Chronic coronary syndromes: diagnosis, management and gaps in evidence
REVIEW
Chronic coronary syndromes: diagnosis, management and gaps in evidence
Article Summary
- DOI: 10.24969/hvt.2024.508
- Page(s): 391-410
- CARDIOVASCULAR DISEASES
- Published: 28/08/2024
- Received: 16/08/2024
- Accepted: 17/08/2024
- Views: 20912
- Downloads: 4056
- Keywords: Coronary artery disease, chronic coronary syndromes, atherosclerosis
Address for Correspondence: Dario Mafrica, Department of Clinical, Internal Medicine, Anesthesiology and Cardiovascular Sciences, Sapienza University of Rome, Rome, Italy
Email: dariomafrica@gmail.com
ORCID: Giuseppe Biondi-Zoccai – 0000-0001-6103-8510, Marco Bernardi – 0000-0001-9269-8829
Dario Mafrica1, Giuseppe Franculli1, Antonio Esposito2, Gianmarco Sarto2, Pierre Sabouret3,4, Giuseppe Biondi-Zoccai5,6, Marco Bernardi5
1Department of Clinical, Internal Medicine, Anesthesiology and Cardiovascular Sciences,
Sapienza University of Rome, Rome, Italy
2ICOT Istituto Marco Pasquali, Latina, Italy
3Heart Institute and Action Group, Pitié-Salpétrière, Sorbonne University, Paris, France.
4National College of French Cardiologists, Paris, France
5Department of Medical-Surgical Sciences and Biotechnologies, Sapienza University of
Rome, Latina, Italy.
6Maria Cecilia Hospital, GVM Care & Research, Cotignola, Italy.
Abstract
Chronic coronary syndromes (CCS) comprehend a wide spectrum of conditions related to coronary artery disease (CAD). CCS definition includes either those patients who experienced an acute coronary syndrome such as myocardial infarction (MI) or unstable angina (UA), and the ones in which CAD has been detected through screening. This aspect creates a potential issue in differentiating medical treatments and modalities and timing for follow up. Another critical aspect is represented by the gap between American and European guidelines, which have been most recently updated respectively in 2023 and 2019. Furthermore, it is not clear what kind of patient must be screened for the detection for CAD and how to manage the eventual follow-up. Lastly, the latest guidelines are not clear for the choice of medical therapy and which are the patients who benefit the most from the eventual revascularization.
The aim of this review is to analyze the issues related to the incongruities about CCS and propose potential options for adequate treatment and follow up.
Key words: Coronary artery disease, chronic coronary syndromes, atherosclerosis
Introduction
Coronary artery disease (CAD) represents a healthcare issue worldwide. It is estimated to be the third leading cause of mortality (1). The main risk factors for CAD are smoking, diabetes mellitus, dyslipidemia, and hypertension.
CAD may present acutely in form of myocardial infarction (ST-elevation myocardial infarction, STEMI; or non-ST elevation myocardial infarction, NSTEMI), stable or unstable angina.
Myocardial infarction is described by the fourth universal myocardial infarction (MI) definition as “the presence of acute myocardial injury detected by abnormal cardiac biomarkers in the setting of evidence of acute myocardial ischemia (2)”. It has a worse long-term prognosis than stable and unstable angina because it determines death of myocardial cells, leading to a potential loss of ventricular function, cardiac remodeling, and fibrosis.
While the acute setting of CAD is well defined in literature, it is not the same for chronic coronary syndromes (CCS). Furthermore, many patients who are classified as CCS experienced an acute coronary syndrome (ACS), relevant guidelines have been recently updated. This aspect created the necessity to update the CCS guidelines.
Graphical abstract
(Prepared using Biorender.com)
The latest European guidelines for the management of CCS have been most recently updated 5 years ago (2019) (3), while latest American guidelines have been released by American Heart Association (AHA) and American College of Cardiology (ACC) in 2023 (4), leading to a potential gap in clinical practice between physicians of different countries.
CCS are defined by the latest European Guidelines as a form of CAD, underlying the dynamic process of atherosclerosis, which may present either in the acute forms mentioned before, or as a chronic disease (previously defined as “Stable CAD”), which may evolve in different scenarios. ESC guidelines define six types of patients:
1) patients with suspected CAD and ‘stable’ anginal symptoms, and/or dyspnea;
2) patients with new onset of heart failure (HF) or left ventricular (LV) dysfunction and suspected CAD;
3) asymptomatic and symptomatic patients with stabilized symptoms <1 year after an ACS or patients with recent revascularization;
4) asymptomatic and symptomatic patients >1 year after initial diagnosis or revascularization;
5) patients with angina and suspected vasospastic or microvascular disease;
6) asymptomatic subjects in whom CAD is detected at screening.
In this context, CCS includes a wide spectrum of patients with heterogeneous features, which may need different treatments, while ESC guidelines define all patients in the same group, as CCS.
This aspect raised some controversies between experts, especially for the management of these category of patients.
In fact, the most recent guidelines suggest the same medical therapy for those patients who experienced an ACS and the ones in whom CAD has been detected during screening (e.g. echocardiography or computed tomography (CT) scans).
About medical therapy, the management of antiplatelet and anticoagulant medications represents another issue. Moreover, these therapies should be re-calibrated in the elderly, group that represents most of the patients (5).
Dual antiplatelet therapy (DAPT) is always indicated in those patients who had an ACS and in those in whom a percutaneous coronary intervention (PCI) has been performed. The first antiplatelet drug is acetylsalicylic acid (ASA, aspirin), which is used with a second drug in DAPT. The options for the second antiplatelet drug rely on P2Y12 inhibitors - clopidogrel, prasugrel and ticagrelor. The choice and the duration of the therapy with the second antiplatelet drug in CCS is not clear. The clinical consensus suggests 3-6 months of DAPT, most frequently with ASA and clopidogrel. The issue with this agreement is that there are no robust clinical trials demonstrating the effective superiority of this association in CCS over the use of more potent P2Y12 inhibitors.
Moreover, the clinical consensus on duration and choice of DAPT is based on studies and guidelines that unify patients with different features under the same definition. In fact, patients who experienced an ACS are classified as CCS. These patients may benefit longer DAPT duration (6). On the other hand, patients who did experience an ACS do not show the same benefit. However, some of the patients who did not experience an ACS still have a high ischemic risk (multiple PCIs, long stents especially in the proximal left anterior descending artery (LAD), evidence of chronic total occlusions (CTO)), but they are still classified under the same group, and there is not a differentiation for the medical therapy.
Lastly, latest ESC guidelines do not clarify how to manage situations such as CTO or intra-stent restenosis, when to switch to a more potent P2Y12 inhibitor or evaluate to a longer DAPT.
The aim of this review is to examine the pitfalls of the ESC guidelines on CCS and propose potential solutions.
The main points we are going to discuss in this review are the management of risk factors, diagnostic assessment, interventional and medical therapy and long-term follow up in the spectrum of CCS (Graphical abstract).
Diagnosis and CAD assessment (Table 1)
The table 1 outlines the indications, advantages, and disadvantages of various diagnostic tests for coronary artery disease.
Table 1. Diagnostic tests for chronic coronary syndromes:
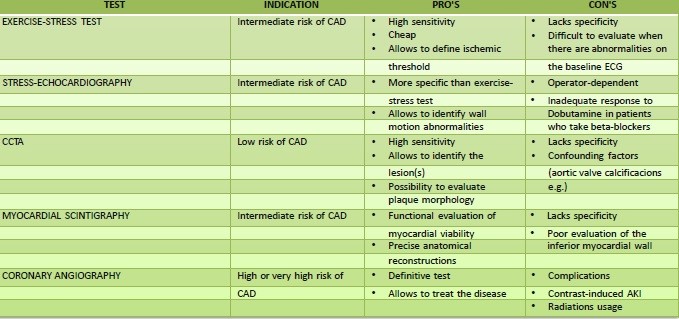
AKI - acute kidney injury, CAD - coronary artery disease, CCTA - coronary computed tomography angiography. ECG - electrocardiography
Testing in patients unknown for CAD remains highly discussed. The actual guidelines suggest performing CAD assessment based on the patient’s risk.
Patients are divided in three classes of risk of CAD: low, intermediate, or high risk.
For those patients with low risk of CAD, the actual guidelines suggest performing coronary computed tomography angiography. It is a non-invasive imaging technique, which uses X-Rays and IV iodine contrast. Its benefits are the high negative predictive value which makes it a good test to exclude CAD when the probability is low (7).
Provocative tests such as exercise-stress test or stress echocardiography are indicated in those patients at intermediate risk.
When CAD risk is high or very high, patients may have indications for coronary angiography without intermediate test, especially those who already have anamnesis for CAD (previous ACS e.g.).
In this context, the main problem is defining the patient’s risk. The main risk factors for CAD are smoking, hypertension, dyslipidemia, and diabetes mellitus. Most of the clinicians evaluate cardiovascular (CV) risk empirically, even though there are scores validated by the ESC such as SCORE-2 and SCORE2-OP (re-calibrated on elderly), which are based on age, blood pressure, and cholesterol levels. Recently, the ESC proposed to stratify the CV risk in the different regions, considering the different demographic incidence of CAD.
These scores have been recently improved including diabetes in the risk calculator, in form of Hb1Ac %, age at the diagnosis of diabetes and estimated glomerular filtration rate (eGFR). This aspect is crucial because patients affected by diabetes mellitus have a higher incidence of CAD, so they need a dedicated score for the risk assessment.
The issue with CAD risk assessment is represented by the discretion of the clinicians to use scores and evaluating empirically instead. This aspect contributes to the lack of consensus for the management of CCS.
Exercise-stress test and stress-echocardiography
The principles of exercise-stress test and stress-echocardiography for the detection of CAD are based on the evaluation of the coronary flow reserve, which is defined as the ratio of the maximal or hyperemic flow down a coronary vessel to the resting flow (8). It is useful to evaluate those patients who experience symptoms related to a possible CAD such as angina or dyspnea during exercise, but not at rest. While exercising, several vasodilators are released: the main coronary vasodilator is adenosine (9) which derives from the hydrolysis of adenosine triphosphate (ATP), which generates adenosine diphosphate and a phosphoric group. The additional hydrolysis of ADP can generate two ulterior phosphoric groups, and adenosine. Adenosine interacts with its receptor A2A, which through the mediator cyclic adenosine mono-phosphate, determinates coronary artery vasodilation. If the vessels are not affected by CAD, this results in an increase in coronary blood flow and oxygen delivery to the myocardium; otherwise, if there is obstructive CAD (where a hemodynamically significant stenosis is defined by a >70% vessel obstruction), there will be discrepancy between oxygen demand and oxygen delivery, causing ischemia.
The same metabolic pathway may be reproduced by stress-echocardiography through the administration of drugs such as dipyridamole or, more frequently, dobutamine.
Dipyridamole acts inhibiting the enzymes phosphodiesterase and adenosine deaminase that increase adenosine levels, determine the accumulation of secondary messengers of the prostacylin/PGD2 pathway, causing vasodilatory effects on the coronary arteries (10).
If CAD is present, the vasodilatory effect will be more pronounced in those vessels, which are not involved, showing a better endothelial function and response to vasodilatory agents. This leads to a “steal” of blood to the vessels with stenoses, causing abnormalities in wall motion which become evident on echocardiography of the area supplied by the vessel involved.
During exercise stress test, it is possible to reproduce the ischemic stress through physical exercise. The physician evaluates symptoms, exercise tolerance, blood pressure profile, and ECG changes.
The amount of work required to have a proper evaluation is typically based on the maximum predicted heart rate (MPHR), which should be >85% of MPHR.
The main ECG changes suggestive for ischemia are ST depressions during exercise, which are evaluated at 60 to 80 ms from the J point. The test is considered positive for ischemia if there is an ST - depression of 2 mm or more with rapid up-sloping shape, or ST depression of 1.5 mm with slowly up-sloping shape, or a 1 mm or more horizontal or down-sloping ST depression. These last two patterns are the most frequently associated with CAD, while the others may be more frequently falsely positive (11)
Another very specific ECG change for CAD is left bundle branch block absent at rest. It is considered suggestive for inducible ischemia and its presence is an indication for coronary angiography (12).
The main issues with exercise stress test are represented by those patients who are unable to complete the test because of orthopedic conditions, deconditioning, and marked baseline ECG abnormalities at rest such as inverted T-waves or ST depressions that make difficult to evaluate possible modifications. Moreover, exercise stress test does not allow localizing with enough specificity the ischemic area and the eventual coronary artery involved. In fact, even though it represents a sensitive test for screening in CAD, it lacks on specificity.
Stress-echocardiography is based on the same metabolic principles explained before. The metabolic stress may be induced by the administration of dobutamine, or less frequently adenosine or dipyridamole or with a physical exercise, typically cycling on the ergometer (13).
It is a more specific test than exercise stress test and allows to localize the eventual lesion, identifying wall motion abnormalities induced by the ischemia.
The main issues of stress-echocardiography are related to the operator because echocardiography is an operator-dependent examination, and to the patient, especially those who take beta-blockers and may not have an adequate response to dobutamine.
Coronary computed tomography angiography
Coronary computed tomography angiography (CCTA) is widely used in clinical practice to detect CAD, especially in those patients who have low probability of being affected. In fact, it has a high sensitivity but lacks in specificity (14) because the physical principles of CT, which intensifies denser tissues, allow to detect easily atherosclerotic plaques, especially those which have a high calcific burden (15). This may be a problem in the elderly, who show calcifications without having significant plaques, which may overestimate the stenosis.
The ESC guidelines for CCS recommend the use of CCTA in those patients who have intermediate-low risk of CAD, or in those in which stress testing resulted inconclusive. The most widely used score is coronary artery calcium (CAC) score, which estimates the amount of calcium in the coronary arteries. Even if it represents a validated tool, CAC score is affected by the amount of calcium that does not participate to the atherosclerotic plaque, like in those patients who have very calcific aortic valves.
Nowadays, it is possible to evaluate the coronary stenoses with functional studies even on ct, similarly to coronary angiography (16). In fact, coronary physiology assessment can be performed without performing invasive coronary angiography, using tools such as virtual functional flow reserve (FFR) or quantitative flow ratio (QFR) based on CCTA. These imaging techniques allow to assess CAD avoiding hospitalizations and complications related to the procedure, such as bleeding and coronary dissections (17).
The limitations of this kind of assessment are mostly related to the CT technique (radiations, excessive enhancement of calcium with overestimation of the stenoses, imaging artifacts).
Myocardial scintigraphy
It is an examination, which uses the principles of single photon emission computed tomography (SPECT) or positron emission tomography (PET). The images are acquired using photons (gamma-rays) emitted by radiopharmaceuticals which bind very selectively the myocardium, such as Thallium-201, which binds the Na+/K+ ATPase, or Sestamibi and Tetrofosmin, which are technetium-based agents, and bind the myocyte’s mitochondria (18).
The myocardial scintigraphy produces perfusion images before and after a stress, which may be pharmacological or physical. The principles are the same we previously described for exercise-stress test and stress-echocardiography. The administration of dypiridamole or the physical stress increases adenosine levels at the endothelium level, causing vasodilation of the coronary arteries, supplying more blood flow to the myocardium. If there is significant obstruction, these areas will be less perfused, showing a lower uptake of the radiopharmaceutical.
The patient must suspend with an adequate washout the therapies with beta-blockers, Ca2+ -channel blockers and nitrates before the examination.
Myocardial scintigraphy is characterized by a high sensitivity and specificity (19). Unlike exercise stress test, it has an anatomical correspondence, which makes it a more specific test. The latest software allows very precise anatomical reconstruction.
The issues with this technique are related to the very long time of acquisition; in fact, it consists in a baseline acquisition, then a pharmacological stress or exercise is administrated, and lastly a second acquisition after stress. This time is further prolonged by the time in which the patient must eat a fatty meal after the stress to increase the emptying of the bladder, which may hold the radiopharmaceutical.
Other confusing factors with myocardial scintigraphy are related to the diaphragm, whose movements may alter the visualization of the inferior wall of the myocardium, often leading to false positive for hypocaptation.
Coronary angiography
Following AHA/ACC and ESC latest guidelines, coronary angiography should be performed without passing through an intermediate test in those patients who have very high probability of CAD. However, the “high-risk phenotype” is not well defined by validated scores. In clinical practice, the indication is frequently given to those patients who are known to be affected by CAD and experience new-onset or worsening angina (which are defined as unstable) or show modifications of the resting ECG or new-onset wall motion abnormalities on echocardiography.
Guidelines suggest to perform coronary angiography In patients known to be affected by CAD, who survived a cardiac arrest or show life-threatening arrhythmias (level of evidence B); who show signs or symptoms of heart failure (level of evidence B); and patients with severe or disabling angina (class III or IV) or with high-profile risk, especially if non-responders to medical therapy (level of evidence C).
Medical therapy (Table 2)
Within the scope of CCS medical therapy, there are two main goals to achieve:
- Controlling CAD progression and prevention of cardiovascular events;
- Reduction of symptoms and ischemic burden.
This table 2 summarizes the effects and mechanisms of various pharmacological agents used in CCS.
Table 2. Pharmacological agents used in chronic coronary syndromes
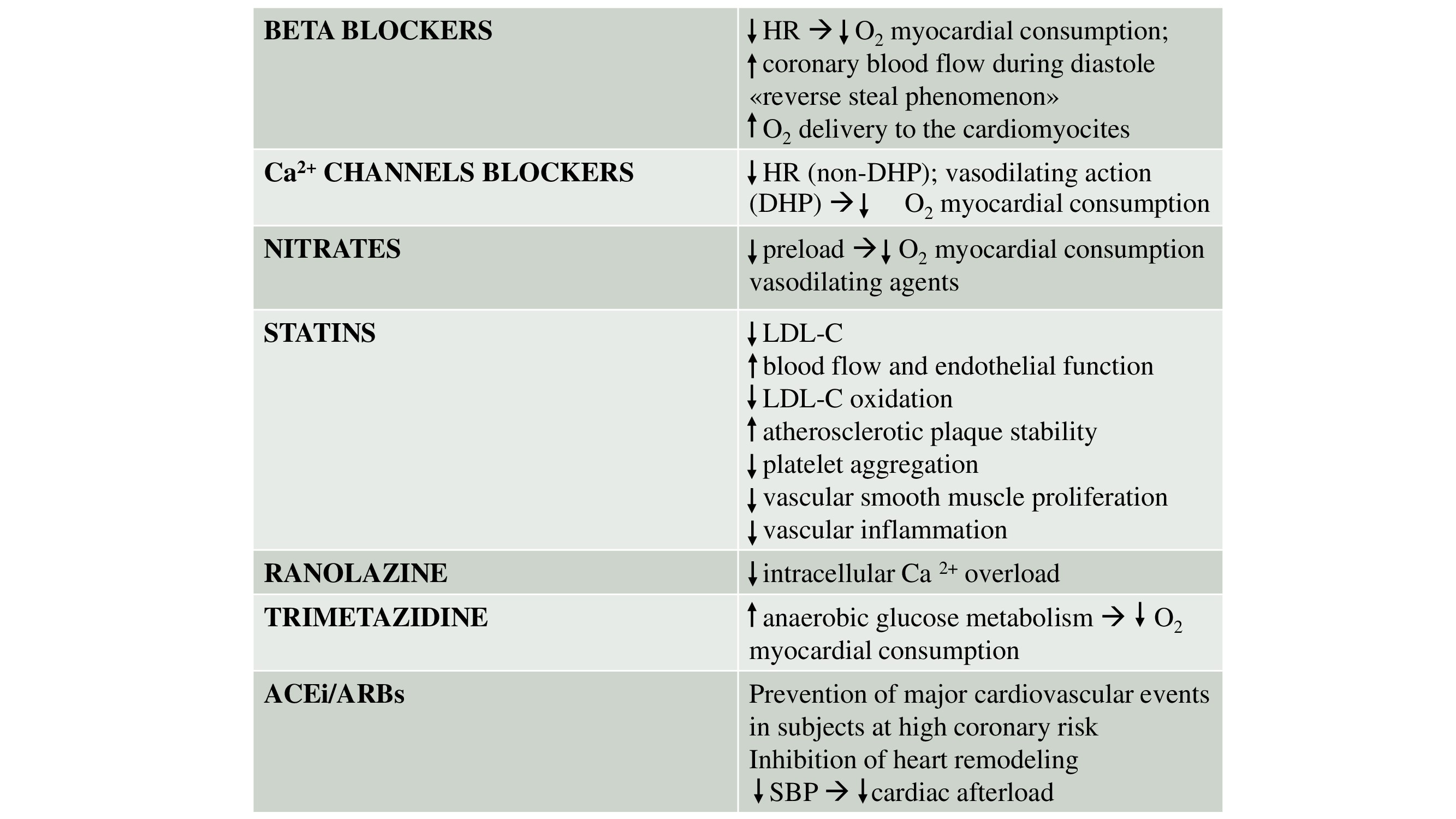
ACEi - angiotensin-converting enzyme inhibitors, ARBs - angiotensin receptor blockers, Ca²⁺ calcium ions, DHP - dihydropyridine, HR - heart rate, LDL-C - low-density lipoprotein cholesterol, O₂ - oxygen, SBP - systolic blood pressure
Drugs for the prevention of CV events
Antithrombotic therapy
Platelet aggregation represents the main mechanism underlying coronary thrombosis (20), justifying the role of antiplatelet drugs in preventing the progression of CAD. The use of these drugs is more aggressive in the acute phase, in which there is an ongoing thrombotic stimulus, characterized by persistent platelet activation and thrombin generation (21). Nevertheless, even months or years after the acute event, as shown by biochemical studies, hyperactivity, and elevation of markers of the coagulation system persist (22).
This process plays an important role in the genesis of recurrent ischemic events.
Primary prevention
The role of aspirin in primary prevention is currently discussed and controversial. Meta-analyses revealed a slight effect of low-dose aspirin in preventing the first heart attack or stroke, at the expense of a significant increase in bleeding risk (23).
The ACC and the AHS propose low-dose aspirin (75 to 100 mg/day) for the primary prevention of atherosclerotic CV disease (CVD) in subjects between 40 and 70 years old who are at higher CVD risk but not at elevated risk of bleeding. However, this approach is not applicable for older people or those who are at higher risk of bleeding (23).
According to the European Society of Cardiology, low-dose aspirin may be taken as a primary preventive measure in people with a very high risk of CVD (24)
Secondary prevention
Patients with CCS without indication for oral anticoagulant therapy (OAC)
The role of antithrombotic therapy in patients with documented CAD is more established. Most of coronaropathic patients, outside of the post-PCI period, are on single antithrombotic therapy. A daily dose of 75-100 mg of Aspirin is recommended by ESC Guidelines for prevention of ischemic events in patients with a previous MI or revascularization (Class I, Level A), while it may be considered in patients without a history of MI or revascularization, but with definitive evidence of CAD on imaging (Class II, Level B) (25). The role of Clopidogrel in this scenario is limited to aspirin-intolerant patients (IA) and based on the CAPRIE study, to those with either peripheral arterial disease (PAD) or a history of ischemic stroke or transient ischemic attack (IIb, B) (26).
The presence of complex CAD and CV risk factors outlines the ischemic risk profile of the individual patient. The ESC does not provide a precise definition of complex CAD, leaving room for clinical judgment based on the patient's CV history and coronary anatomy; the task of future guidelines will be to offer readily available risk-stratification tools that include anatomical and non-anatomical variables, to formulate more standardized treatment plans both in the field of medical therapy and correct interventional management. In subjects with a high risk of ischemic events, defined as: “diffuse multivessel CAD with at least one of the following: diabetes mellitus requiring medication, recurrent MI, PAD, or chronic kidney disease with eGFR 15-59 mL/min/1.73 m2)”, and without high bleeding risk, addition of a second antithrombotic drug should be considered (Class IIa, Level A), with a lower class of recommendation (Class IIb, Level A) in patients with a moderately increased risk of ischemic events, defined as “at least one of the following: multivessel/diffuse CAD, diabetes mellitus requiring medication, recurrent MI, PAD, HF, or CKD with eGFR 15-59 mL/min/1.73 m2” (Class IIb, Level A) (26). There are several treatment options for DAPT in combination with aspirin 75-100 mg daily, such as clopidogrel 75 mg, ticagrelor 60 mg b.i.d. (27) or rivaroxaban 2.5 mg b.i.d. The use of P2Y12 inhibitors is mainly indicated in patients who have tolerated 12 months of DAPT following MI. Factor Xa inhibitor, on the other hand, finds its place in the context of the patient with diabetes, (28) chronic kidney failure (29), but especially in patients with peripheral arteriopathy, in which peripheral CV events are reduced in addition to major CV events (30).
Patients with CCS and indication for OAC
Secondary prevention of CV events is pursued with the non-vitamin K oral anticoagulant alone in patients who have indication for an oral anticoagulant; however, despite the lack of specific data, dual therapy with an OAC and a single antiplatelet agent like aspirin or clopidogrel may be considered in highly selected cases with high ischemic risk (Class IIb, Level B) (31).
Therapeutic management of the post-PCI phase
In patients undergoing coronary angiography for stable angina, evidence of CAD on CCTA or ischemia by functional noninvasive tests, stent implantation is followed by more aggressive antithrombotic therapy. The type and duration options of these therapies are various, based on the patient’s features.
Patients without indication for OAC
After angioplasty, DAPT is provided. The standard therapy involves the combination of aspirin 75-100 mg and Clopidogrel 75 mg daily for 6 months (Class I, Level A). In cases in which the risk of hemorrhage is considerable, it is possible to reduce the DAPT duration to 1-3 months (32).
The unlicensed use of ticagrelor or prasugrel in stable patients having elective PCI who are at high-risk of stent thrombosis is supported by a small number of pharmacodynamic trials; nevertheless, the safety/efficacy balance of this method in comparison to clopidogrel has not been established (33). Another possible scenario in which to hypothesize the use of these drugs is aspirin intolerance.
Prasugrel or ticagrelor can find a rationale as second antiplatelet agent, at least as first therapy, in certain high-risk elective stenting scenarios, like numerous implanted stents, vascular tree with diffuse lesions, total stent length > 60 mm, difficult left main stem stenting, history of intra-stent thrombosis on antiplatelet treatment, inadequate stent deployment, or other procedural characteristics associated with high risk of stent thrombosis. The choice to embrace this therapeutic strategy is weighted based on the specific profile of the individual patient, always taking in consideration his bleeding risk.
The main data we have about the comparison between prasugrel and ticagrelor are in the context of ACS and are mostly indirect data derived from three major studies, the Disperse -2 (34) and PLATO (35) (ticagrelor vs clopidogrel) and TRITON - TIMI 38 (36) prasugrel vs clopidogrel).
In a meta-analysis, Biondi-Zoccai et al. performed a head-to-head comparison between prasugrel and ticagrelor with the results of these three studies and demonstrated no difference in the risk of overall death, non-fatal MI, non-fatal stroke or their composite endpoints. However, prasugrel seems to be more effective than ticagrelor in preventing stent thrombosis, but without having other clinical benefits, and while it may increase the risk of bleeding (37).
Therefore, in clinical practice ticagrelor appears to be more widely used in view of its better safety profile from the bleeding point of view especially in elderly patients (> 75 years), weighing less than 60 kg, or with a history of cerebral ischemic event in whom the use of prasugrel is not recommended and also in view of its shorter half-life, which results in an acceptable bleeding rate if the patient undergoes cardiac surgery during the same hospitalization. Instead, prasugrel has a place especially in patients with development of intra-stent restenosis during antiplatelet therapy with ticagrelor or with complex CAD associated with a low bleeding risk.
Patients with indication for OAC
The most frequent scenario are those patients with CCS and atrial fibrillation, present in 5-8% of patients undergoing PCI (38) but there are also other contexts in which anticoagulant therapy is required.
In this setting personalized antithrombotic therapy is needed, both in terms of composition and duration. The decision is related to a complex and subtle balance between the individual patient-specific hemorrhagic risk (added to the intrinsic one in the nature of the antithrombotic drugs) and the combined coronary and embolic ischemic one. There are several scores and parameters that help us to quantify these: the HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly) score, the PRECISE DAPT SCORE and Academic Research Consortium for high bleeding risk (ARC-HBR) criteria regarding bleeding risk, the latter having greater sensitivity than the others (39); the CHA2DS2-VASc score regarding cardioembolic risk in atrial fibrillation patients.
In relation to the risk of intra-stent thrombosis, this is not defined by any validated score, however a variety of factors that participate in this risk are considered in clinical practice, including: complex PCI (stenting of left main stem, proximal LAD or last coronary artery; implantation of two stents on bifurcation, etc.), previous intra-stent thrombosis in adequate therapy, procedural factors (stent length > 60 mm, etc.) and comorbidities (diabetes mellitus, chronic kidney disease, etc.) (40).
According to European guidelines, the standard protocol provides the combination of aspirin, clopidogrel, and OAC for one month (Class IIa, Level B) (41), after which clopidogrel and OAC are continued for the next six months, then leaving the patient on OAC monotherapy lifelong or possibly in association with a single antiplatelet drug in selected patients (as discussed above).
The choice on the duration of triple antithrombotic therapy depends on the balance between bleeding and the intra-stent thrombosis risk, both determined by the parameters and scores named above.
In consideration of what has just been said, the triple therapy should be reduced to one week in patients where the hemorrhagic risk prevails (Class IIa, Level B) (42), conversely, if the ischemic risk of intra-stent thrombosis is higher, this pharmacological combination should be prolonged over a month up to six months (Class IIa, Level C) (41).
In addition to paying attention to the management of antithrombotic therapy, there are other pharmacological and nonpharmacological measures that can be taken to reduce bleeding events, which occur more in elderly patients with a history of previous bleeding, often gastrointestinal. Proton pump inhibitors (PPIs), which are recommended in all patients at risk of gastrointestinal bleeding who are taking aspirin, DAPT, or an OAC, play a key role in this regard because of their various protective effects at the gastrointestinal mucosal level (43); This class of drugs, such as esomeprazole and omeprazole, may interact with CYP2C19, which metabolizes the inactive form of clopidogrel, reducing its pharmacodynamic effect (44), so co-administration of these should be avoided, even if it has not been demonstrated to impact the risk of ischemic events or stent thrombosis.
Other preventive measures include the choice of a radial artery approach as default vascular access and proper adjustment of anticoagulant dosage in relation to the patient's weight, age, and renal function. A more invasive measure is left auricle closure, which may be considered in patients who have an absolute contraindication to anticoagulation, such as may be the high risk of potentially fatal bleeding.
Lipid-lowering therapy
Another cornerstone of the prevention of CV events is represented by lipid-lowering therapy. Scientific literature has consistently shown that lowering cholesterol linked to low-density lipoproteins (LDL-C) is associated with a lower risk of CV events, the magnitude of which is proportionate to lowering plasma LDL-C concentrations.
LDL oxidation plays a significant pathogenic role in atherosclerosis due to several specific biological properties in vitro and in vivo, such as the formation from macrophages of foam cells, which play a critical role in lesion progression. Indeed, scientific evidence shows a correlation between plasma and plaque levels of oxidized LDL and vulnerability to rupture of atherosclerotic lesions (45).
Despite the levels of LDL-C, lipid-lowering medications are necessary for patients with established CAD, since they are deemed to be at very high risk for CV events. Lowering LDL-C by at least 50% from baseline and to <1.4 mmol/L (<55 mg/dL) is the aim of therapy, while individuals who have experienced a second vascular incident within the last two years may be eligible for a lower target LDL-C of <1.0 mmol/L (<40 mg/dL) (46).
The first therapeutic choice is certainly a statin at the maximum tolerated dosage. In addition to reducing serum cholesterol, statins have numerous other pleiotropic effects. These other features include improving blood flow and endothelial function, reducing LDL-C oxidation, increasing atherosclerotic plaque stability, reducing platelet aggregation, vascular smooth muscle proliferation and vascular inflammation (47).
High doses of atorvastatin have been demonstrated to lower the incidence of peri-procedural events in patients undergoing PCI, in both patients on chronic statin treatment and statin-naive patients (48).
In case of failure to achieve the pre-established cholesterol levels, even using the maximum tolerated dose of statins, the addition of other compounds is necessary.
Ezetimibe is one of the molecules of choice; it acts at the intestinal level and selectively lowers cholesterol absorption. When used as monotherapy, it reduces LDL-C levels from 15% to 22% of baseline values. While the role of ezetimibe as monotherapy in patients with elevated LDL-C levels is limited, its activity is complementary to statins. In fact, while statins reduce cholesterol biosynthesis, increasing its absorption at the intestinal level. Ezetimibe inhibits intestinal absorption of cholesterol, increasing its biosynthesis at the hepatic level. Thus, combining these two mechanisms of action, ezetimibe in combination with a statin can result in an additional reduction of LDL-C (regardless of the statin used and its dosage) by 15%-20%.
This drug has been shown to lower cholesterol and CV events in post-ACS patients (49) and in those with diabetes, (50) with no further effect on mortality.
Another weapon at our disposal is represented by the inhibition of proprotein convertase subtilisin-kexin type 9 (PCSK9), which can be obtained through different mechanisms: starting from the use of monoclonal antibodies, such as evolocumab and alirocumab, which have been shown to be effective in reducing CV and primarily ischemic events (51), up to gene silencing through small interfering RNA, such as inclisiran, which effectiveness in reducing LDL levels is well established (52) but studies are still underway to evaluate the impact of this therapy on CV outcomes.
A further therapeutic option is represented by bempedoic acid.
This drug acts by inhibiting ATP citrate lyase (ACLY), a cytosolic enzyme located within the enzymatic cascade leading to cholesterol synthesis, upstream of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR) (53).
Therefore, although at a different level, bempedoic acid acts on the same metabolic route of statins. This suggests that this drug may have pleiotropic effects related to manipulation of the mevalonic pathway, such as reduction of coenzyme Q production or prenylation regulatory proteins (small GTPases) (54).
Potential muscle-related adverse events, such as myalgia and myopathy should be less common because skeletal muscle does not contain this enzyme.
In addition to confirm its effectiveness in terms of reducing LDL-C levels (24% as monotherapy, 18% when in addition to statin, 38% in combination with ezetimibe), bempedoic acid has been demonstrated to reduce major adverse cardiovascular events (55).
Renin-angiotensin-aldosterone system inhibition
In individuals with prior vascular disease (56), high-risk diabetes (57) or LV dysfunction (58), angiotensin-converting enzyme (ACE) inhibitors demonstrated interesting outcomes in terms of mortality, MI, stroke and HF. Specifically, ramipril and perindopril have been shown to prevent major CV events in subjects at high coronary risk (dyslipidemia, diabetic, and patients with previous vascular events) (58).
Unless there is a contraindication (such as significant renal impairment, hyperkaliemia, etc.), it is advised that patients with CCS who also have concomitant hypertension, diabetes, LV ejection fraction < 40%, or chronic kidney disease should be treated with ACE inhibitors or angiotensin receptor blockers (ARBs) in situations of intolerance. ACE inhibitors have been shown in certain trials to lower CV mortality, non-fatal MI, stroke, HF, and all-cause death in individuals with atherosclerosis who do not have reduced LV function (56) therefore it finds a space, although with weaker recommendation, even in this class of patients.
Beta -blockers
Beta-blockers are considered the primary choice in long-term maintenance drug therapy in patients with CAD, based on positive evidence for improving clinical outcomes in patients with acute MI (59) or HF (60). However, the use of beta-blocker in post-infarction period is supported by randomized clinical trials (61) that have been conducted before the extraordinary development and spread of percutaneous revascularization techniques, and before the systematic implementation of statins and modern antiplatelet therapies. Thus, these are studies conducted mainly in patients with extensive MIs and residual ventricular dysfunction.
Since the beginning of the PCI era, the number of patients with MI and preserved ventricular function increased, and the value of beta-blocker therapy in this setting has been questioned. Until recently, only observational studies on this subject were available, which provided conflicting results (62) (63).
The randomized evaluation of decreased usage of beta-blockers after acute myocardial infarction (REDUCE-AMI) trial is a large, randomized control trial that represents the first modern study of the benefits of beta-blockers and highlights the lack of efficacy of this therapy in reducing the risk of death or re-infarction in MI subjects treated with coronary angioplasty who do not show LV dysfunction (64).
Beta-blockers prevent ventricular remodeling by reducing myocardial oxygen consumption, protecting against fearsome arrhythmias particularly in the first 3 months after reperfusion, and finally reducing anginal episodes. However, early revascularization, which has become increasingly common and widespread, has been shown to be equally a strong inhibitor of sympathetic activity mimicking the action of beta-blockers.
Considering the actual data from scientific evidence, it seems reasonable to start treatment with beta-blockers in the most vulnerable post-MI period, i.e. the first 3 months in all patients with the possibility of extending this period probably to 1 year, reserving the long-term treatment only for those patients with ventricular dysfunction, which are classified as HF, a condition in which beta blockade should be a cornerstone of treatment.
In the context of CCS, therefore, long-term beta-blocker use finds its place in controlling anginal symptoms and preventing CV events in patients with ventricular dysfunction, as emphasized by European guidelines.
Drugs for the reduction of symptoms and ischemic burden
Nitrates
Nitrates are the most effective drugs to stop angina episodes. These substances work through two different mechanisms: firstly, lowering myocardial oxygen consumption through a decrease in ventricular preload, acting as a venous vasodilator; secondly, improving oxygen supply inducing coronary vasodilation through the delivery of nitric oxide (NO), which is the main endothelial vasodilator. Intact endothelium releases NO, which activates guanilate cyclase (65). Many pathological conditions, such as smoking, hypertension, diabetes mellitus, hypercholesterolemia and HF are linked with endothelial dysfunction, characterized by a reduced release of NO into the arterial wall either because of impaired synthesis or excessive oxidative degradation (66). Nitrates bypass the need for an intact endothelium by directly stimulating NO production, mostly at the level of vascular smooth muscle cells. This action requires the activity of the enzyme glutathione-S-transferase and the presence of thiol groups, the depletion of which would underlie the development of habituation during prolonged therapy (67).
It is advised to utilize sublingual formulations or sprays for this purpose, because of their quick start of action and absorption.
In the context of chronic therapy, long-acting nitrates should be taken into consideration as a second-line treatment in cases where beta-blocker and/or non-dihydropyridine calcium channel blockers (non-DHP-CCB) initial therapy is contraindicated, poorly tolerated, or insufficient to manage angina symptoms. A nitrate-free or nitrate-low gap of 10–14 hours must be provided since long-acting nitrates lead to tolerance and loss of effectiveness when administered over a longer period (68). The most common side effect is headache, which in 5-10% of cases is of such magnitude that the drug is discontinued. Nitrate-induced hypotension is common but often asymptomatic. Rarely, nitrates cause coronary steal and myocardial ischemia. When using intermittent nitroglycerin patch therapy, patients may develop nocturnal anginal episodes because of nitrate rebound (69).
Also because of the risk of the side effects described above, nitrates are to be considered as second/third-line therapy when other treatments are contraindicated, poorly tolerated or insufficient to control symptoms. In patients with hypertrophic obstructive cardiomyopathy (70) or those receiving phosphodiesterase inhibitors concurrently (71), nitrates are not advised.
Beta-blockers
While the role of beta blockers is questioned for the prevention of CV events, their role is central in controlling ischemia-related symptoms, for which they represent a first-line therapy.
The anti-ischemic mechanisms are multiple (72) and mainly mediated by the reduction of heart rate, which results in a reduction in myocardial oxygen consumption and an increase in effective coronary flow through the extension of the duration of the diastole. Additional auxiliary processes include the reverse steal phenomenon (73), which increases perfusion in stenotic disease regions through vasoconstriction of the epicardial coronaries, and improvement of oxygen release to the myocardium, by modifying the hemoglobin dissociation curve (38).
Beta-blockers also increase the tolerability of other drug classes indicated for angina (e.g., dihydropyridine calcium channel blockers (DHP-CCB), nitrates, and nicorandil) by reducing reflex tachycardia.
In patients with a significant decrease in coronary reserve and elevated heart rate, blood pressure, or both, the therapeutic effectiveness of this family of medications is particularly evident in raising the ischemia threshold; in these cases, beta-blockers are clearly superior to calcium blockers (74).
The association with a DHP-CCB is a valid option in case of lack of control of the symptoms, as it should be considered from the beginning in patients with hypertensive phenotype.
The main contraindications to the use of this category of drugs are represented by bronchial asthma, depressive state and major bradyarrhythmic disorders.
Calcium channel blockers
Calcium channel blockers are equally effective drugs in controlling angina symptoms, due to the predominantly bradycardic action of the nondihydropyridine agents and the vasodilating action of the dihydropyridines; in both cases, the result is a reduction in cardiac work and reduction in oxygen demand (75).
On the other hand, while the anti-ischemic efficacy of these drugs is well established, there are no data to support a benefit on mortality or reduction of major cardiovascular events in patients with CCS (76).
Non-dihydropyridine agents (heart rate-lowering calcium channel blockers)
Non-dihydropyridines include the medicines verapamil and diltiazem. The main issues with these drugs are their less predictable bradycardic effect compared with beta-blockers with possible development of marked bradycardia and conduction defects that may lead to discontinuation of the drug and their negative inotropic effect, such that their use in patients with LV dysfunction is detrimental.
No comparison studies between verapamil and diltiazem are available. The former has indication in a greater spectrum of anginal pictures (exertional, vasospastic, and unstable angina) with efficacy in controlling ischemic symptoms shown to be similar to metoprolol (76). In contrast, the second has a place in exertional angina, with a lower rate of side effects.
Because of their chronotropic and negative inotropic actions, these drugs should be used with extreme caution in combination with beta-blockers to control ischemic symptoms. If it becomes necessary to use this combination, it is advisable to prefer the use of diltiazem because of its lower inotropic and negative chronotropic action (77).
Dihydropyridine agents
In contrast to non-DHP CCBs, dihydropyridines at therapeutic doses induce a more pronounced reduction in peripheral resistance and thus in arterial blood pressure, through a relaxing action of arteriolar smooth muscle. This occurs without inducing detectable cardiac depression, a result of their marked selectivity for the vessel musculature. Even if it has been documented a reduction in myocardial contractility in vitro, however, the effect found in clinical practice in terms of inotropism and chronotropism is that of sympathetic stimulation mediated by baroreceptor reflexes. This cardiac stimulation makes these drugs less attractive in the setting of chronic coronary syndrome, as they are associated with worsening angina to even the onset of myocardial infarction or sudden death (78).
The solution to this problem is longer-acting and slow-release preparations, because of their slower onset of effect, which allows restoration of the baroreflex. Another remedy is undoubtedly the concomitant administration of a beta-blocker, often considered in clinical practice to be the winning combination.
The most widely used and most evidence-based drugs in this class are nifedipine long-acting and amlodipine.
The former was shown in the ACTION study to be a safe and effective drug in addition to beta-blocker therapy in hypertensive anginal patients and to reduce the need for coronary angiography and CV interventions; however, it was not effective in reducing CV events and improving survival (79).
Amlodipine is a very effective and safe antihypertensive and antianginal drug thanks to its very long half-life. This drug was shown to be more effective than atenolol in reducing ischemia during exercise (80) and in reducing revascularization operations and hospitalizations due to angina at 24 months in the CAMELOT trial (81).
In clinical practice, the role of calcium channel blockers in therapy for the control of ischemic symptoms is limited to vasospastic angina and as an alternative to beta-blockers (class IA). The combination with the latter remains a valid option in case of lack of control of symptoms (class IIaC) or even as initial first-line therapy (class IIaB), considering the greater risk of hypotension and bradycardia, the latter reduced by preferring association with DHP-CCBs. Overall, the 2019 ESC guidelines provide weak indications on this class of drugs supported by dated studies.
Evaluation of borderline stenoses
When performing coronary angiography, it can be tricky to differentiate subcritical lesions and critical lesions, which need to be treated with PCI. In borderline situations, it is possible to apply functional tools to resolve doubts, overcoming the limits of mere angiography.
The main diagnostic tools we can perform in the catheterization laboratory are coronary intravascular imaging (intravascular ultrasound, IVUS and optical coherence tomography, OCT), and coronary physiology (istantaneous wave-free ratio, iFR and FFR).
Coronary Intravascular Imaging
The main methods for coronary intravascular imaging are IVUS and OCT. Both methods consist of using a small probe (in IVUS) or a small tomograph (in OCT), placed on a guidewire that has been previously advanced across the coronary area of interest. These diagnostic tools are useful to determine significance of a coronary lesion: left main lesions with minimum lumen area (MLA) >6.0 mm² by IVUS or >5.4 mm² by OCT do not require revascularization (however, assessment of ostial left main lesions can be challenging with OCT and there are no outcomes data with OCT of the left main). In non-left main lesions, the MLA cut off are 4 mm2 by IVUS, while regarding OCT, no specific cut-off has been established. However, in these cases, the use of intravascular imaging for determining their hemodynamic severity and need for revascularization has less evidence. Moreover, these methods allow to evaluate angiographically ambiguous lesions, such as suspected dissection, thrombus, and calcified nodule.
As for the main differences between those two processes, OCT has 10-fold higher resolution (10-15 µm compared with 100 µm for IVUS), but requires blood clearing, which is usually achieved by contrast injection and has low penetration. OCT works better in detecting thrombus, dissection, and assessing plaque morphology. The choice between the methods depends both on the differential diagnosis and the patient’s features (acute kidney injury and chronic kidney disease, e.g.).
Coronary physiology
The invasive physiological assessment of intermediate coronary artery stenosis with FFR has been shown to represent a valid method for severity evaluation (82). These methods allow to assess the hemodynamic significance of intermediate lesions by measuring the pressure drop distally to the lesion. It can be performed in a resting situation, by iFR and several other nonhyperemic indices that have been developed (such as the resting full cycle ratio (RFR), the diastolic hyperemia-free ratio (DFR), and the diastolic pressure ratio (DPR), and in hyperemic situations, by using the adenosine FFR.
Several studies have shown that PCI of lesions with FFR >0.80 or lesions with non-hyperemic indices that do not show ischemia such iFR >0.89 can be safely deferred without increasing the incidence of adverse outcomes. This applies to left main lesions too (83).
Two main trials, the FAME and FAME II trials have demonstrated the clinical benefits of FFR-guided PCI in patients with stable CAD compared to angiographically guided PCI and optimized medical therapy alone, respectively (84, 85).
Useful information can be detected when a pullback measurement is performed in the target vessel. This allows to differentiate between focal or diffuse CAD, which are associated with different clinical scenarios. The hypothesis, currently under investigation, is that focal stenoses are associated with a more vulnerable plaque (mainly because of turbulent flow and shear-stress forces), being more prone to cause acute clinical events. These lesions would most likely benefit from a PCI. On the other hand, a diffuse atherosclerotic pattern consists more of chronic, stable plaques, with less lipid pools and necrotic cores, suggesting a less aggressive condition, less prone to generate ACS.
Advanced techniques of plaque characterization may contribute to recognize which lesions present instability features that could determine a higher risk of acute events in patients with stable CAD. Invasive physiology assessment and intravascular imaging may help to identify which patients might obtain more clinical benefit from PCI and play an essential role in PCI optimization (86).
Regarding FFR evaluation for PCI optimization, the FFR SEARCH and TARGET-FFR studies highlighted that more than half of patients presented a suboptimal post-PCI FFR (FFR ≤ 0.90) (87, 88). Other studies showed that lower FFR values after PCI were associated with increased adverse clinical events (89) and higher post-PCI FFR values were related to a higher rate of angina symptoms relief and a lower number of clinical events (90). One of the described mechanisms leading to lower post-PCI FFR values was the presence of residual diffuse coronary atherosclerosis, which may not be detected on coronary angiography (91).
Management of chronic total occlusions
A CTO is defined as a completely occluded artery with no anterograde flow (TIMI 0) with an estimated time > 3 months. When approaching a CTO, planning is mandatory. The key indication to perform a CTO PCI is ischemic symptom relief and quality of life improvement (92).
Since performing a CTO-PCI could be very time-consuming, requiring high contrast and radiation doses, it is mandatory to consider several aspects. A CCTA can be very useful for evaluating the CTO segment and planning, helping to choose the best angiographic projections. Several CCTA-based score have been created (mainly CT-RECTOR and the Korean Multicenter CTO CT Registry score), mostly based on the number of the lesions and their length, the bending angle, the entity of calcification and the duration of the CTO (93, 94).
The other aspect to assess is the myocardial viability, which can be evaluated by integrating different tests, such as ECG and echocardiography, both at rest and during stress, and cardiac magnetic resonance imaging (CMR). Once obtained a complete picture, assessed the risks and benefits and the patient’s preferences, CTO PCI can be performed.
CTO PCI has some typical features that make it a technically risky procedure: First, dual (or sometimes triple) arterial access is commonly required to allow dual angiography. The use of combinations of bi-femoral, femoral-radial, or bi-radial accesses will depend on the team's preference, the availability of the needed materials, the patient's characteristics, the procedure, and the anatomy (95). The vessel engagement is obtained by using a larger profile catheter (7 or 8 Fr) to guarantee a better support. A CTO PCI can be performed both through an anterograde and through retrograde approaches. The choice is based on the length, features, and morphology of the plaque and the collateral vessels.
To make better planning of CTOs’ treatment, the “hybrid algorithm” (96) has been developed, which allowed to maximize success and reduce the time of the procedure, radiation doses and contrast erogation. The core of this algorithm is the rapid identification of the inapplicability of one strategy followed by immediate exchange for another type of technique.
The algorithm or hybrid approach consists of two paths (anterograde and retrograde), and two ways of crossing CTO: through true lumen or the subintimal space (dissection and then re-entry technique). The definition of which path to use and how to cross the occlusion is determined by 4 factors:
- proximal cap anatomy
- occlusion length
- presence of a disease-free zone for reentry in the distal vessel
- presence of usable collaterals.
For the anterograde dissection and reentry technique, dissection and reentry are related to the intentional and controlled use of the subintimal space to cross the occlusion. This should be considered when CTO extension is >2 cm. Strategies to induce limited and controlled dissections seem to have better short- and long-term results compared to those that cause extensive dissections (97, 98). Controlled dissection is achieved with dedicated micro catheters that create a limited dissection plane. The reentry is obtained with the help of a specific balloon. This is related to lower rates of major cardiovascular events (MACE) (4.3 vs. 15.4%, p = 0.02) and target revascularization (3.1 vs. 15.5%, p = 0.02) when compared to older techniques (99).
The retrograde approach to CTO crossing can significantly increase success rates, particularly in more complex lesions. This is the first line strategy when the proximal cap is ambiguous, the antegrade reentry zone is not adequate or the distal cap ends at a bifurcation. Retrograde crossing by grafts (especially venous grafts) and by septal collaterals are preferred to epicardial collaterals because they are easier to engage and cross and, mainly, present lower risk of tamponade in case of perforation (100, 101).
The guidewire proceeds to the distal region of the occlusion passing through a collateral. From this point, the CTO is crossed in the opposite direction to the blood flow (102). The rational of retrograde technique is that crossing by the true lumen is easier, because the distal lumen tends to have more favorable (softer, pencil-like, less ambiguous) characteristics than the proximal one. If true lumen crossing is not available, dissection and re-entry techniques, other than anterograde techniques, may be applied.
Performing a complex procedure involves a significant delivery of contrast and radiation. Protocols dedicated to CTO interventions, more modern equipment, and operators’ expertise significantly impacts these concerns (103, 104). The decision to interrupt the procedure should be evaluated case by case. There are not guidelines suggesting when to stop the intervention. The final decision depends on the operator’s evaluation and must consider factors such as contrast and radiation erogation and risk/benefit ratio.
Intra- and post-hospital care should be the same as any other complex PCI, considering the complications that occurred during the procedure and the amounts of contrast and radiation used.
Successful CTO recanalization is associated with clinical benefits, such as improved angina, quality of life and physical limitation, improved ventricular function, decreased mortality and incidence of clinical events when compared to patients whose recanalization was not successful.
Sapontis et al. (105) evaluated the quality of life of 1,000 patients submitted to CTO PCI. One-month follow-up showed a significant improvement in all domains of the Seattle angina questionnaire (SAQ), Rose dyspnea scale and PHQ-8 scores. Several observational studies show a relationship of CTO recanalization in the reduction of clinical events. Jang et al. (106) compared CTO revascularization (by PCI or by surgery) with drug therapy in 738 patients with well-developed collaterals. The combined prognostic analysis at 42 months showed a 73% reduction in the incidence of cardiac death (108).
The Italian CTO Registry (107) assessed the clinical outcomes of 1,777 patients, showing lower cardiac mortality (1.4, 4.7 and 6.3%, p<0.001) and MACE at one year (2.6, 8.2 and 6.9%, p<0.001) in patients treated with PCI when compared to clinical treatment or surgery. In this study, the group receiving optimized medical treatment presented higher rates of MACE, death, and re-hospitalization (107).
When to perform PCI and when to choose medical therapy? Pitfalls of ISCHEMIA trial:
he ISCHEMIA trial, whose results were published in 2020, randomized more than five thousand patients with moderate or severe ischemia on functional testing to an initial invasive (coronary angiography and eventual revascularization) vs. an initial conservative strategy (optimal medical therapy alone) in roughly equal proportions. Myocardial ischemia was proven in 75.5% with stress imaging methods (nuclear, echocardiography and CMR) and in 24.5% with an exercise tolerance test. After ischemia was confirmed, CCTA was performed to rule out left main stenosis or non-obstructive CAD (108, 109). After a median follow-up of 3.2 years, there was no significant difference between the two strategies in the primary endpoint, a composite of cardiac death, MI and hospitalization for unstable angina, HF or resuscitated cardiac arrest (110). Unlike other trials, the need for revascularization was left out of the primary outcome, as the trial tested the initial strategy, rather than clinical benefit of revascularization itself. Within the conservative arm, 26% of patients eventually underwent coronary angiography (“cross-over”), 21% revascularization and 15% were revascularized before the occurrence of an event. Furthermore, 26% of the revascularizations in the invasive arm were performed surgically. These results have been interpreted as there is not a clear advantage of an early invasive strategy for the reduction of major clinical endpoints in patients with CCS. Nevertheless, the invasive arm reported significantly better prognosis Quoad valetudinem than the conservative arm, particularly in symptomatic patients at the time of randomization.
Some critics have been moved to this trial. Several groups of patients have been excluded:
• CCS and left stenosis stenosis;
• CCS with no proof of myocardial ischemia;
• CCS with very severe ischemia (e.g. fall in blood pressure, very limited functional capacity) were not likely enrolled by trials;
• CCS with an unacceptable degree of angina;
• CCS and LV ejection fraction < 35%;
• CCS and HF New York Heart Association (NYHA) III/IV;
• CCS and valvular disease.
The study was also characterized by a slow recruitment and a lower-than-expected incidence of events.
This is usually related to a selection bias that overshadows the results. In fact, the initial sample size calculated that 8000 patients were required to reach the primary endpoint but had to be reduced to almost 5000 due to difficulties with recruitment.
Furthermore, several critical variables were not equally distributed between the groups, in favor of less risk within the non-invasive arm.
In fact, the invasive arm had numerically more HF (4.3% vs. 3.6%, p=0.207), stroke (3.2% vs. 2.6%, p=0.219), cerebrovascular disease (7.8% vs. 6.8%, p=0.194), PAD (4.5% vs. 3.4%, p=0.049) and was characterized by more symptomatic patients (evaluated by recurrent angina at randomization 21.7% vs. 18.9%, p=0.049). This aspect corroborates the hypothesis of the presence of selection bias and has obviously a potential impact on the outcome, disadvantaging the invasive arm.
The most important argument is about the evolution of the event-curves and is related to the incidence of periprocedural MI. These events, in fact, show the higher price paid earlier by the invasive strategy, and, on the other hand, promotes the conservative approach. The slope of the curve flattens in the invasive arm compared with the conservative arm, and the curves intersect at approximately 2 years of follow-up, with continued divergence to the 4th year of follow-up when the differences in the primary outcome become statistically significant: 13.3% vs. 15.5% in the invasive and conservative arms, respectively. However, the final report is considered non-significant. This cannot be justified by the number of events. The most plausible explanation for the final lack of statistical significance seems to be the reduction of the sample size. To explain this, we must consider that the primary endpoint is reported as Cox regression, and the median follow-up is 3.2 years (the study was planned for 4 years). Therefore, the primary endpoint in ISCHEMIA reports just a Kaplan-Meier estimate, instead of a truncated follow-up, for all patients. Because of this, the appendix of the hazard rates of all endpoints continues diverging in favor of the invasive strategy.
In conclusion, a larger number of patients and a longer follow-up are needed to report a statistically significant difference in favor of the invasive strategy. Even considering the importance of the periprocedural MI, the invasive management confers an advantage for the event-free survivors over those treated with a conservative strategy.
Gaps in evidence and future directions
One of the main issues with CCS is the lack of actual consensus between specialists in terms of diagnosis, management and long-term follow up. These problems derive also from a gap between American and European guidelines, which have been lastly released respectively in 2023 and 2019, with contents differing not only in terms of therapeutic management, but also in the diagnostic process.
The timing gap between the latest ESC guidelines for acute coronary syndromes (2023) and CCS (2019) creates a further difficulty for management, because those patients who experience an ACS are classified as CCS following the latest ESC guidelines.
As we already discussed, there is not a clear consensus for the type and duration of antiplatelet therapy. This happens because the patients who are classified as CCS represent a wide spectrum, with different clinical features (e.g., patients with previous STEMI vs. patients who undergo elective angioplasty for stable angina a positive screening test for CAD); but despite that, the medical therapy is not differentiated. The latest guidelines from ESC for ACS discuss the possibility of reducing the duration time of DAPT in patients with lower thrombotic risk. This is also present in 2023 ACC/AHA guidelines for CCS but not in ESC guidelines, which are not updated to the latest ACS guidelines.
As we already discussed, the use of beta-blockers represents one of the most important points in medical therapy for CCS patients. On the other hand, ACC/AHA does not recommend the use of beta-blockers to improve outcomes in patients with CCS in the absence of MI in the past year, left ventricular ejection fraction ≤50%, or another primary indication for beta-blocker therapy. Meanwhile, CCB are considered as a first-line antianginal therapy, while in ESC guidelines these medications are poorly represented if compared with other therapeutic solutions, as we discussed before.
Another important point raised by ACC/AHA guidelines is represented by sodium glucose cotransporter 2 inhibitors (SGLT2i), which have been recently added in the guideline-directed medical therapy for HF, and glucagon-like peptide-1 receptor agonists (GLP1ra), which are suggested for those patients with type 2 diabetes mellitus and high risk or documented atherosclerotic CV disease (ASCVD) (111). These categories of medications are not included in ESC guidelines for CCS.
Another crucial point in CCS is long-term follow up, especially in the functional and anatomic assessment of the coronary blood flow. In fact, ESC guidelines are not very clear about testing in patients known for CAD, and do not differentiate the follow-up times and modalities between the six phenotypes included in the CCS spectrum. The 2019 ESC guidelines suggest performing early echocardiography at rest after revascularization, which must be repeated periodically (every 3-5 years). Stress tests for inducible ischemia are indicated early after revascularization (1-3 months), and “as necessary” in those patients who are symptomatic even after optimization of medical therapy, or periodically (every 3-5 years) to reassess ischemia. Invasive coronary angiography IC) is indicated in those patients known for CAD which are considered as “high risk”.
On the other hand, ACC/AHA guidelines suggest deferring testing after the optimization of medical therapy, and do not recommend routine periodic anatomic or ischemic testing without a change in clinical or functional status in patients known with CCS.
Invasive coronary angiography is indicated in those patients with CCS and a change in symptoms or functional capacity that persists despite guideline directed medical therapy (Class I LOE B-R), regardless of the ASCVD risk.
On the other hand, ESC guidelines propose performing invasive coronary angiography in those patients who are known for CCS which are considered as “high risk profile”, or those patients known as CCS, which result positive for inducible ischemia during a stress test.
The point is that ESC guidelines tend to be less prone to perform coronary angiography if the patient does not have a high-risk profile, but at the same time, there is not a clear definition for “high risk CAD”. Instead, ACC/AHA has defined in 2023 guidelines with more accuracy the potential features associated with a higher risk of MACE among patients with CCS and proposed a definition of “very-high risk” of future ASCVD events. A “very-high risk” patient is defined as someone who experienced multiple ASCVD events, or one major ASCVD event (recent ACS, MI, ischemic stroke, PAD) in addition to at least two high-risk conditions (age >65 years, familial hypercholesterolemia, history of previous coronary bypass surgery or PCI, diabetes, hypertension, chronic kidney disease, smoking, persistently high levels of LDL-C (>100 mg/dL) despite maximally tolerated statin therapy and ezetimibe, history of congestive HF).
Conclusions
CCS represents a wide spectrum of diseases, which consequently incorporates different kind of patients. However, different clinical situations are still classified as one, even if they have significant differences. The indications for medical therapy, revascularization and long-term management should be differentiated based on the features of the patients.
We need for the new ESC guidelines for CCS to clarify the risk profile of the patients and to precisely direct the patients to specific therapeutic paths and follow-ups. Furthermore, we think that it would be more appropriate to differentiate the six phenotypes described by ESC guidelines in terms of therapeutic management and follow-up.
Peer-review: External and internal
Conflict of interest: Giuseppe Biondi-Zoccai has consulted for Aleph, Amarin, Balmed, Cardionovum,
Crannmedical, Endocore Lab, Eukon, Guidotti, Innovheart, Meditrial, Menarini, Microport, Opsens
Medical, Terumo, and Translumina, outside the present work. All other authors report no conflict of interest.
Authorship: D. M., G. F., A.E., G. S., P.S., G.B.-Z., and M.B. equally contributed to the manuscript preparation and fulfilled authorship criteria
Acknowledgements and funding: None to declare
Statement on A.I.-assisted technologies use: Authors declare that they did not use AI-assisted technologies in preparation of this manuscript
References
| 1.Brown JC, Gerhardt, TE, Kwon E, Risk factors for coronary artery disease. 2023 Jam 23. In: Statpearls. Treasure Island (FL); Statpearls publishing: 2024. | ||||
| 2.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019; 40: 237-69. Doi: 10.1093/eurheartj/ehy462 https://doi.org/10.1093/eurheartj/ehy462 PMid:30165617 |
||||
| 3.Knuuti, J., Wijns, W., Saraste, A., Capodanno, D., Barbato, E., Funck-Brentano, C., et al. (2020). 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020; 41: 407-77. Doi: 10.1093/eurheartj/ehz425 https://doi.org/10.1093/eurheartj/ehz425 PMid:31504439 |
||||
| 4.Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, Demeter SH, et al. AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023; 148: doi:.1161/CIR.0000000000001168 https://doi.org/10.1161/CIR.0000000000001195 |
||||
| 5.Cacciatore S, Spadafora L, Bernardi M, Galli M, Betti M, et al. Management of coronary artery disease in older adults: recent advances and gaps in evidence. J Clin Med 2023; 12: 5233. Doi: 10.3390/jcm12165233 https://doi.org/10.3390/jcm12165233 PMid:37629275 PMCid:PMC10455820 |
||||
| 6.Yeh RW, Kereiakes DJ, Steg PG, Windecker S, Rinaldi MJ, Gershlick AH, Cutlip DE, et al; DAPT Study Investigators. Benefits and risks of extended duration dual antiplatelet therapy after PCI in patients with and without acute myocardial infarction. J Am Coll Cardiol 2015; 65: 2211-21. doi: 10.1016/j.jacc.2015.03.003 https://doi.org/10.1016/j.jacc.2015.03.003 PMid:25787199 PMCid:PMC4678101 |
||||
| 7.Perone F, Bernardi M, Redheuil A, Mafrica D, Conte E, Spadafora L, et al. Role of cardiovascular imaging in risk assessment: recent advances, gaps in evidence, and future directions. J Clin Med 2024; 12: 5563. 10.3390/jcm12175563 https://doi.org/10.3390/jcm12175563 PMid:37685628 PMCid:PMC10487991 |
||||
| 8.Tremmel JA, Fearon FW. Is post percutaneous coronary intervention fractional flow reserve of value in chronic total occlusions? Circ Cardiovasc Res 2018; 11: e700360. https://doi.org/10.1161/CIRCINTERVENTIONS.118.007360 PMid:30571221 |
||||
| 9.Guieu R, Deharo J-C, Maille B, Crotti L, Torresani E, Brignole M, et al. . Adenosine and the cardiovascular system: the good and the bad. J Clin Med 2020; 9: 1366. Doi: 10.3390/jcm9051366 https://doi.org/10.3390/jcm9051366 PMid:32384746 PMCid:PMC7290927 |
||||
| 10.Kerndt CC, Nagalli S. Dipyridamole. (Updated 2023 Jul 3). In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2024. Available from: URL: https://www.ncbi.nlm.nih.gov/books/NBK554455/ | ||||
| 11.Lim YC, Teo S-G, Poh K-K. ST-segment changes with exercise stress. Singapore Med J 2016; 57: 347-53. doi: 10.11622/smedj.2016116 https://doi.org/10.11622/smedj.2016116 PMid:27440279 PMCid:PMC4958709 |
||||
| 12.Hamilton MD, Ezeh E, Suliman M, Saylor J, Thompson E. Stress test-induced left bundle branch block. Cureus 2021; 13: e17384. Doi: 10.7759/cureus.17384 https://doi.org/10.7759/cureus.17384 |
||||
| 13.Kosaraju A, Muppidi V, Makaryus AN. Stress echocardiography. (Updated 2023 Jul 25). In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2024 . Available from: URL: https://www.ncbi.nlm.nih.gov/books/NBK448062/. | ||||
| 14.Yoshida K, Tanabe Y, Hosokawa T, Morikawa T, Fukuyama N, Kobayashi Y, et al. Coronary computed tomography angiography for clinical practice. Japan J Radiol 2024; 42: 555-80. Doi: 10.1007/s11604-024-01543-1 https://doi.org/10.1007/s11604-024-01543-1 PMid:38453814 PMCid:PMC11139719 |
||||
| 15.Giusca S, Schütz M, Kronbach F, Wolf D, Nunninger P, Korosoglou G. Coronary computer tomography angiography in 2021-acquisition protocols, tips and tricks and heading beyond the possible. Diagnostics (Basel, Switzerland) 2021; 11: doi: 10.3390/diagnostics11061072 https://doi.org/10.3390/diagnostics11061072 PMid:34200866 PMCid:PMC8230532 |
||||
| 16.Mafrica D, Spadafora, L, Galanti K, Biondi-Zoccai G, Bernardi M. The role of coronary physiology in the management of percutaneous coronary intervention: Insights from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Consensus. Heart Vessels Transplant 2023; 7: doi: 10.24969/hvt.2023.416 https://doi.org/10.24969/hvt.2023.416 |
||||
| 17.Manda YR, Baradhi KM. Cardiac catheterization risks and complications. (Updated 2023 Jun 5). In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2024. Available from: URL: https://www.ncbi.nlm.nih.gov/books/NBK531461/. | ||||
| 18.Notghi A, Low CS. Myocardial perfusion scintigraphy: past, present and future. Br J Radiol 2011; 84 (Spec Iss 3): S229-36. Doi: 10.1259/bjr/14625142 https://doi.org/10.1259/bjr/14625142 PMid:22723530 PMCid:PMC3473917 |
||||
| 19.Koumna S, Yiannakkaras C, Avraamides P, Demetriadou O. Specificity and sensitivity of SPECT myocardial perfusion studies at the Nuclear Medicine Department of the Limassol General Hospital in Cyprus. J Physics: Conference Series, 2011; 317: 012024. doi: 10.1088/1742-6596/317/1/012024 https://doi.org/10.1088/1742-6596/317/1/012024 |
||||
| 20.Ross R. The pathogenesis of atherosclerosis - an update. N Engl J Med 1986; 314: 488-500. Doi: 10.1056/NEJM198602203140806 https://doi.org/10.1056/NEJM198602203140806 PMid:3511384 |
||||
| 21.Mehta SR, Yusuf S. Short- and long-term oral antiplatelet therapy in acute coronary syndromes and percutaneous coronary intervention. J Am Coll CArdiol 2003; 41: S79-S88. Doi: 10.1016/S0735-1097(02)02831-0 https://doi.org/10.1016/S0735-1097(02)02831-0 PMid:12644345 |
||||
| 22.Trip MD, Cats VM, van Capelle FJL, Vreeken J. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N Engl J Med 1990; 322: 1549-54. doi:10.1056/NEJM199005313222201 https://doi.org/10.1056/NEJM199005313222201 PMid:2336086 |
||||
| 23.Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events. JAMA 2019; 321: 277. doi:10.1001/jama.2018.20578 https://doi.org/10.1001/jama.2018.20578 PMid:30667501 PMCid:PMC6439678 |
||||
| 24.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. Explanation of postprocedural fractional flow reserve below 0.85: A comprehensive ultrasound analysis of the FFR SEARCH Registry. Circ Cardiovasc Interv 2019; 12: e007030. doi: 10.1161/CIRCINTERVENTIONS.118.007030 https://doi.org/10.1161/CIRCINTERVENTIONS.118.007030 PMid:30732469 |
||||
| 25.Antithrombotic Trialists' (ATT) Collaboration. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373: 1849-60. doi:10.1016/S0140-6736(09)60503-1 https://doi.org/10.1016/S0140-6736(09)60503-1 PMid:19482214 |
||||
| 26.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996; 348: 1329-39. doi:10.1016/s0140-6736(96)09457-3 https://doi.org/10.1016/S0140-6736(96)09457-3 PMid:8918275 |
||||
| 27.Mauri L, Kereiakes, DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, et al. DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371: 2155-66. doi:10.1056/NEJMoa1409312 https://doi.org/10.1056/NEJMoa1409312 PMid:25399658 PMCid:PMC4481318 |
||||
| 28.Storey RF, Angiolillo DJ, Bonaca MP, Thomas MR, Judge HM, Rollini F, et al. Platelet Inhibition With Ticagrelor 60 mg Versus 90 mg Twice Daily in the PEGASUS-TIMI 54 Trial. J Am Coll Cardiol 2016; 67: 1145-54. doi:10.1016/j.jacc.2015.12.062 https://doi.org/10.1016/j.jacc.2015.12.062 PMid:26965534 |
||||
| 29.Bhatt DL, Eikelboom JW, Connolly SJ, Steg PG, Anand SS, Verma S, et al. COMPASS Steering Committee and Investigators. Role of combination antiplatelet and anticoagulation therapy in diabetes mellitus and cardiovascular disease: Insights from the COMPASS Trial. Circulation 2020; 141: 1841-54. doi:10.1161/CIRCULATIONAHA.120.046448 https://doi.org/10.1161/CIRCULATIONAHA.120.046448 PMid:32223318 PMCid:PMC7314494 |
||||
| 30.Fox KAA, Eikelboom JW, Shestakovska O, Connolly SJ, Metsarinne K P, & Yusuf, S. Rivaroxaban Plus Aspirin in Patients With Vascular Disease and Renal Dysfunction: From the COMPASS Trial. J Am Coll Cardiol 2019; 73: 2243-50. doi:10.1016/j.jacc.2019.02.048 https://doi.org/10.1016/j.jacc.2019.02.048 PMid:31072566 |
||||
| 31.Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, et al. COMPASS Investigators. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med 2017; 377: 1319-30. doi:10.1056/NEJMoa1709118 https://doi.org/10.1056/NEJMoa1709118 PMid:28844192 |
||||
| 32.Lip GYH, Collet JP, Haude M, Byrne R, Chung EH, Fauchier L, et al. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Europace 2019; 21: 192 -3. https://doi.org/10.1093/europace/euy174 PMid:30052888 |
||||
| 33.Orme RC, Parker WAE, Thomas MR, Judge HM, Baster K, Sumaya W, et al. Study of Two Dose Regimens of Ticagrelor Compared With Clopidogrel in Patients Undergoing Percutaneous Coronary Intervention for Stable Coronary Artery Disease. Circulation 2018; 138: 1290-300. doi:10.1161/CIRCULATIONAHA.118.034790 https://doi.org/10.1161/CIRCULATIONAHA.118.034790 PMid:29930021 PMCid:PMC6159686 |
||||
| 34.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. IMPROVE-IT Investigators. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med 2015; 372: 2387-97. doi:10.1056/NEJMoa1410489 https://doi.org/10.1056/NEJMoa1410489 PMid:26039521 |
||||
| 35.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. PLATO Investigators, Freij, A, & Thorsén, Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2019; 361: 1045-57. doi:10.1056/NEJMoa0904327 https://doi.org/10.1056/NEJMoa0904327 PMid:19717846 |
||||
| 36.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357: 2001-15. doi:10.1056/NEJMoa0706482 https://doi.org/10.1056/NEJMoa0706482 PMid:17982182 |
||||
| 37.Biondi-Zoccai G, Lotrionte M, Agostoni P, Abbate A, Romagnoli E, Sangiorgi G, et al. Adjusted indirect comparison meta-analysis of prasugrel versus ticagrelor for patients with acute coronary syndromes. Int J Cardiol2011; 150: 325-31. doi:10.1016/j.ijcard.2010.08.035 https://doi.org/10.1016/j.ijcard.2010.08.035 PMid:20828843 |
||||
| 38. Korkushko O, Ivanov LA, Pisaruk AV, LAsistsaTS. Mechanisms of the effect of propranolol on the oxygen transport function of the blood in elderly patients with ischemic heart diseases. Kardiologiia 1986. 26, 54-7. | ||||
| 39.Kralev S, Schneider K, Lang S, Süselbeck T, Borggrefe M.. Incidence and severity of coronary artery disease in patients with atrial fibrillation undergoing first-time coronary angiography. PloS One 2011; 6: e24964. doi:10.1371/journal.pone.0024964 https://doi.org/10.1371/journal.pone.0024964 PMid:21957469 PMCid:PMC3177852 |
||||
| 40.Ueki Y, Bär S, Losdat S, Otsuka T, Zanchin C, Zanchin T, et al. Validation of the Academic Research Consortium for High Bleeding Risk (ARC-HBR) criteria in patients undergoing percutaneous coronary intervention and comparison with contemporary bleeding risk scores. EuroIntervention 2020; 16: 371-9. doi:10.4244/EIJ-D-20-00052 https://doi.org/10.4244/EIJ-D-20-00052 PMid:32065586 |
||||
| 41.Uren NG, Schwarzacher SP, Metz JA, Lee DP, Honda Y, Yeung AC, et al. POST Registry Investigators. Predictors and outcomes of stent thrombosis: an intravascular ultrasound registry. Eur Heart J 2002; 23: 124-32. doi:10.1053/euhj.2001.2707 https://doi.org/10.1053/euhj.2001.2707 PMid:11785994 |
||||
| 42.Fiedler KA, Maeng M, Mehilli J, Schulz-Schüpke S, Byrne RA, Sibbing D, et al. Duration of Triple Therapy in Patients Requiring Oral Anticoagulation After Drug-Eluting Stent Implantation: The ISAR-TRIPLE Trial. J Am Coll Cardiol 2015: 65: 1619-29. doi:10.1016/j.jacc.2015.02.050 https://doi.org/10.1016/j.jacc.2015.02.050 PMid:25908066 |
||||
| 43.Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, et al. AUGUSTUS Investigators. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med 2019; 380: 1509-24. doi:10.1056/NEJMoa1817083 https://doi.org/10.1056/NEJMoa1817083 PMid:30883055 |
||||
| 44.Agewall S, Cattaneo M, Collet JP, Andreotti F, Lip GYH, Verheugt FWA, et al. ESC Working Group on Cardiovascular Pharmacology and Drug Therapy and ESC Working Group on Thrombosis. Expert position paper on the use of proton pump inhibitors in patients with cardiovascular disease and antithrombotic therapy. Eur Heart J 2013; 34: 1708-13. doi:10.1093/eurheartj/eht042 https://doi.org/10.1093/eurheartj/eht042 PMid:23425521 |
||||
| 45.Abukhalil AD, Al Sheikh T, Muallem S, Al-Shami N, Naseef HA. Prevalence and safety of prescribing PPIS with clopidogrel in palestine. Patient Pref Adher 2023; 17: 749-59. doi:10.2147/PPA.S404139 https://doi.org/10.2147/PPA.S404139 PMid:36970301 PMCid:PMC10038207 |
||||
| 46.Nishi K, Itabe H, Uno M, Kitazato KT, Horiguchi H, Shinno K, et al. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler Thromb Vasc Biol 2022; 22, 1649-54. doi:10.1161/01.ATV.0000033829.14012.18 https://doi.org/10.1161/01.ATV.0000033829.14012.18 PMid:12377744 |
||||
| 47.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020; 41: 111-88. doi:10.1093/eurheartj/ehz455 https://doi.org/10.1093/eurheartj/ehz455 PMid:31504418 |
||||
| 48.Liao JK, Laufs U. Pleiotropic effects of statins. Ann Rev Pharmacol Toxicol 2005; 45: 89-118. doi:10.1146/annurev.pharmtox.45.120403.095748 https://doi.org/10.1146/annurev.pharmtox.45.120403.095748 PMid:15822172 PMCid:PMC2694580 |
||||
| 49. Zhai C, Cong H, Liu Y, Zhang Y, Liu X, Zhang H, Ren Z. Effect of high-dose statin pretreatment on the incidence of periprocedural myocardial infarction in patients undergoing percutaneous coronary intervention: Grading the evidence through a cumulative meta-analysis. Clin Cardiol2015; 38: 668-78. doi:10.1002/clc.22471 https://doi.org/10.1002/clc.22471 PMid:26442621 PMCid:PMC6490761 |
||||
| 50.Cannon CP, Husted S, Harrington RA, Scirica BM, Emanuelsson H, Peters G, et al. DISPERSE-2 Investigators. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol 2007; 50: 1844-51. doi:10.1016/j.jacc.2007.07.053 https://doi.org/10.1016/j.jacc.2007.07.053 PMid:17980250 |
||||
| 51.Giugliano RP, Cannon CP, Blazing MA, Nicolau JC, Corbalán R, Špinar J, et al. IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) Investigators. Benefit of adding ezetimibe to statin therapy on cardiovascular outcomes and safety in patients with versus without diabetes mellitus: Results from IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation 2018; 137: 1571-82. doi:10.1161/CIRCULATIONAHA.117.030950 https://doi.org/10.1161/CIRCULATIONAHA.117.030950 PMid:29263150 |
||||
| 52.Schmidt AF, Carter, JPL, Pearce LS, Wilkins JT, Overington JP, Hingorani AD, et al. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane System Rev 2020; 10: CD011748. doi:10.1002/14651858.CD011748.pub3 https://doi.org/10.1002/14651858.CD011748.pub3 PMid:33078867 PMCid:PMC8094613 |
||||
| 53.Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med 2020; 382: 1507-19. doi:10.1056/NEJMoa1912387 https://doi.org/10.1056/NEJMoa1912387 PMid:32187462 |
||||
| 54.Ruscica M, Banach M, Sahebkar A, Corsini A, Sirtori CR. ETC-1002 (Bempedoic acid) for the management of hyperlipidemia: from preclinical studies to phase 3 trials. Expert Opin Pharmacother 2019; 20: 791-803. doi: 10.1080/14656566.2019.1583209 https://doi.org/10.1080/14656566.2019.1583209 PMid:30810432 |
||||
| 55.Petrosyan AS, Rud RS, Polyakov PP, Kade AKh, Zanin SA. The pathogenetic basis of the action of bempedoic acid. Ration Pharmacother Cardiol 2023; 18: 734-41. doi: 10.20996/1819-6446-2022-12-11 https://doi.org/10.20996/1819-6446-2022-12-11 |
||||
| 56.Banach M, Penson PE, Farnier M, Fras Z, Latkovskis G, Laufs U, et al. Bempedoic acid in the management of lipid disorders and cardiovascular risk. 2023 position paper of the International Lipid Expert Panel (ILEP). Progress Cardiovasc Dis 2023; 79, 2-11. doi:10.1016/j.pcad.2023.03.001 https://doi.org/10.1016/j.pcad.2023.03.001 PMid:36889490 |
||||
| 57.Patel A. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370: 829-40. doi:10.1016/S0140-673661303-8 https://doi.org/10.1016/S0140-6736(07)61303-8 PMid:17765963 |
||||
| 58.Flather MD, Yusuf S, Køber L, Pfeffer M, Hall A, Murray G, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. Lancet 2000; 355: 1575-81. doi:10.1016/S0140-673602212-1 https://doi.org/10.1016/S0140-6736(00)02212-1 PMid:10821360 |
||||
| 59.Dagenais GR, Pogue J, Fox K, Simoons ML, Yusuf S. Angiotensin-converting-enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: a combined analysis of three trials. Lancet 2006; 368: 581-8. doi:10.1016/S0140-673669201-5 https://doi.org/10.1016/S0140-6736(06)69201-5 PMid:16905022 |
||||
| 60.Freemantle N, Cleland J, Young P, Mason J, Harrison J. Beta-blockade after myocardial infarction: systematic review and meta regression analysis. BMJ 1999; 318: 1730-7. doi:10.1136/bmj.318.7200.1730 https://doi.org/10.1136/bmj.318.7200.1730 PMid:10381708 PMCid:PMC31101 |
||||
| 61.Leizorovicz A, Lechat P, Cucherat M, Bugnard F. Bisoprolol for the treatment of chronic heart failure: A meta-analysis on individual data of two placebo-controlled studies-CIBIS and CIBIS II. Am Heart J 2002; 143: 301. doi:10.1067/mhj.2002.120768 https://doi.org/10.1067/mhj.2002.120768 PMid:11835035 |
||||
| 62.The beta-blocker heart attack trial. Beta-Blocker Heart Attack Study Group. JAMA 1981; 246: 2073-4. https://doi.org/10.1001/jama.246.18.2073 https://doi.org/10.1001/jama.1981.03320180063037 |
||||
| 63.Kaddoura R, Madurasinghe V, Chapra A, Abushanab D, Al-Badriyeh D, Patel A. Beta-blocker therapy in heart failure with preserved ejection fraction (B-HFpEF): A systematic review and meta-analysis. Curr Probl Cardiol 2024; 49: 102376. doi:10.1016/j.cpcardiol.2024.102376 https://doi.org/10.1016/j.cpcardiol.2024.102376 PMid:38184132 |
||||
| 64.Liu F, Chen Y, Feng X, Teng Z, Yuan Y, Bin J. Effects of beta-blockers on heart failure with preserved ejection fraction: A meta-analysis. PLoS ONE 2014; 9: e90555. doi:10.1371/journal.pone.0090555 https://doi.org/10.1371/journal.pone.0090555 PMid:24599093 PMCid:PMC3944014 |
||||
| 65.Yndigegn T, Lindahl B, Mars K, Alfredsson J, Benatar J, Brandin L, et al. Beta-blockers after myocardial infarction and preserved ejection fraction. N Engl J Med 2024; 390: 1372-81. doi:10.1056/NEJMoa2401479 https://doi.org/10.1056/NEJMoa2401479 PMid:38587241 |
||||
| 66.Tulloh R. Etiology, diagnosis, and pharmacologic treatment of pediatric pulmonary hypertension. Pediatr Drugs 2009; 11: 115-28. doi:10.2165/00148581-200911020-00003 https://doi.org/10.2165/00148581-200911020-00003 PMid:19301933 |
||||
| 67.Tousoulis D, Kampoli AM, Tentolouris Nikolaos Papageorgiou C, Stefanadis C. The Role of nitric oxide on endothelial function. Curr Vasc Pharmacol 2012; 10: 4-18. doi:10.2174/157016112798829760 https://doi.org/10.2174/157016112798829760 PMid:22112350 |
||||
| 68.Thadani U, Lipicky RJ. Short and long-acting oral nitrates for stable angina pectoris. Cardiovasc Drugs Ther 1994; 8: 611-23. doi:10.1007/BF00877415 https://doi.org/10.1007/BF00877415 PMid:7848896 |
||||
| 69.Wei J, Wu T, Yang Q, Chen M, Ni J, Huang D. . Nitrates for stable angina: A systematic review and meta-analysis of randomized clinical trials. Int J Cardiol 2011; 146: 4-12. doi:10.1016/j.ijcard.2010.05.019 https://doi.org/10.1016/j.ijcard.2010.05.019 PMid:20557963 |
||||
| 70.Thadani U, Rodgers T. Side effects of using nitrates to treat angina. Exp Opinion Drug Safety 2006; 5: 667-74. doi:10.1517/14740338.5.5.667 https://doi.org/10.1517/14740338.5.5.667 PMid:16907656 |
||||
| 71.Badran HM, Ibrahim WA, Faheem N, Yassin R, Alashkar T, Yacoub M. Provocation of left ventricular outflow tract obstruction using nitrate inhalation in hypertrophic cardiomyopathy: Relation to electromechanical delay. Global Cardiol Sci Pract 2015; 15: doi:10.5339/gcsp.2015.15 https://doi.org/10.5339/gcsp.2015.15 PMid:26779503 PMCid:PMC4448073 |
||||
| 72. Webb DJ, Muirhead GJ, Wulff M, Sutton JA, Levi R, Dinsmore WW. Sildenafil citrate potentiates the hypotensive effects of nitric oxide donor drugs in male patients with stable angina. J Am Coll Cardiol 2000; 36: 25-31. doi:10.1016/S0735-109700705-1 https://doi.org/10.1016/S0735-1097(00)00705-1 PMid:10898408 |
||||
| 73.Boudonas GE. β-Blockers in coronary artery disease management. Hippokratia 2010; 14: 231-5. | ||||
| 74.Chiariello M, Ribeiro LG, Davis MA, Maroko PR. "Reverse coronary steal" induced by coronary vasoconstriction following coronary artery occlusion in dogs. Circulation 1977; 56: 809-15. doi:10.1161/01.CIR.56.5.809 https://doi.org/10.1161/01.CIR.56.5.809 PMid:912843 |
||||
| 75.Ardissino D, Savonitto S, Egstrup K, Rasmussen K, Bae EA, Omland T, et al. Selection of medical treatment in stable angina pectoris: Results of the International Multicenter Angina Exercise (IMAGE) study. J Am Coll Cardiol 1995; 25, 1516-21. doi:10.1016/0735-109700042-3 https://doi.org/10.1016/0735-1097(95)00042-3 PMid:7759701 |
||||
| 76.Sueta D, Tabata N, Hokimoto S. Clinical roles of calcium channel blockers in ischemic heart diseases. Hypert Res 2017; 40: 423-8. doi:10.1038/hr.2016.183 https://doi.org/10.1038/hr.2016.183 PMid:28123178 |
||||
| 77.Rehnqvist N, Hjemdahl P, Billing E, Bjorkander I, Eriksson SV, Forslund L, et al. Effects of metoprolol vs verapamil in patients with stable angina pectoris: The angina prognosis study in Stockholm (APSIS). Eur Heart J 1996; 17: 76-81. doi:10.1093/oxfordjournals.eurheartj.a014695 https://doi.org/10.1093/oxfordjournals.eurheartj.a014695 PMid:8682134 |
||||
| 78.Wing LMH. Calcium channel antagonists. Austral Prescr 1997; 20: 5-8. doi:10.18773/austprescr.1997.004 https://doi.org/10.18773/austprescr.1997.004 |
||||
| 70.Kannam JAJGB. Calcium channel blockers in the management of stable angina pectoris. UpToDate. 2010. | ||||
| 80.Poole-Wilson PA, Lubsen J, Kirwan B-A, van Dalen FJ, Wagener G, Danchin N, et al. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet 2004; 364: 849-57. doi:10.1016/S0140-673616980-8 https://doi.org/10.1016/S0140-6736(04)16980-8 PMid:15351192 |
||||
| 81.Frishman WH, Glasser S, Stone P, Deedwania PC, Johnson M, Fakouhi TD. Comparison of controlled-onset, extended-release verapamil with amlodipine and amlodipine plus atenolol on exercise performance and ambulatory ischemia in patients with chronic stable angina pectoris. Am J Cardiol 1999; 83: 507-14. doi:10.1016/S0002-914900904-7 https://doi.org/10.1016/S0002-9149(98)00904-7 PMid:10073852 |
||||
| 82.Nissen SE, Tuzcu EM, Libby P, Thompson PD, Ghali M, Garza D, et al. CAMELOT Investigators. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure. jAMA 2004; 292: 2217. doi:10.1001/jama.292.18.2217 https://doi.org/10.1001/jama.292.18.2217 PMid:15536108 |
||||
| 83.Pijls NH, de Bruyne B, Peels K, van der Voort PH, Bonnier HJ, Bartunek J, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 1996; 334: 1703-8. doi: 10.1056/NEJM199606273342604 https://doi.org/10.1056/NEJM199606273342604 PMid:8637515 |
||||
| 84.Tonino PAL, Siebert U, Manoharan G, MacCarthy PA. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009; 360: 213-24. doi:10.1056/NEJMoa0807611 https://doi.org/10.1056/NEJMoa0807611 PMid:19144937 |
||||
| 85.Pham V, Moroni A, Gall E, Benedetti A, Zivelonghi C, Picard F. Revascularization and medical therapy for chronic coronary syndromes: Lessons learnt from recent trials, a literature review. J Clin Med 2023; 12: 28-33. doi: 10.3390/jcm12082833 https://doi.org/10.3390/jcm12082833 PMid:37109169 PMCid:PMC10141707 |
||||
| 86.Xaplanteris P, Fournier S, Pijls NH, Fearon WF, Barbato E, Tonino PA, et al. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med 2018; 379: 250-9. doi: 10.1056/NEJMoa1803538 https://doi.org/10.1056/NEJMoa1803538 PMid:29785878 |
||||
| 87.Collison D, Didagelos M, Aetesam-Ur-Rahman M, Copt S, McDade R, McCartney P, et al. Post-stenting fractional flow reserve vs coronary angiography for optimization of percutaneous coronary intervention (TARGET-FFR). Eur Heart J 2021; 42: 4656-68. doi: 10.1093/eurheartj/ehab449 https://doi.org/10.1093/eurheartj/ehab449 PMid:34279606 PMCid:PMC8634564 |
||||
| 88.Piroth Z, Toth GG, Tonino PA, Barbato E, Aghlmandi S, Curzen N, et al. Prognostic value of fractional flow reserve measured immediately after drug-eluting stent implantation. Circ Cardiovasc Interv 2017; 10: e005233. doi: 10.1161/CIRCINTERVENTIONS.116.005233 https://doi.org/10.1161/CIRCINTERVENTIONS.116.005233 PMid:28790165 |
||||
| 89.Fournier S, Ciccarelli G, Toth GG, Milkas A, Xaplanteris P, Tonino PAL, et al. Association of improvement in fractional flow reserve with outcomes, including symptomatic relief, after percutaneous coronary intervention. JAMA Cardiol 2019; 4: 370-4. doi: 10.1001/jamacardio.2019.0175 https://doi.org/10.1001/jamacardio.2019.0175 PMid:30840026 PMCid:PMC6484787 |
||||
| 90.van Bommel RJ, Masdjedi K, Diletti R, Lemmert ME, van Zandvoort L, Wilschut J, et al. Routine fractional flow reserve measurement after percutaneous coronary intervention: The FFR-SEARCH Study. Circ Cardiovasc Interv 2019; 12: e007428. doi: 10.1161/CIRCINTERVENTIONS.118.007428 https://doi.org/10.1161/CIRCINTERVENTIONS.118.007428 PMid:31018666 |
||||
| 91.Hamzaraj K, Kammerlander A, Gyöngyösi M, Frey B, Distelmaier K, Graf S. Patient selection and clinical indication for chronic total occlusion revascularization-a workflow focusing on non-invasive cardiac imaging. Life 2022; 13: doi:10.3390/life13010004 https://doi.org/10.3390/life13010004 PMid:36675954 PMCid:PMC9864679 |
||||
| 92.Opolski MP, Achenbach S, Schuhbäck A, Rolf A, Möllmann H, Nef H, et al. Coronary computed tomographic prediction rule for time-efficient guidewire crossing through chronic total occlusion: insights from the CT-RECTOR multicenter registry (Computed Tomography Registry of Chronic Total Occlusion Revascularization). JACC Cardiovasc Interv 2015; 8: 257-67. doi:10.1016/j.jcin.2014.07.031 https://doi.org/10.1016/j.jcin.2014.07.031 PMid:25700748 |
||||
| 93. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, et al. ESC National Cardiac Societies. ESC Scientific documents group. ESC guidelines on cardiovascular prevention in clinical practice. Heart J 2021; 42: 3227-337. doi:10.1093/eurheartj/ehab484 https://doi.org/10.1093/eurheartj/ehab484 PMid:34458905 |
||||
| 94.Yu C-W, Lee H-J, Suh J, Lee N-H, Park S-M, Park TK, et al. Coronary Computed tomography angiography predicts guidewire crossing and success of percutaneous intervention for chronic total occlusion: Korean multicenter CTO CT registry score as a tool for assessing difficulty in chronic total occlusion percutaneous coronary intervention. Circulation Cardiovasc Imag 2017; 10: doi:10.1161/CIRCIMAGING.116.005800 https://doi.org/10.1161/CIRCIMAGING.116.005800 PMid:28389507 |
||||
| 95.Rinfret S, Ribeiro HB, Nguyen CM, Nombela-Franco L, Urena M, Rodes-Cabau J. Dissection and re-entry techniques and longer-term outcomes following successful percutaneous coronary intervention of chronic total occlusion. Am J Cardiol 2014; 114: 1354-60. https://doi.org/10.1016/j.amjcard.2014.07.067 PMid:25242364 |
||||
| 96.Rangan BV, Kotsia A, Christopoulos G, Spratt J, Rinfret S, Banerjee S, Brilakis ES. The hybrid approach to intervention of chronic total occlusions. Curr Cardiol Rev 2015; 11: 299-304. doi:10.2174/1573403X11666150909113026 https://doi.org/10.2174/1573403X11666150909113026 PMid:26354507 PMCid:PMC4774633 |
||||
| 97.Valenti R, Vergara R, Migliorini A, Parodi G, Carrabba N, Cerisano G, et al. Predictors of reocclusion after successful drug-eluting stent-supported percutaneous coronary intervention of chronic total occlusion. J Am Coll Cardiol 2013; 61: 545-50. https://doi.org/10.1016/j.jacc.2012.10.036 PMid:23273395 |
||||
| 98.Rinfret S, Dautov R. Radial or femoral approach for chronic total occlusion revascularization?: The answer is both. JACC Cardiovasc Interv 2017; 10: 244-46. https://doi.org/10.1016/j.jcin.2016.12.023 PMid:28183465 |
||||
| 99.Azzalini L, Dautov R, Brilakis ES, Ojeda S, Benincasa S, Bellini B, et al. Impact of crossing strategy on mid-term outcomes following percutaneous revascularisation of coronary chronic total occlusions. EuroIntervention 2017; 13: 978-85. https://doi.org/10.4244/EIJ-D-16-01010 PMid:28242587 |
||||
| 100.Dautov R, Nguyen CM, Altisent O, Gibrat C, Rinfret S. Recanalization of chronic total occlusions in patients with previous coronary bypass surgery and consideration of retrograde access via saphenous vein grafts. Circ Cardiovasc Interv 2016; 9: e003515. https://doi.org/10.1161/CIRCINTERVENTIONS.115.003515 PMid:27418611 |
||||
| 101.Dautov R, Urena M, Nguyen CM, Gibrat C, Rinfret S. Safety and effectiveness of the surfing technique to cross septal collateral channels during retrograde chronic total occlusion percutaneous coronary intervention. EuroIntervention 2017; 12: e1859-67. https://doi.org/10.4244/EIJ-D-16-00650 PMid:27802929 |
||||
| 102.Joyal D, Thompson CA, Grantham JA, Buller CE, Rinfret S. The retrograde technique for recanalization of chronic total occlusions: a step-by-step approach. JACC Cardiovasc Interv 2012; 5: 1-11. https://doi.org/10.1016/j.jcin.2011.10.011 PMid:22230144 |
||||
| 103.Christakopoulos GE, Christopoulos G, Karmpaliotis D, Alaswad K, Yeh RW, Jaffer FA, et al. Predictors of excess patient radiation exposure during chronic total occlusion coronary intervention: insights from a contemporary multicentre registry. Can J Cardiol 2017; 33: 478-84. https://doi.org/10.1016/j.cjca.2016.11.002 PMid:28169091 PMCid:PMC5367931 |
||||
| 104.Balter S, Brinkman M, Kalra S, Nazif T, Parikh M, Kirtane AJ, et al. Novel radiation dose reduction fluoroscopic technology facilitates chronic total occlusion percutaneous coronary interventions. EuroIntervention 2017; 13: e1468-74. https://doi.org/10.4244/EIJ-D-16-00216 PMid:28741573 |
||||
| 105. Sapontis J. Salisbury AC, Yeh RW. Cohen DJ, Hirai T, Lombardi W, et al. Early procedural and health status outcome of chronic total occlusion angioplasty: A report from OPEN-CTO registry (Outcomes, Patients Health Status and Efficiency in Chronic Total Occlusion Hybrid Procedures) JACC Cardiovasc Interv 2017; 10: 1523-34. | ||||
| 106.Jang WJ, Yang JH, Choi SH, Song YB, Hahn JY, Choi JH, et al. Long-term survival benefit of revascularization compared with medical therapy in patients with coronary chronic total occlusion and well-developed collateral circulation. JACC Cardiovasc Interv 2015; 8: 271-9. https://doi.org/10.1016/j.jcin.2014.10.010 PMid:25700750 |
||||
| 107.Tomasello SD, Boukhris M, Giubilato S, Marza F, Garbo R, Contegiacomo G, et al. Management strategies in patients affected by chronic total occlusions: results from the Italian Registry of Chronic Total Occlusions. Eur Heart J 2015; 36: 3189-98. https://doi.org/10.1093/eurheartj/ehv450 PMid:26333367 |
||||
| 108.Maron DJ, Hochman JS, O'Brien SM, Reynolds HR, Boden WE, Stone GW, et al. ISCHEMIA Trial Research Group. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. Am Heart J 2018; 201: 124-35. doi: 10.1016/j.ahj.2018.04.011 https://doi.org/10.1016/j.ahj.2018.04.011 PMid:29778671 PMCid:PMC6005768 |
||||
| 109.Hochman JS, Reynolds HR, Bangalore S, O'Brien S, Alexander KP, Senior R, et al. ISCHEMIA Research Group. Baseline Characteristics and Risk Profiles of Participants in the ISCHEMIA Randomized Clinical Trial. JAMA Cardiol 2019; 4: 273-86. doi: 10.1001/jamacardio.2019.0014 https://doi.org/10.1001/jamacardio.2019.0014 PMid:30810700 PMCid:PMC6439551 |
||||
| 110.Maron D, Hochman J, Reynolds H, Bangalore S, O1Brien SM, Boden WE, et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med 2020; 382: 1395-407. doi: 10.1056/nejmoa1915922 https://doi.org/10.1056/NEJMoa1915922 PMid:32227755 PMCid:PMC7263833 |
||||
| 111.Khan MS, Fonarow GC, McGuire DK, Hernandez AF, Vaduganathan M, Rosenstock J, et al. Glucagon-like peptide 1 receptor agonists and heart failure. Circulation 2020; 142: 1205-18. doi:10.1161/CIRCULATIONAHA.120.045888 https://doi.org/10.1161/CIRCULATIONAHA.120.045888 PMid:32955939 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

