factors associated with a slower or unsuccessful cardioversion include fetal AFL, SVT with a long VA interval such as atrial ectopic tachycardia or permanent junctional reciprocating tachycardia, fetal hydrops, incessant tachycardia, and the choice of treatment.
SVT with no hydrops should be treated initially with flecainide (1st line treatment). If no conversion to normal rhythm achieved flecainide in combination with digoxin or sotalol with digoxin (or sotalol itself) could be started (2nd line). If no conversion achieved again flecainide in combination with sotalol or amiodarone alone is an option (3rd line).
SVT complicated by hydrops should be treated initially with flecainide in combination with digoxin (1st line treatment). If no conversion to normal rhythm achieved sotalol with digoxin or flecainide with sotalol could be prescribed (2nd line). If no conversion achieved again amiodarone alone or in combination with digoxin or at the same time a direct treatment can be considered as an option (3rd line).
AFL with no hydrops should be treated initially with sotalol (1st line treatment). If no conversion to normal rhythm achieved sotalol with digoxin could be started (2nd line). If no conversion achieved again sotalol plus flecainide or amiodarone alone is an option (3rd line).
AFL complicated by hydrops should be treated initially with sotalol in combination with digoxin (1st line treatment). if no conversion to normal rhythm achieved sotalol with flecainide or amiodarone could be prescribed (2nd line). if no conversion achieved again amiodarone in combination with digoxin or at the same time a direct treatment can be considered as an option (3rd line).
Clinical use information of fetal antiarrhythmic agents is summarized in Table 2.
|
Table 2. Fetal antiarrhythmic agents |
||||||
|
Drug |
Maternal oral dose |
Fetal- maternal ratio |
ECG effects |
Drug interactions |
Adverse effects |
Maternal contraindications |
|
Digoxin |
LD: 0.5 mg twice daily for 2 d MD: 0.375–0.75 mg/d |
0.8–1 |
ST segment |
Flecainide, amiodarone |
↓ Birth weight |
WPW, high-degree AVB, HCM |
|
Flecainide |
200–400 mg/d in 2–3 doses |
0.8–0.95 |
↑ QRS |
Digoxin, amiodarone |
Visual disturbances, obstetric cholestasis |
Congestive heart failure, Brugada syndrome, coronary heart disease |
|
Sotalol |
160–480 mg/d in 2–3 doses |
0.7–2.9 |
↑ PR, ↑ QTc |
|
Hypoglycemia ↓ birth weight |
Bradycardia, hypotension, asthma |
|
Amiodarone |
LD: 1.2–2.4 g/d for 2–7 d |
0.1–0.28 |
↑ QTc |
|
|
Thyroid disorders, abnormal neurodevelopment |
|
ECG p electrocardiogram,LD – loading dose, MD – maintenance dose |
||||||
Monotherapy
In the absence of hydrops, tachyarrhythmia-related mortality rate is low (<5%) even if cardioversion fails. For this reason, it is safe to initiate transplacental therapy with digoxin, flecainide, or sotalol and then escalate treatment if no response is obtained or if the fetal state deteriorates.
Combination therapy, amiodarone, and direct treatment
Combinations of either flecainide or sotalol with digoxin are used as primary treatment of poorly tolerated arrhythmias or as second-line treatment of a resistant arrhythmia. Intramuscular digoxin has also been reported to be effective for SVT in the fetus with a poor biophysical profile or refractory hydrops. Because of the higher risk of fetal-maternal adverse events, transplacental or direct fetal injections of amiodarone are used mainly when fetal death without rapid in utero cardioversion is anticipated.
Fetal conversion rates with antiarrhythmic medication
Retrospective studies report variable success rates for all antiarrhythmic agents. Adjusting drug dosing, switching to a different agent, or adding a medication is often required to eventually achieve prenatal conversion to sinus rhythm or adequate rate control in most cases with fetal SVT or AFL. Of note, limited data in the literature compare the efficacy and safety of multiple antiarrhythmic agents as first- and second-line treatment.
In a meta-analysis by Hill et al. (2) of 21 published retrospective studies, first-line monotherapy with flecainide or sotalol was superior to digoxin in converting fetal SVT and AFL to sinus rhythm. In fetuses presenting with hydrops, this benefit was even more notable. First-line monotherapy with flecainide was more efficient than sotalol or digoxin for treating AVRT.
With a treatment protocol of digoxin as first-line and digoxin plus sotalol or flecainide as second-line medication for AFL without hydrops, cardioversion was obtained in 59% with digoxin but in 93% with combination treatment in the prospective study by Miyoshi et al. (3). According to the literature, flecainide and sotalol, rather than digoxin, are increasingly preferred as first-line therapies for SVT and AFL, respectively, in fetuses without hydrops.
Ventricular Tachycardia
Treatments for VT in the fetus have included flecainide, sotalol, and amiodarone.
Conversely, episodes of an accelerated idioventricular rhythm generally do not require treatment unless sustained and associated with hemodynamic compromise.
Bradyarrhythmias
Cardiac neonatal lupus erythematosus refers to a spectrum of maternal anti-Ro antibody–mediated fetal and neonatal disorders, including third-degree AVB. Beta-stimulation with oral maternal salbutamol or terbutaline is advised for third-degree AVB with ventricular rates <50 bpm or when there is significantly reduced cardiac contractility. More controversial is prenatal treatment with fluorinated steroids (dexamethasone, betamethasone) and intravenous immunoglobulins because the treatment will usually not reverse third-degree AVB and may cause potential adverse effects on the developing fetus and co-treated mother and because fetal survival is often attained without prenatal intervention.
Treatment of third-degree AVB with steroids with or without venous immunoglobulins has been reported to increase fetal, neonatal, and 1-year survival to 95%, 93%, and 89% respectively, and to decrease the incidence of dilated cardiomyopathy to 3%. Treatment of first- and second-degree AVB may prevent the progression to third-degree heart block.
A possible algorithm for immune-mediated AVB when treatment is considered:
-Start with dexamethason 8 mg/d, after 2 weeks → 4 mg/d, after 28 weeks → 2 mg/d continue to birth if antiRo+.
-Add IVIG if endocardial fibroelastosis present or incomplete AVB. Dosing 1 g/kg to a maximal dose 70 g every 3-4 weeks.
-Add β-mimetic if ventricular rate < 50 bpm: salbutamol 10 mg in 3 doses or terbutaline 10-30 mg in 4 doses.
-Weekly assessment of obstetrics and fetal cardiologist is indicated.
-Delivery at tertiary care center.
Specific considerations concerning pharmacology in the fetus
When medications are prescribed for fetal treatment, it is important to take into account the route of administration and the pharmacokinetics of them. Fetal arrhythmia treatment relies on transplacental transfer of drug to the fetus by the mother receives an oral or intravenous antiarrhythmic. Most drugs cross the placenta by simple diffusion, in which the diffusion rate is dependent on the maternal-fetal concentration gradient, lipid solubility, protein binding, molecular weight, and degree of ionization.
Digoxin
Because maternal blood volume and renal clearance of digoxin increase during pregnancy and digoxin has high protein binding, larger and more frequent doses are required to achieve therapeutic levels in the fetus. Digoxin is not routinely used as first-line therapy in hydropic pregnancies because it crosses the hydropic placenta poorly. Digoxin levels are generally obtained at least 6 hours after the first dose to allow time for digoxin to distribute evenly between the serum and tissues.
Flecainide
This medication is lipophilic, does not have high protein binding, and is able to cross the placenta expeditiously. In the nonhydropic fetus, flecainide fetal levels achieve 90% of maternal levels. Trough level goal for flecainide is 0.2 to 1 μg/mL; however, maternal blood levels do not clearly correlate with fetal treatment efficacy.
Sotalol
Sotalol crosses the placenta quickly and almost completely. Because sotalol is excreted into the amniotic fluid rather than metabolized, fetal drug levels may exceed maternal levels and may not clearly correlate with treatment efficacy.
Amiodarone
Amiodarone and its active metabolite, desethylamiodarone, have poor transplacental transfer, with fetal-maternal concentration ratios ranging from 0.1 to 0.28. Amiodarone is metabolized by the cytochrome P3A4 (CYP) pathway, which may be mostly upregulated in pregnancy. Amiodarone has limited clinical utility in the treatment of fetal tachyarrhythmias.
Alternative routes of drug administration
Various alternative routes of administration have been reported for direct fetal treatment: intracordal, intraperitoneal, intra-amniotic, intracardiac, and intramuscular such as injection in the thigh of the fetus. Intraperitoneal, intra-amniotic, and intramuscular drug administration theoretically provides a sustained release of medication; however, this may be limited by unpredictable drug delivery. Intracordal injection is effective but requires multiple injections and has a significant risk of procedural complication.
Role of the maternal-fetal medicine specialist
Fetal arrhythmias are most often diagnosed at an early gestational age when the morbidities and mortality of preterm delivery would worsen the outcome compared with treatment in utero to prolong gestation. Although maternal administration of antiarrhythmics accomplishes effective transplacental passage, significantly elevated maternal doses are required for adequate fetal bioavailability and effective treatment.
The initial maternal evaluation requires special attention to medications and family history of cardiac disease, sudden death, or a history of long QT syndrome. Laboratory tests should be obtained to evaluate maternal organ function and to rule out secondary causes of fetal arrhythmia. Co-management with cardio-obstetric subspecialists (fetal cardiologist, maternal-fetal medicine, pediatric and adult electrophysiologist) is indicated to monitor for maternal arrhythmias, heart block, and prolongation of the QT interval.
Daily maternal ECGs until a steady state is reached may guide drug dosing and avoid maternal drug toxicity. A baseline maternal echocardiogram may be useful to assess both anatomy and function, especially if flecainide is being considered.
Direct monitoring of the fetus for drug toxicity is difficult, although serial Doppler measurements of the AV interval may demonstrate 10 to 30 milliseconds of prolongation or intermittent AVB. Once drug levels are therapeutic, the fetal heart rhythm is controlled, weekly fetal heart rate monitoring, and echocardiograms at 2- to 4-week intervals are suggested.
The vaginal delivery of a term nonhydropic fetus in sinus rhythm is an achievable goal of maternal-fetal arrhythmia management. According to published data, the preterm birth rate should be no higher than 7.6%. Delivery by Caesarean section is associated with a substantial list of maternal risks, with correlations that typically favor vaginal delivery, including shorter hospital stays, less maternal morbidity and lower risks for adverse obstetric and perinatal outcomes for next births.
Risk of postnatal arrhythmia recurrence
Prenatal factors that predict postnatal recurrence of arrhythmia include hydrops, delayed or lack of prenatal cardioversion, prenatal treatment with multiple arrhythmias, and a fetal long VA tachycardia. At least one-half of patients diagnosed with fetal SVT will experience recurrence after birth and typically during the first 2 to 3 days of life.
It is, therefore advised to closely monitor the newborn for any evidence of SVT recurrence and to initiate antiarrhythmic treatment if tachycardia recurs. At the same time, observational studies have consistently found that postnatal recurrence of AFL is rare regardless of the use of preventive treatment in the absence of structural heart disease or another substrate for tachycardia.
Arrhythmias in neonates
Classification and diagnoses of neonatal arrhythmias
Significant neonatal arrhythmias are estimated to occur in 1:4000 live births. The most common sustained arrhythmia is AVRT using either a manifest (Wolff-Parkinson-White syndrome) or a concealed accessory AV pathway. Atrial tachycardias, including AFL, atrial ectopic tachycardia, and multifocal or chaotic atrial tachycardia, represent less common types of neonatal SVT, whereas permanent junctional reciprocating tachycardia, congenital JET, and VT are even less common.
Pharmacological treatments of neonatal arrhythmias
Initial SVT treatments
The usual initial pharmacological treatment of sustained SVT is intravenous adenosine, although vagal maneuvers can be attempted. The standard initial dose of 0.1 mg/kg fails in many infants, so starting with a higher dose of 0.2 mg/kg is reasonable. If AVB (even transient) is not achieved, higher doses may be required. If adenosine results in termination but with prompt reinitiation of SVT, repeat doses may increase catecholamine levels, making termination more difficult. In such cases, the patient may need to receive additional antiarrhythmic medication before repeating adenosine administration.
Intravenous esmolol can be effective for the initial termination of SVT, especially when SVT recurs after adenosine. Clinical experience suggests that if intravenous adenosine is repeated after administration of a bolus of intravenous esmolol, there is a lesser chance of reinitiation. The onset of action of intravenous esmolol is within 60 seconds, and steady state is achieved in 2 minutes. Dexmedetomidine 1 μg/kg over 20 seconds is also effective for the termination of SVT, although it is not typically used as first line treatment.
Intravenous amiodarone and procainamide can be used as therapies for refractory SVT. Neonates may be more prone to side effects with these medications such as thyroid dysfunction with amiodarone treatment.
Intravenous sotalol is a newer option, with limited pediatric experience to date. Recent studies have shown that intravenous sotalol is effective for refractory SVT. An initial treatment dose of 30 mg/m2 given over 15 minutes provides effective cardioversion in most patients.
Emerging data show that ivabradine, a novel selective inhibitor of hyperpolarization-activated cyclic nucleotide-gated channels, appears to be a safe and well-tolerated medication that can induce suppression of SVT and restoration of sinus rhythm in children with refractory SVT. Conversely, verapamil and other intravenous calcium channel blockers are contraindicated in neonates because of a greater reliance on calcium channels and should be avoided in favor of safer alternatives.
Long-Term SVT treatments
First-line therapy for long-term management of SVT in neonates includes oral digoxin or propranolol. Digoxin should not be used in children with ventricular pre-excitation because it may increase risk of ventricular fibrillation. Oral sotalol, propafenone, and flecainide are useful for the treatment of SVT that recurs despite first-line agents. In a study of infants with recurrent SVT, oral flecainide achieved arrhythmia control in 84% of patients, the majority of whom had failed first-line therapy.
The substrate for SVT spontaneously resolves in many infants, so long-term therapy is usually maintained for 6 to 12 months. Shorter durations may be considered in low-risk patients with structurally normal hearts and no recurrence on single drug therapy.
Treatment of other neonatal tachycardias
Sotalol can be useful in neonatal AFL as a 1-time dose for chemical cardioversion, thereby obviating electrical cardioversion or overdrive pacing. Dosing is identical to that described for SVT.
Chaotic atrial tachycardia is particularly responsive to propafenone. Permanent junctional reciprocating tachycardia can be difficult to manage and often requires multiple drugs, including β-blockers, sotalol, and flecainide for rhythm control. Congenital JET often requires combinations with β-blockers and amiodarone being the most widely used agents. Ivabradine has been shown to be an effective monotherapy for congenital JET, even after failure of combination therapies.
Ventricular tachycardias are rare in the neonate and are usually secondary to cardiac tumors, cardiomyopathies, or genetic channelopathies. Therefore, treatment is dictated by the suspected underlying cause.
Drug-drug interactions involving antiarrhythmic agents in neonates
The likelihood of a clinically relevant interaction between amiodarone and flecainide or propranolol is small. Drug-drug interactions involving digoxin have been reported in neonates. Cases of digoxin intoxication with concomitant administration of carvedilol have been reported in neonates. This interaction is likely mediated by carvedilol inhibition of P-glycoprotein, enhancing the absorption and reducing the renal secretion of digoxin, thus increasing serum digoxin concentrations.
Recent advances and future research
Major changes since the 2014 fetal cardiac disease scientific statement include a greater appreciation of the pathology that can be associated with sinus bradycardia, treatment rather than delivery of the term and near-term fetus with SVT, and weaning of antiarrhythmic medication doses after sustained conversion to sinus rhythm in the fetus and neonate.
Currently, efforts to establish systematic treatment protocols for fetal and neonatal arrhythmias have begun, largely through multicenter approaches. The objectives of these prospective studies are to define best practices for initial arrhythmia treatment and to reduce the potentially serious adverse effects of these pharmacological treatments.
The FAST Therapy Trial (Fetal AFL and SVT) is a prospective multicenter trial addressing the knowledge gap of medication efficacy and adverse effects on the pregnant patient and fetus (FAST RCT Prospective randomized clinical trial of fetal atrial flutter and supraventricular tachycardia therapy. ClinicalTrials.gov identifier NCT02624765).
The medical community and our patients are waiting for the outcomes of 2 studies that will lead to the necessary evidence-based guidelines on the risk of fetal AVB resulting from maternal anti-Ro/SSA antibodies. The first is the ongoing prospective AVB study STOP BLOQ (Surveillance and Treatment to Prevent Fetal Atrioventricular Block Likely to Occur Quickly; ClinicalTrials.gov identifier NCT04474223); the second is the Slow Heart Registry of Fetal Immune-Mediated High Degree Heart Block (ClinicalTrials.gov identifier NCT04559425).
With respect to neonates, because of the variables of dynamic enzyme expression, intracellular versus extracellular drug distribution, and altered drug clearance, research is needed to delineate the pharmacokinetic properties of the various antiarrhythmic drugs specifically for this age group. Other unanswered questions are the best method(s) to predict neonatal arrhythmia recurrence when fetal arrhythmias have occurred and the indications and optimal duration of pharmacological treatment for neonatal SVT.
Peer-review: Internal
Conflict of interest: None to declare
Authorship: Y.I. and N.L. equally contributed to the preparation for manuscript, and fulfilled authorship criteria
Acknowledgements and funding: None to declare
Statement on A.I.-assisted technologies use: We declare that we did not use AI-assisted technologies in preparation of this manuscript
References
- 1.Batra AS, Silka MJ, Borquez A, Cuneo B, Dechert B, Jaeggi E, et al. ; on behalf of the American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology, Council on Basic Cardiovascular Sciences, Council on Cardiovascular and Stroke Nursing, Council on Genomic and Precision Medicine, and Council on Lifelong Congenital Heart Disease and Heart Health in the Young; Endorsed by the Pediatric & Congenital Electrophysiology Society (PACES). Pharmacological Management of Cardiac Arrhythmias in the Fetal and Neonatal Periods: A Scientific Statement From the American Heart Association. Circulation 2024; 149: e937-52. :
- 2. Hill GD, Kovach JR, Saudek DE, Singh AK, Wehrheim K, Frommeit MA. Transplacental treatment of fetal tachycardia: a systematic review and meat-analysis. Prenat Diagn 2017: 37: 1076-83.
- 3. Myoshi T, Maeno Y, Hamasaki T, Inamura N, Yasukochi S, Kawataki M, et al. Antenatal therapy for fetal supraventricular tachyarrhythmias. Multicenter trial. J Am Coll Cardiol 2019; 74: 874- 85.
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
AUTHOR'S CORNER

