Catheter-related right atrial thrombosis: a case series
ORIGINAL RESEARCH ARTICLE
Catheter-related right atrial thrombosis: a case series
Article Summary
- DOI: 10.24969/hvt.2024.516
- CARDIOVASCULAR DISEASES
- Published: 28/09/2024
- Received: 14/07/2024
- Revised: 10/09/2024
- Accepted: 11/09/2024
- Views: 6158
- Downloads: 2854
- Keywords: venous catheterization, right atrium, thrombosis, management, surgery, anticoagulation
Address for Correspondence: Raul Cruz Palomera, Instituto Mexicano Del Seguro Social, 2 Norte 2004 Col Centro, Puebla, Puebla, México
Email: Raulcp777@gmail.com Phone: 222 242 4520
ORCID: Raul Cruz Palomera - 0000-0002-1347-7858, Valencia González José Darío - 0009-0008-7529-9584
Twitter X: Raul Cruz Palomera @raulcp777_raul
Juan Francisco Rodríguez Alvarado, Raul Cruz Palomera, Eduardo Sánchez Cortés, Jorge Guillermo Arenas Fonseca, José Darío Valencia González
Instituto Mexicano Del Seguro Social, Puebla, Mexico
Abstract
Objective: Complications associated with central venous catheters (CVC), such as right atrial thrombosis, are a common but poorly recognized complication. Therefore, it is important to describe the follow-up and the various forms of treatment along with the responses associated with it. Delayed approach leads to a higher rate of complications, morbidity, and mortality. This case series presents three patients with catheter-related thrombosis.
Case Presentation: The initial patient was a 55-year-old male with short bowel syndrome who required parenteral nutrition through a CVC. Unfortunately, this was complicated by thrombosis. During the approach, the patient's condition remained stable, and he was managed with anticoagulation, with an adequate response.
The 2nd case involved a 56-year-old woman with chronic kidney disease (CKD) who underwent renal replacement therapy (RRT) with hemodialysis. However, her condition deteriorated due to thrombus formation in the Mahurkar catheter, resulting in obstruction of the tricuspid valve. Consequently, she underwent surgical thrombectomy, which yielded a favorable outcome.
The 3rd patient was a 31-year-old male with CKD and RRT via hemodialysis, complicated by thrombus formation in the Mahurkar catheter. He remained hemodynamically stable and was treated with anticoagulation therapy alone, with a favorable resolution.
Conclusions: Despite the absence of a uniform approach to treatment, the selection of an appropriate strategy and the management of these patients must be based on the experience and expertise of the medical center, the characteristics of the thrombus, and the patient's hemodynamic status. It is therefore essential to highlight the use of various treatments in our series, which were adapted to the characteristics of the cases and led to satisfactory clinical outcomes.
Graphical abstract
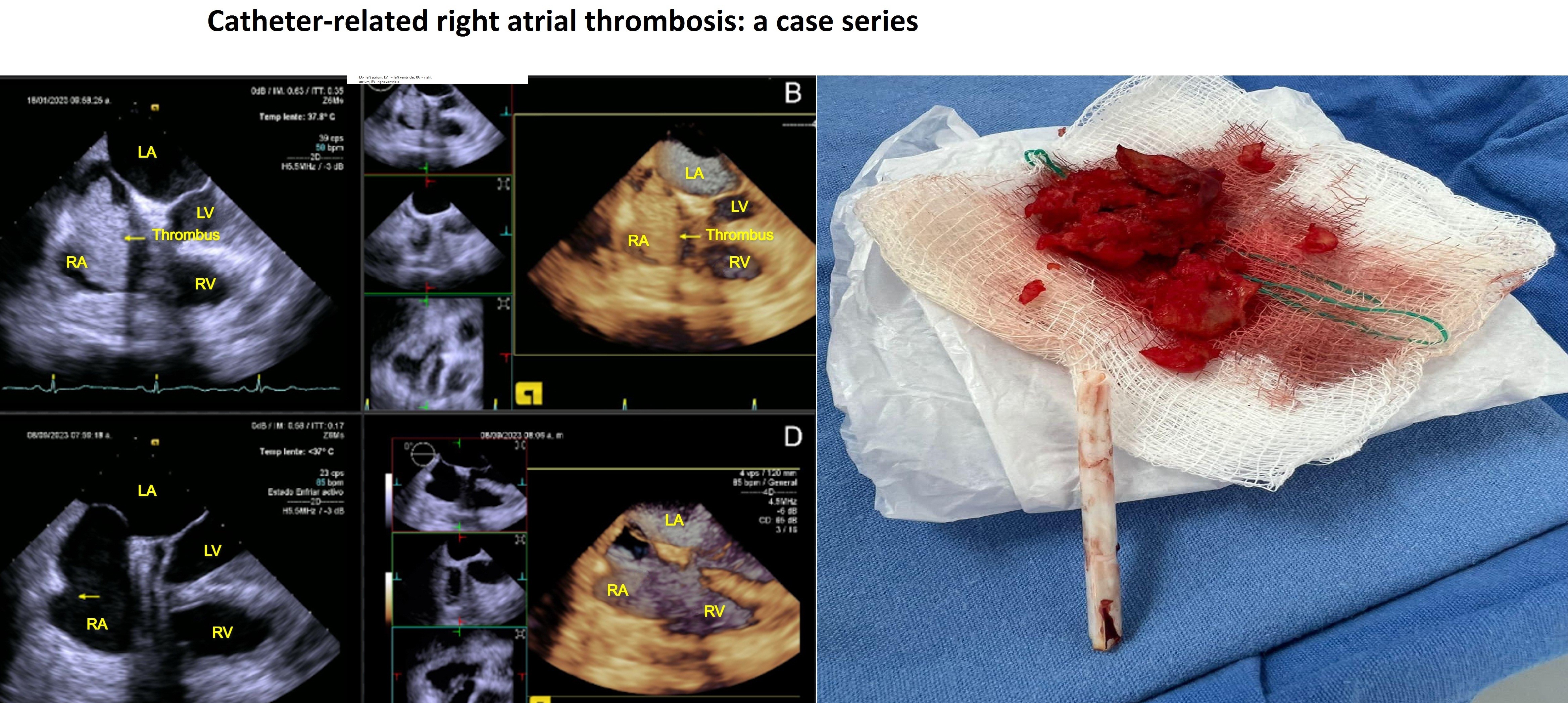
Key words: venous catheterization, right atrium, thrombosis, management, surgery, anticoagulation
Introduction
Central vascular access is required in many critically ill, oncological and renal patients. Complications may include soft tissue infections, endocarditis, catheter occlusion, deep vein thrombosis, central venous stenosis, pulmonary embolism, and thrombus formation in the right heart (1, 2).
Malfunction of the central vascular access may be indicative of an early sign of secondary obstruction from a potential thrombus (3, 4). The incidence of a thrombus in the right atrium (RA) level due to a central vascular access is estimated to be 12.5% to 29% in vivo and 32% on autopsies (1--6).
The aim of this case series is to present different clinical scenarios of catheter-related thrombosis furthermore different options of treatment and its results.
Methods
This is a retrospective study of consecutive cases with catheter-related thrombus treated at our hospital.
All included patients provided informed consent for procedures. Ethic Committee approval was not required for retrospective study.
We collected demographic, medical history, physical examination, clinical, laboratory, and treatment data about patients. All patients underwent computed tomography, transthoracic and transesophageal echocardiography (TEE). Treatment included surgery and/or anticoagulation.
Results
Clinical characteristics of patients with catheter-related thrombus are presented in Table 1.
|
Table 1. Clinical features |
|||||
|
|
Sex |
NYHA Class |
Age |
Treatment |
Reason for central vascular access |
|
Patient 1 |
Male |
Class I |
55 years |
Apixaban 5mg s.i.d. |
Parenteral nNutrition |
|
Patient 2 |
Female |
Class II |
56 years |
Surgery (thrombectomy) |
CKD KDIGO V |
|
Patient 3 |
Male |
Class I |
31 years |
Rivaroxaban 5 b.i.d. |
CKD KDIGO V |
|
CKD KDIGO V – chronic kidney disease stage V |
|||||
Case 1
A 55-year-old male with a single cardiovascular risk factor, smoking, and cardiovascular history of deep venous thrombosis was admitted to our hospital. The patient's surgical history includes open reduction of a maxillary fracture, laparoscopic cholecystectomy, intestinal resection with ileostomy, and restitution of intestinal transit. The patient developed short bowel syndrome, necessitating the placement of a central venous catheter for parenteral nutrition. Two weeks later, the patient developed fever and was admitted to the general surgery service. Upon admission, blood cultures were obtained, but no pathogens were isolated. The physical examination (PE) did not yield any pertinent findings. The diagnostic protocol commenced with computed tomography, which identified a thrombus in the RA, and TEE, which revealed an immobile mural thrombus (type B) measuring 20 x 25 mm in diameter (Fig. 1, A-D).
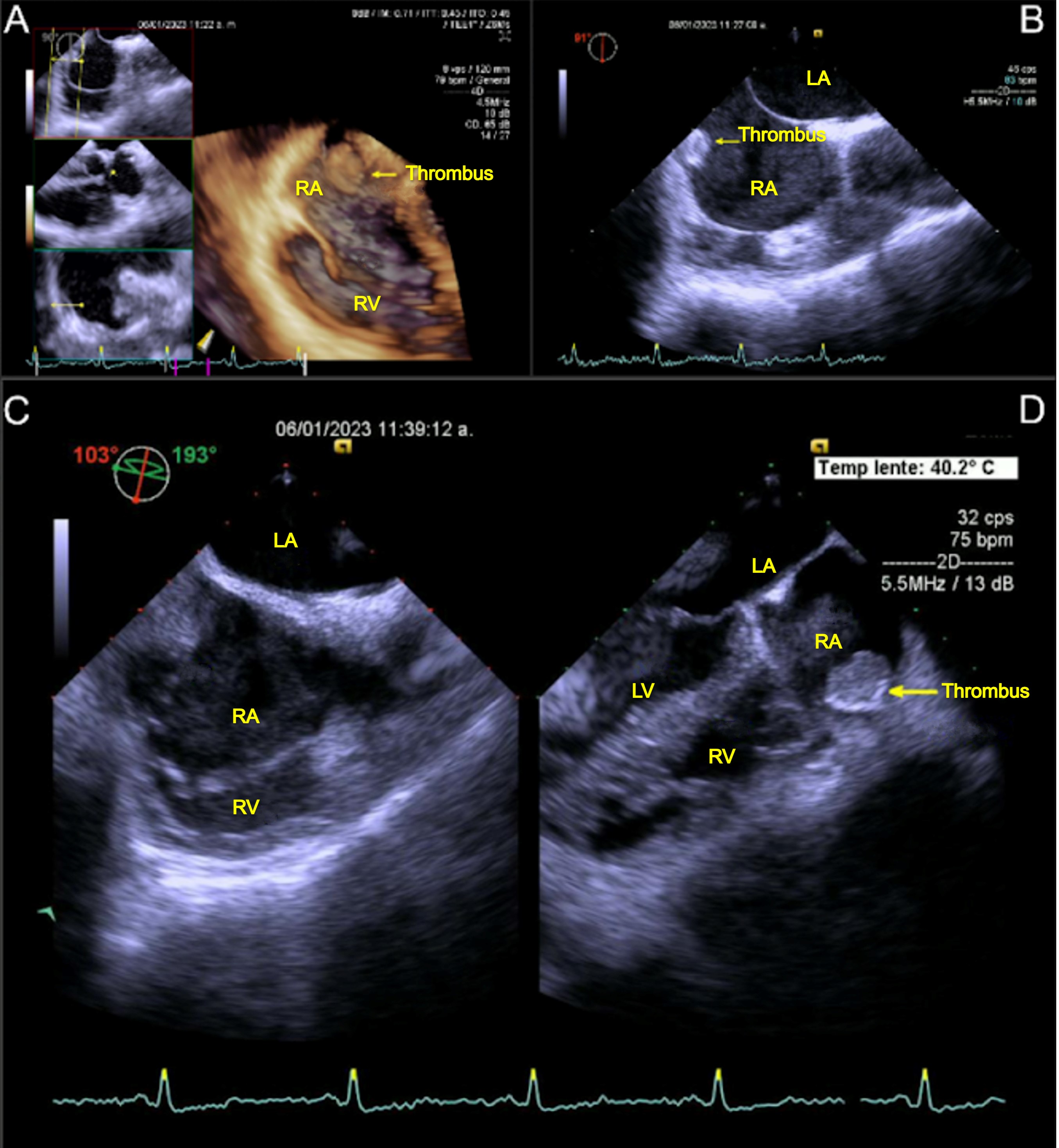
Figure 1. Transesophageal echocardiography views (A-D) of a mobile mass (arrow) in the RA in patient 1
LA- left atrium, LV – left ventricle, RA- righ atrium, RV- right ventricle
The patient was clinically stable. For this reason, a conservative medical treatment was chosen. He was initiated on a treatment regimen comprising of antibiotics - vancomycin, gentamicin, and anticoagulant - apixaban 5 mg every 24 hours. A subsequent echocardiogram demonstrated the absence of residual thrombus and an adequate response to anticoagulant treatment, with complete resolution of the thrombus.
Case 2
A 56-year-old woman with multiple cardiovascular risk factors, including type 2 diabetes mellitus, obesity, menopause, dyslipidemia, hypertension, and chronic kidney disease (CKD), is presented. The patient denied any previous cardiovascular history. The patient has a history of stage V CKD requiring hemodialysis, with a superimposed SARS-CoV-2 infection that resolved without apparent sequelae. Subsequently, she developed dyspnea NYHA class II, without fever. She underwent a stress test and transthoracic echocardiography and TEE in the cardiology service. TEE revealed an ovoid mass with irregular borders, homogenous and attached to the lateral and posterior wall of the RA, near the inferior vena cava orifice. Additionally, a mobile mural thrombus of type B, measuring 52 x 33 mm, was observed, causing partial obstruction. It was noted that the catheter from the superior vena cava (SVC) extended 3 cm beyond the junction between the SVC and the RA, with the latter being visible inside the RA (see Figure 2 A-B).
Consequently, a cardiac team session was convened to determine the optimal thrombectomy strategy, considering the size of the thrombus and its hemodynamic implications and the high risk for embolism. The surgical procedure was successfully completed without complications. During the procedure, the presence of the thrombus adhered to the catheter during its removal was observed (see Fig. 3). A subsequent echocardiogram confirmed the absence of thrombus remnants following thrombectomy (see Fig.2 C-D).
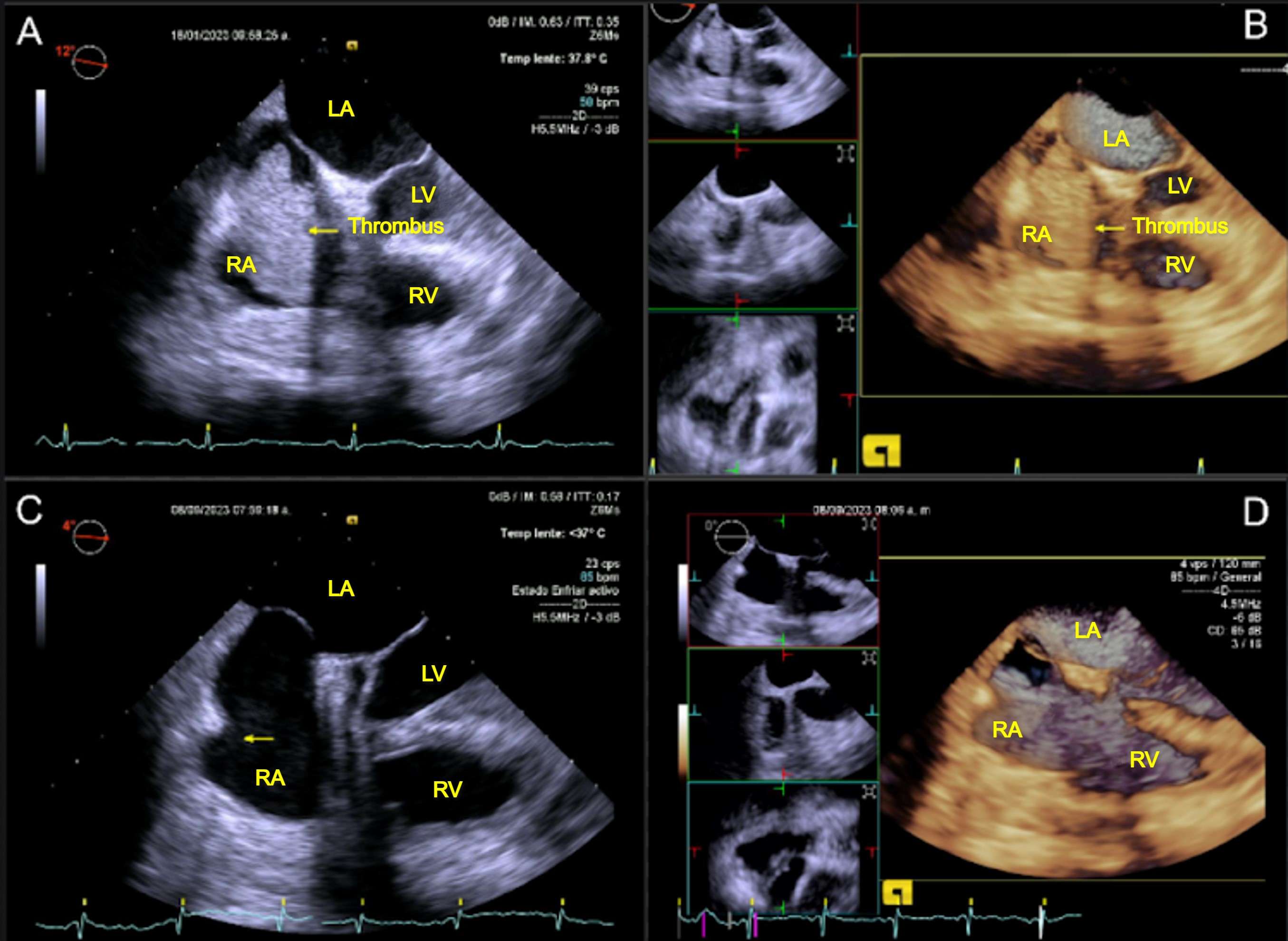
Figure 2. Transesophageal echocardiogram views: (A-B) of mobile mass in the RA, and (C-D) echocardiographic review after thrombectomy
AI - , AD - , VD -, VI-
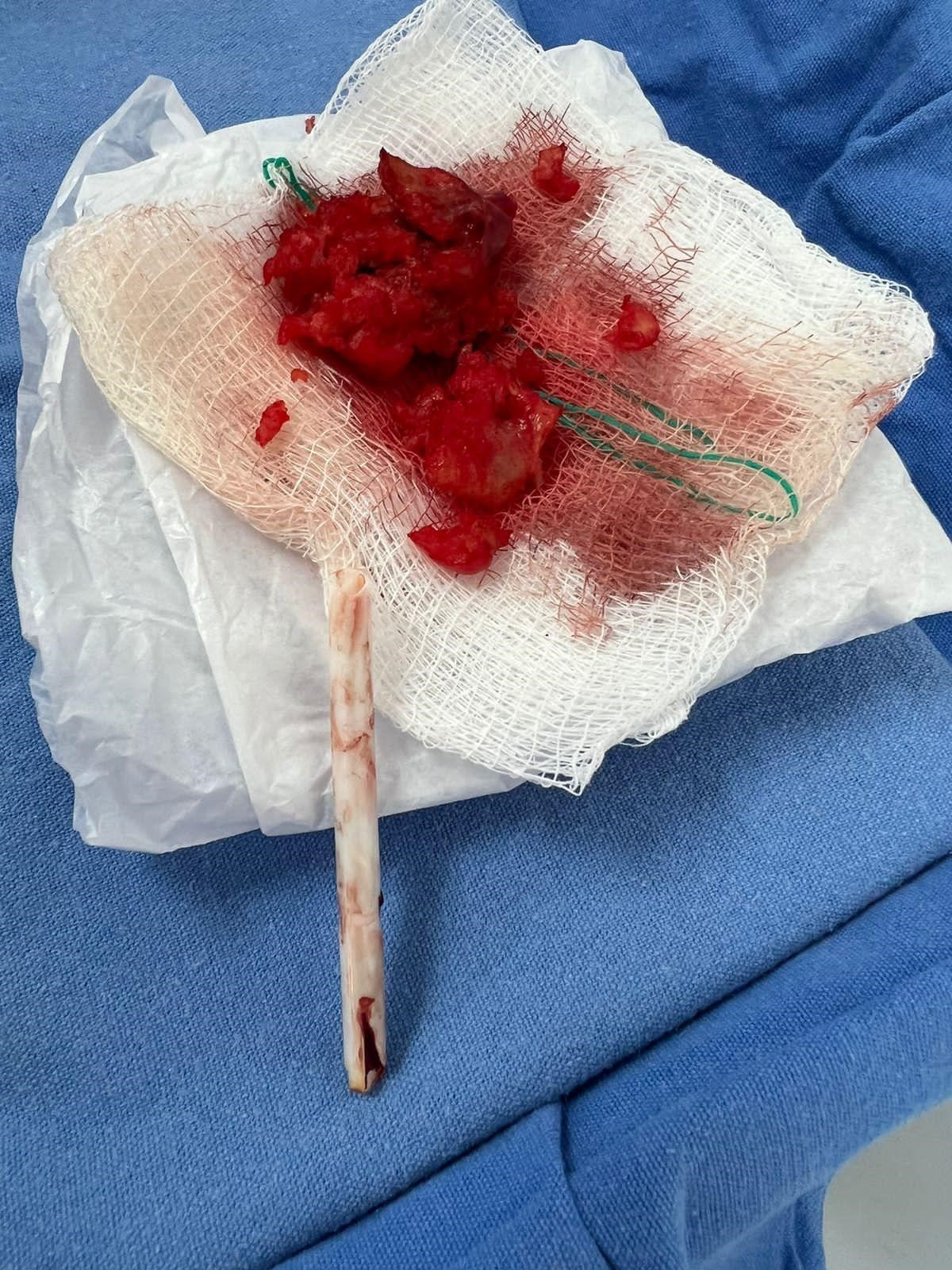
Figure 3. Gross pathology of excised inferior vena cava mass removed post-thrombectomy: Thrombus in the middle third of the Mahurkar catheter
Case 3
A 31-year-old male with cardiovascular risk factors, including arterial hypertension secondary to CKD, presented with no prior cardiovascular history. The patient has a history of allergy to vancomycin and a history of end-stage CKD, treated kidney transplant from a living donor. Subsequently, he experienced graft dysfunction, necessitating renal replacement therapy with peritoneal dialysis, which was complicated by peritonitis, requiring a change in the modality of renal replacement therapy to hemodialysis.
During the follow-up period for the protocol of a new kidney transplant, the patient reported hemodialysis catheter dysfunction without concurrent cardiovascular symptoms. A physical examination yielded no abnormalities. Consequently, a transthoracic echocardiogram was conducted, which revealed moderate eccentric hypertrophy with a left ventricular ejection fraction of 31% and a thrombi compatible lesion. TEE was then performed to further investigate this lesion. A mural thrombus (type B) measuring 19x19 mm was identified in the posterolateral wall of the right atrium in the vicinity of the coronary sinus (Fig. 4 A-B). The thrombus was the result of friction between the central venous catheter and the right atrial wall.
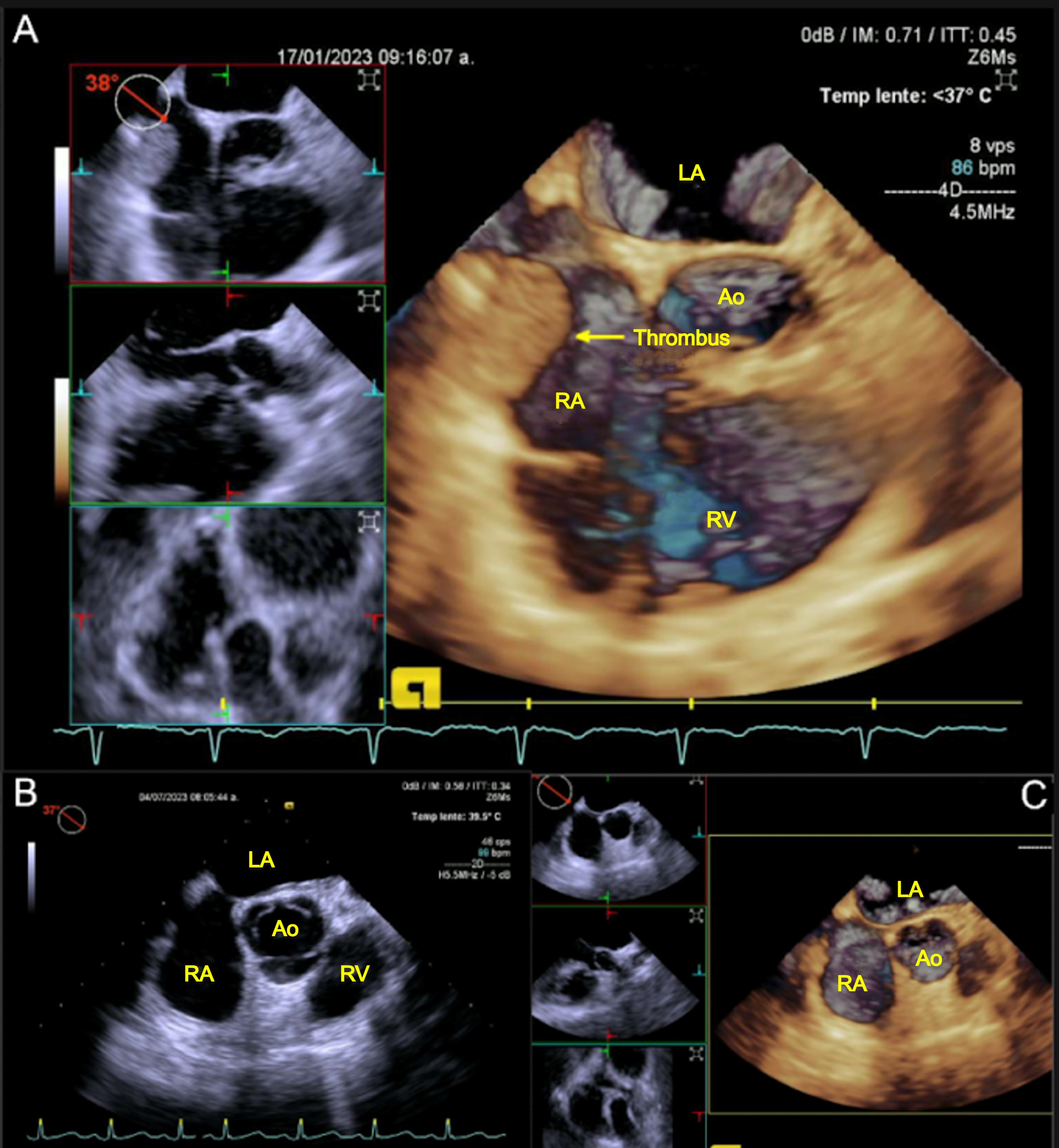
Figure 4. Transesophageal echocardiogram view: (A-B) of a mobile mass in the RA, (C-D) echocardiographic review after anticoagulation
AI -, AD -, AO - VD -
The patients was stable, perhaps he required the placement of a catheter for hemodialysis for this reason the nephrologist chose conservative treatment with anticoagulation.
A new Mahurkar catheter was temporarily placed in a different site, and treatment with rivaroxaban 5 mg every 12 hours was initiated, with a follow-up scheduled for three months. A subsequent echocardiogram demonstrated the absence of residual thrombus, thereby confirming an adequate response to anticoagulation treatment (Fig. 4 C-D).
Discussion
The diagnosis of intracardiac thrombi at the RA level is typically accomplished through imaging studies, such as TEE, which exhibit higher sensitivity and specificity than transthoracic echocardiography (1). These thrombi are classified into two categories: Type A and Type B. Type A is the most prevalent and appears elongated and worm-like, originating in peripheral veins, frequently in the context of deep venous thrombosis. It may be observed passing through a permeable foramen ovale and is associated with a higher embolization rate. Type B thrombi are oval-shaped and originate within a right atrium with an abnormal structure. Typically, they adhere to the chamber wall and are associated with factors such as central vascular access or intracardiac wires (3-5). In our case series, we only encountered Type B thrombi and we do not have records of thrombi Type A.
Type B thrombus, also known as catheter-related thrombus in RA, results from mechanical irritation of the atrial wall caused by movement of the catheter tip and sustained high flows, leading to endothelial damage (3). Another mechanism is low atrial pressure, which increases the prothrombotic state, promotes platelet aggregation, and activates the coagulation cascade (3). Risk factors for thrombus formation include hypercoagulability, number of catheters, and previous thrombus formation (7).
Type B thrombi may present clinically with fever and sepsis (30.9%), without any symptoms (23.5%), or with symptoms of pulmonary thromboembolism (16.2%). Advanced age and the presence of fever/sepsis have been identified as factors associated with higher mortality rates. The mortality rate is 19.1% (1, 4, 6), which increases to 45% when accompanied by arrhythmias, pulmonary embolism, or systemic embolism in the case of patent foramen ovale (4, 7).
At present, there is no established algorithm for the treatment of Type A and B thrombi. Nevertheless, case reports indicate that primary treatments may include anticoagulants, surgical thrombectomy, and thrombolysis, contingent on the patient's circumstances (1). In hemodynamically stable patients, anticoagulation can be initiated for up to six months or until complete thrombus resolution is achieved. In the event that anticoagulant therapy is unsuccessful or the thrombus fails to reduce in size, surgical thrombectomy is considered to be an appropriate course of action (1, 3, 7). In our case series, all patients were hemodynamically stable, two were treated with oral anticoagulation and demonstrated a favorable response.
The anticoagulation therapy used was direct oral anticoagulants with a success rate of 100% for thrombus removal. The systemic thrombolysis should be considered in cases complicated with massive pulmonary embolism or if anticoagulation fails (3).
Surgical thrombectomy is the preferred option for primary or rescue treatment in cases where anticoagulation is contraindicated, anticoagulation therapy fails, the thrombus is 2-6 cm in size, there are cardiac anomalies, or there is endocarditis requiring surgery (1, 3, 4, 5, 7). Studies have not demonstrated a mortality benefit between patients treated with systemic anticoagulation and surgical embolectomy because there are not randomized controlled trials that directly compared the available treatment methods and evidence is limited to case reports (3). One patient underwent thrombectomy due to thrombus size and obstruction.
The decision to remove the catheter remains controversial. While catheter removal may lead to spontaneous thrombus resolution in some cases, systemic anticoagulation should precede extraction to prevent embolization during manipulation. Systemic thrombolysis has been used in cases complicated by massive pulmonary embolism or if anticoagulation fails. Catheter extraction may prevent recurrence of atrial thrombi, thromboembolism, and septic complications (5, 7, 8).
Study limitations
The limitation for this study is the absence of randomized controlled trials that comparing the different treatment options. Further studies are required to compare the effectiveness of different anticoagulants and studies comparing surgery versus medical treatment.
Conclusion
The decision regarding the most appropriate treatment modality for a given patient is dependent on a number of factors, including the experience and expertise of the medical center, the specific clinical scenario, and the patient's individual characteristics. In some cases, anticoagulants may be employed, while in others, thrombectomy may be the preferred approach.
In our case series, the criteria for thrombectomy were based on the size and partial obstruction of the tricuspid valve. In the other cases, primary treatment with anticoagulation was administered. All patients in the series demonstrated appropriate resolution.
Take home message: This case study offers crucial insights for primary care physicians and cardiologists. Firstly, it is of paramount importance to prevent central vascular access from reaching the cardiac chambers, with particular attention to the atrial level.
Secondly, the malfunction of central vascular access and the presence of fever may suggest the presence RA thrombosis.
Thirdly, close follow-up through imaging studies such as echocardiography is necessary to determine the proper evolution of the patient and to inform decision-making regarding the modification or continuation of treatment.
Ethics: The informed consent of patients for all procedure and publication of their cases were obtained. The approval of Ethical Committee was not required for this retrospective study.
Peer-review: External and Internal
Conflict of interest: None to declare
Authorship: J.F.R. A., R.C.P., E.S.C., J.G.A.F., and J.D.V.G. equally contributed to the study, management of patients and manuscript preparation, and fulfilled authorship criteria.
Acknowledgements and funding: None to declare
Statement on A.I.-assisted technologies use: We declare that we did not use AI-assisted technologies in preparation of this manuscript
References
| 1.Tran M-H, Wilcox T, Tran PN. Catheter-related right atrial thrombosis. J Vasc Access 2019; 21: 300-7. doi:10.1177/1129729819873851 https://doi.org/10.1177/1129729819873851 |
||||
| 2.Ducatman BS. Catheter-induced lesions of the right side of the heart. JAMA 1985; 253: 791. doi:10.1001/jama.1985.03350300079024 https://doi.org/10.1001/jama.1985.03350300079024 |
||||
| 3.Akanya DT, Parekh J, Abraham S, Uche S, Lancaster G. Catheter-related right atrial thrombus requiring surgical embolectomy. Cureus 2021; doi:10.7759/cureus.17641 https://doi.org/10.7759/cureus.17641 |
||||
| 4.Hussain N, Shattuck PE, Senussi MH, Velasquez Kho E, Mohammedabdul M, Sanghavi DK, et al. Large right atrial thrombus associated with central venous catheter requiring open heart surgery. Case Reports Med 2012; 2012:1-4. doi:10.1155/2012/501303 https://doi.org/10.1155/2012/501303 |
||||
| 5.Burns KE, McLaren A. Catheter-related right atrial thrombus and pulmonary embolism: A case report and systematic review of the literature. Can Respir J 2009; 16: 163-5. doi:10.1155/2009/751507. https://doi.org/10.1155/2009/751507 |
||||
| 6.Gilon D, Schechter D, Rein AJJT, Gimmon Z, Or R, Rozenman Y, et al. Right atrial thrombi are related to indwelling central venous catheter position: Insights into time course and possible mechanism of formation. Am Heart J 1998; 135: 457-62. doi:10.1016/s0002-8703(98)70322-9 https://doi.org/10.1016/S0002-8703(98)70322-9 |
||||
| 7.Rossi L, Covella B, Libutti P, Teutonico A, Casucci F, Lomonte C. How to manage catheter-related right atrial thrombosis: Our Conservative approach. J Vasc Access 2020; 22: 480-4. doi:10.1177/1129729820922703 https://doi.org/10.1177/1129729820922703 |
||||
| 8. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G-J, Harjola V-P, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2019; 41: 543-603. doi:10.1093/eurheartj/ehz405 https://doi.org/10.1093/eurheartj/ehz405 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

