Massive pericardial effusion as a manifestation of cardiac angiosarcoma
CASE REPORT
Massive pericardial effusion as a manifestation of cardiac angiosarcoma
Article Summary
- DOI: 10.24969/hvt.2024.518
- CARDIOVASCULAR DISEASES
- Published: 02/10/2024
- Received: 12/11/2023
- Revised: 07/09/2024
- Accepted: 08/09/2024
- Views: 4063
- Downloads: 2486
- Keywords: Pericardial effusion, angiosarcoma, cardiac computer tomography imaging, heart, heart neoplasms, coronary vessels
Address for Correspondence: Ihor Romaniuk, Ukrainian-Polish Heart Center "Lviv", Lviv, Ukraine
Email: zahloba91@gmail.com Phone: +38(099)0930037
ORCID: Ihor Romaniuk - 0009-0005-2811-651X; Andriy Mangov - 0000-0002-1094-0704;
Sofia Vyshynska - 0000-0002-0135-5941; Uliana Pidvalna - 0000-0001-7360-8111
Facebook: Ihor Romaniuk - @ihor.romaniuk; Andriy Mangov - @andrii.mangov; Sofia Vyshynska - @sophia.vyshynska.3; Oleh Kobziev-100007948473757; Uliana Pidvalna - @uliana.pidvalna
Ukrainian-Polish Heart Center "Lviv" - @heartcenterlviv; Lviv Regional Clinical Hospital - @LvivRegionalClinicalHospital; Department of cardiac surgery - @LvivCardiosurgeryGroup; Danylo Halytsky Lviv National Medical University - @meduniv.lviv.ua; Yuri Semenyuk Rivne regional clinical hospital - @rokl.rv.ua
Twitter X: Ihor Romaniuk @ihorRomaniuk_xr; Uliana Pidvalna - @UlianaPidvalna
Ihor Romaniuk1, Andriy Mangov2,3, Sofia Vyshynska4, Oleh Kobziev5, Uliana Pidvalna1,6
1 Ukrainian-Polish Heart Center "Lviv", Lviv, Ukraine
2 National Technical University «Kharkiv Polytechnic Institute», Kharkiv, Ukraine
3 European Radiological Center, Kharkiv, Ukraine
4 Lviv Regional Clinical Hospital, Lviv, Ukraine
5 Yuri Semenyuk Rivne regional clinical hospital, Rivne, Ukraine
6 Danylo Halytsky Lviv National Medical University, Lviv, Ukraine
Abstract
Objective: Pericardial effusion, resulting from various conditions including infections, malignancies, and post-operative complications, can lead to significant diagnostic challenges, especially in cases where hemopericardium is present.
The purpose of presenting this clinical case is to increase awareness and alertness among medical professionals to exclude neoplastic involvement of the heart when hydro- or hemopericardium is detected.
Case presentation: A 39-year-old female patient was admitted with a diagnosis of chronic exudative pericarditis for subtotal pericardiectomy, presenting with shortness of breath, dizziness, general weakness, and chest pain. Her medical history included two prior pericardial punctures yielding 1200 ml of hemorrhagic exudate each. Preoperative imaging revealed a massive hemopericardium and a multi-nodular pericardial mass originating from the right atrium, with involvement of the right coronary artery and adjacent structures. Based on the imaging findings, a preliminary diagnosis of cardiac angiosarcoma was made. Surgical cytoreduction on a beating heart with cardiopulmonary bypass was performed, followed by successful tumor debulking. Postoperative recovery was marked by significant clinical improvement. Histological and immunohistochemical analysis confirmed low-grade angiosarcoma. The patient was referred for adjuvant chemotherapy. This case underscores the complexity of managing cardiac angiosarcoma with pericardial involvement and highlights the role of multimodal treatment in such rare malignancies.
Conclusion: This case highlights the urgent need for early detection of neoplastic involvement in patients presenting with pericardial effusion. While hydropericardium is often associated with conditions such as pericarditis, heart failure, and infections, it can also indicate underlying malignancies like primary cardiac angiosarcoma, which may present as massive hemopericardium. Comprehensive imaging, particularly CT scans with intravenous contrast, is crucial for identifying potential tumors and assessing their extent, thereby guiding surgical planning. Timely diagnosis is essential not only for effective management but also for improving patient outcomes in cases of cardiac tumors.
Keywords: Pericardial effusion, angiosarcoma, cardiac computer tomography imaging, heart, heart neoplasms, coronary vessels
Graphical abstract
Introduction
Pericardial effusion (PE) can occur in inflammatory and infectious diseases; postoperatively, in pulmonary arterial hypertension, renal failure, as well as in malignant diseases that can affect the pericardium and manifest as a paraneoplastic syndrome (1). On the other hand, the presence of blood in the pericardial cavity – hemopericardium – is more commonly seen in aortic dissection, cardiac rupture, trauma, or after invasive procedures (2) and less frequently in malignant cardiac tumors.
The late diagnosis of content in the pericardial cavity, often due to the absence of specific symptoms, can lead to delayed detection and preclude radical treatment in the case of tumors or neoplastic heart lesions. However, early diagnosis and tumor resection can significantly improve patients' condition and allow for life-extending chemotherapy (3). This case highlights the critical importance of early detection in pericardial effusion.
The purpose of presenting this clinical case is to increase awareness and alertness among family doctors, therapists, cardiologists, cardiothoracic surgeons, and radiologists to exclude neoplastic involvement of the heart when hydro- or hemopericardium is detected.
Case report
A 39-year-old woman was admitted to the cardiothoracic department of Lviv Regional Clinical Hospital with a diagnosis of chronic exudative pericarditis for subtotal pericardiectomy. Patient complained of shortness of breath, dizziness, general weakness, and chest pain. In her medical history, two pericardial punctures were performed within a month, yielding 1200 ml of hemorrhagic exudate each time.
During the pre-hospital stage, a non-contrast computed tomography (CT) scan of the chest organs revealed a massive hemopericardium (Fig. 1).
Clinical presentation:
• Dyspnea with slight physical exertion, aggravated in a horizontal position on the back
• Sinus tachycardia with a frequency of 110 beats per minute
• Blood pressure - 90/60 mmHg
• Electrocardiography (Fig. 2) - sinus rhythm, heart rate 73 beats per minute; PQ interval – 73 msec, QRS complex – 82 msec; QT interval – 380 msec, QTc - 403 axis: Р – 68⁰, QRS - 16⁰, T – 58.
• On the chest X-ray, there was an enlargement of the heart contour due to the right and left chambers (Fig. 3).
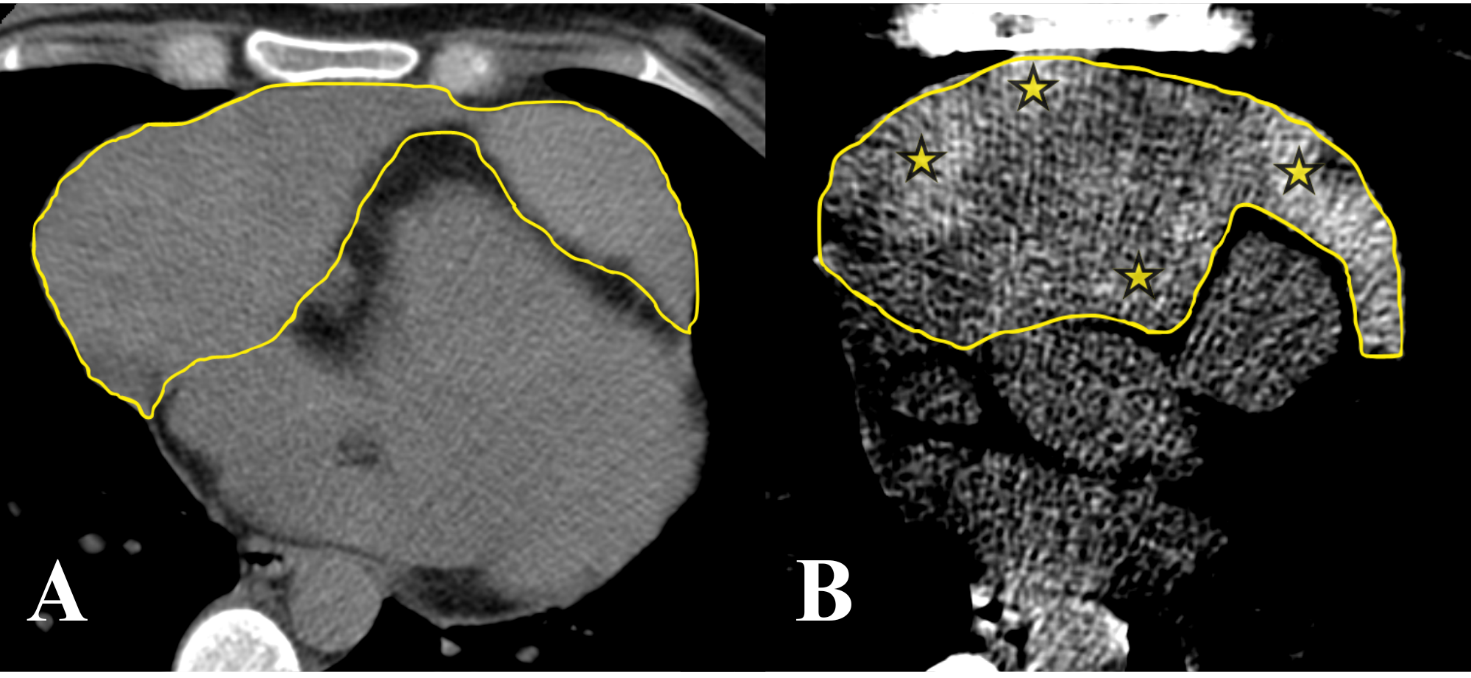
Figure 1. Native images of computer tomography in axial projection. A, visualized massive pericardial content (outlined); B, displayed in a contrast CT (computed tomography) window (WL:50, WW:100) heterogeneity and increased density of pathological content in the pericardial cavity due to blood (*, areas of increased density)
CT – computed tomography
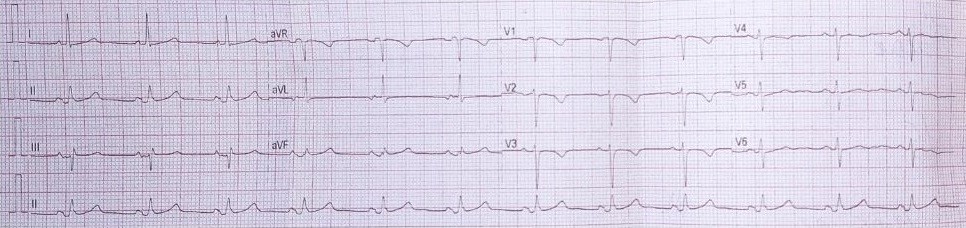
Figure 2. ECG of the 39-years old woman with diagnosis chronic exudative pericarditis, presenting symptoms of shortness of breath, dizziness, general weakness, and chest pain, after two recent pericardial punctures, indicating the need for a subtotal pericardiectomy
ECG – electrocardiogram

Figure 3. Chest X-ray of the 39-years old woman with diagnosis chronic exudative pericarditis, presenting symptoms of shortness of breath, dizziness, general weakness, and chest pain, after two recent pericardial punctures, indicating the need for a subtotal pericardiectomy. X-ray before surgery depicted the enlargement of the heart contour, indicative of enlargement in the right and left chambers
Based on patients history, complaints, CT scan results, ECG and chest X-Ray , indicating massive PE, pericardiocentesis was performed under ultrasound guidance, obtaining 900 ml of hemorrhagic exudate without atypical cells on cytological examination.
Cardiac parameters before and after pericardial puncture are presented by ultrasound examination in Table 1. There was no fluid accumulation after pericardiocentesis and left atrial, ventricular and right ventricular diameters increased.
|
Table 1. Echocardiography parameters before and after pericardial puncture |
|||||||||
|
Variables |
RV |
IVS |
LV |
LVW |
LA |
AO |
EF |
tacc |
PPV |
|
Before pericardial puncture |
2.2 |
1.3 |
3.9 |
1.0 |
3.0 |
3.5 |
60% |
110 |
2.0 |
|
After pericardial puncture |
2.8 |
1.2 |
4.3 |
1.2 |
4.7 |
3.7 |
>60% |
110 |
- |
|
AO – ascending aorta, EF – ejection fraction, IVS – interventricular septum, LA – left atrium, LV – left ventricle, LVW – left ventricular wall, PPV – fluid in the pericardial cavity, RV – right ventricle, tacc – acceleration time on the pulmonary artery valve (dimensions indicated in cm, tass in ms) |
|||||||||
The patient further underwent CT with intravenous contrast enhancement to clarify diagnosis before surgery, and a multi-nodular mass was detected in the pericardial cavity, originating from the right atrium and extending to the folds of the pericardium, forming additional nodes. The pericardial mass was adjacent to the ascending aorta and pulmonary trunk, with local circumferential involvement of the right coronary artery (Fig. 4).
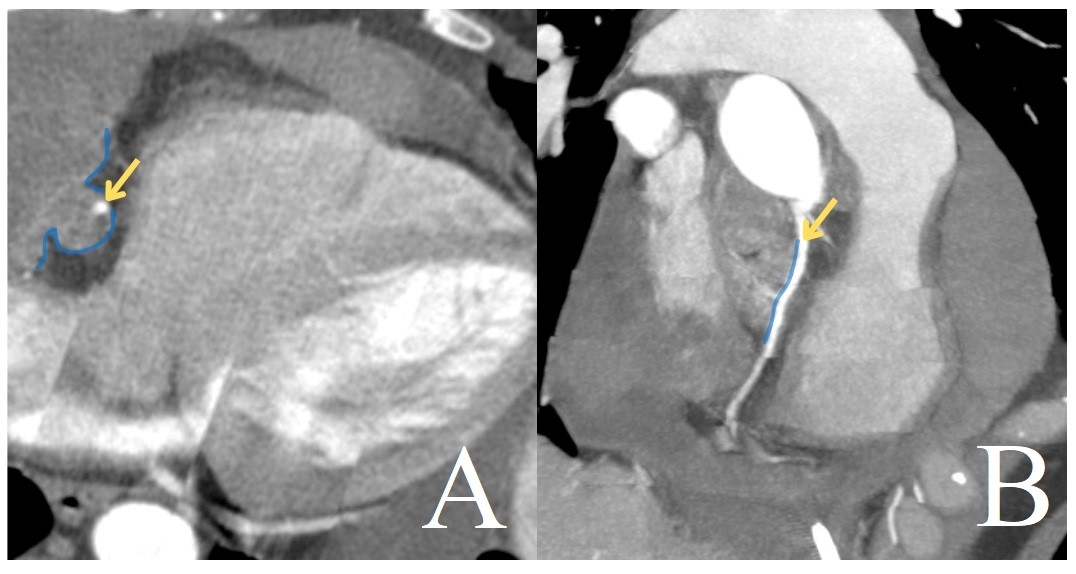
Figure 4. CT of the heart with ECG-synchronization (A, axial projection; B, multiplanar reconstruction, maximum intensity projection). The right coronary artery (arrow) is involved in the mass (line)
CT – computed tomography, ECG – electrocardiogram
The mass showed intense heterogeneous contrast enhancement and slow accumulation of contrast from the periphery to the center (Fig. 5, 6). The maximum intensity enhancement was observed in the venous phase, with further accumulation in delayed scanning (at 4 minutes after contrast administration).
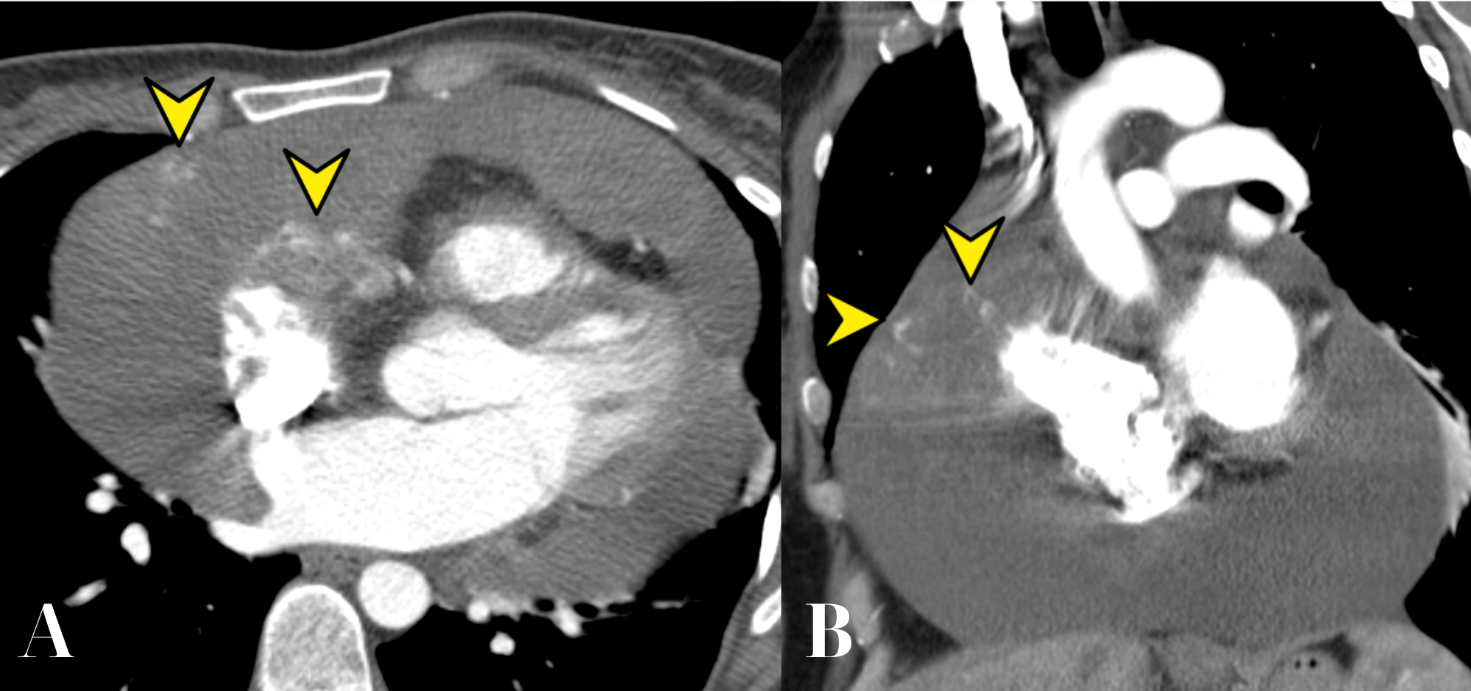
Figure 5. CT of the heart in the early arterial phase (A, axial projection; B, frontal projection). Small areas of intense contrast accumulation are identified (arrowheads).
CT – computed tomography
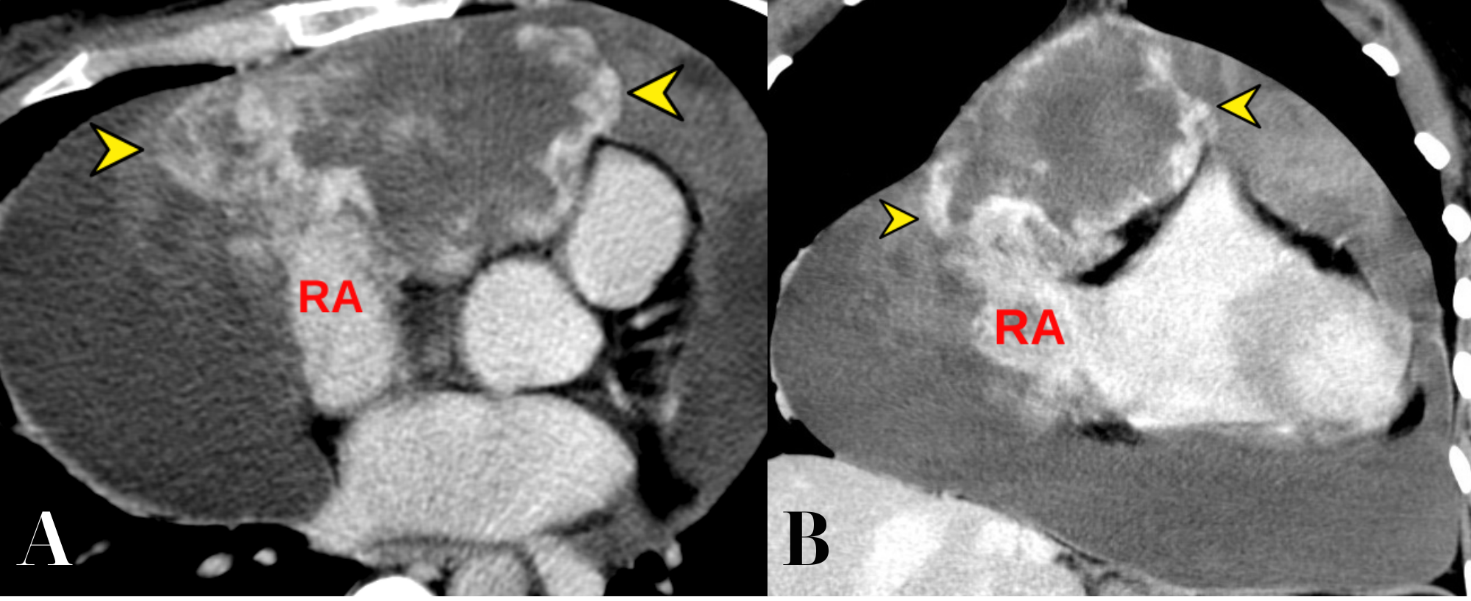
Figure 6. CT of the heart in the venous phase (A, axial projection; B, frontal projection). Tumour mass with intense enhancement are identified (arrowheads), originating from the RA.
CT – computed tomography, RA – right atrium
Based on the typical CT findings (slow and intense contrast enhancement, originates from the right atrium with tumor masses spreading into the pericardial recesses), a preliminary diagnosis of cardiac angiosarcoma with spread into the pericardial cavity and massive hemopericardium has been made.
By the decision of the team consisting of a cardiac surgeon, thoracic oncologist, and radiologist, based on the CT data, specifically the presence of a tumor mass originating from the right atrium with signs of invasion into the right coronary artery and proximity to the ascending aorta and pulmonary trunk, an optimal cytoreductive surgery was performed on a beating heart with cardiopulmonary bypass and parallel perfusion.
The surgical intervention involves optimal tumor cytoreduction (maximal possible removal of visible tumor parts while preserving critical cardiac structures, aiming to leave behind only a small, visibly detectable tumor mass, less than 1 cm).
After a median sternotomy, a pericardiotomy was performed. The heart was not enlarged. Upon attempting to open the pericardium, a large mass was immediately visualized, tightly adhered to the pericardium, with parietal thrombus formation (Fig. 7).

Figure 7. Surgical intervention for cardiac angiosarcoma. Debulking tumour mass on the beating heart. B, Protrusion of a large tumour (*) with the dark-red nodules (arrowheads) into the surgical field
After creating access to the major vessels, cardiopulmonary bypass was established in the usual manner: ascending aorta and both venae cavae. The tumor was removed in stages, resecting it with electrocautery. The attachment site was further treated with diathermy. A portion of the tumor that was fixed to the pulmonary artery stem was separately removed. The tumor had a well-developed blood supply and had deeply invaded the epicardium of the right ventricle and grown invasively into the right atrium. It was resected as much as possible, but due to the proximity of the tumor’s growth to the right ventricle and the right coronary artery, it was not technically feasible to completely remove the tumor from the right atrium, so only the external part of it was excised. The right pleural cavity was opened, creating a pleuro-pericardial window. After stable hemodynamics was achieved, cardiopulmonary bypass was discontinued. Drains were placed in the retrosternal space and the pericardial cavity. After the sternum was stabilized with polyester sutures, the wound was closed in layers.
After the surgical intervention, the patient's condition significantly improved, with complaints of shortness of breath, dizziness, and general weakness disappearing. The patient was subsequently referred to oncologists for chemotherapy.
Histological and immunohistochemical study
The histological and immunohistochemical examination revealed tumor fragments with relatively clear boundaries but without the formation of a fibrous capsule. The tumor consisted of numerous anastomosing cavities of varying sizes and shapes, some being slit-like, while others had papillary structures composed of hyalinized stroma covered by endothelium showing signs of cytological atypia.
The solid proliferation of tumor cells was observed in some areas. Mitotic figures were present in the tumor (4 per 10 high-power fields).
Large hemorrhages were seen in the center of the tumor. Immunohistochemical analysis showed that the tumor cells were positive for ERG, CD31, CD34, and smooth muscle actin alpha. The histological and immunohistochemical characteristics corresponded to a low-grade angiosarcoma.
Discussion
The case at hand demonstrates the difficulty in detecting cardiac angiosarcoma, which manifests as symptoms of PE. Echocardiography revealed PE without visualization of tumor masses, and analysis of the obtained fluid after pericardial puncture also did not reveal atypical cells. Only CT with intravenous contrast convincingly indicated the presence of a large tumor in the pericardial cavity (4).
Angiosarcoma is the most common malignant tumor of the heart, with the most frequent localization being in the wall of the right atrium. The tumor can diffusely spread to involve the pericardium (5). It is encountered in approximately 0.001-0.03% of autopsies (6), with an overall incidence rate of 0.007% (7, 8).
The size and spread of the tumor determine the clinical presentation. During CT with intravenous contrast, the tumor masses demonstrated slow and intense contrast enhancement and the spread of tumor masses into pericardial recesses. Macroscopically, a structure tightly adhered to the pericardium with mural thrombus formation and developed blood supply was found, which invaded the epicardium of the right ventricle and infiltrated into the right atrium; another node was identified that was fixed to the main pulmonary artery. Optimal cytoreduction of the tumor was performed, and since it was not technically feasible to remove the tumor from the right atrium due to its close invasion into the right ventricle and coronary artery, only a portion of it was excised externally. Positive immunohistochemical tests for ERG, CD31, and CD34 indicate the endothelial origin of this tumor.
The primary treatment method for cardiac angiosarcoma is surgery; however, radical resection is usually impossible due to late detection and spread of tumors involving critical anatomical structures (coronary arteries, aorta, main pulmonary artery, massive invasion into atria). Late detection also increases the risk of simultaneous identification of distant metastases, further precluding surgical resection.
Conclusions
This case highlights the urgent need for early detection of neoplastic involvement in patients presenting with pericardial effusion. While hydropericardium is often associated with conditions such as pericarditis, heart failure, and infections, it can also indicate underlying malignancies like primary cardiac angiosarcoma, which may present as massive hemopericardium. Comprehensive imaging, particularly CT scans with intravenous contrast, is crucial for identifying potential tumors and assessing their extent, thereby guiding surgical planning. Timely diagnosis is essential not only for effective management but also for improving patient outcomes in cases of cardiac tumors.
To take home message: In clinical practice, it is critical to evaluate patients with hydropericardium, especially hemopericardium, for the presence of cardiac tumors to ensure accurate diagnosis and appropriate management.
Ethics: Patient consent was obtained prior to the preparation of this report. The Bioethics Committee of Danylo Halytsky Lviv National Medical University approved the study and adhered to ethical principles
Peer-review: External and Internal
Conflict of interest: None to declare
Authorship: I.R., A.M., and U.P. performed diagnostic procedures – radiology assessment of CT images, histological evaluation of specimens, and drafting manuscript; S.V., and O.K. performed the surgery and were involved in writing surgery section. All authors critically reviewed and approved the final version of the manuscript, thus
fulfilled authorship criteria.
Acknowledgement: We thank the admissions department, intensive care unit No. 2, and cardiovascular surgery department of Lviv Regional Clinical Hospital for their medical work and performing oncological cardiac-thoracic intervention
Funding: The project was funded as part of the research work of the «Ukrainian-Polish Heart Center «Lviv»
Statement on A.I.-assisted technologies use: We used Large Language Model (Chat GPT free version) to improve English language
References
| 1. Manea M , Bratu OG, Bacalbasa N, Diaconu CC. Diagnosis and management of pericardial effusion. Journal of Mind and Medical Sciences, 2020, 7.2: 148-55. doi: 10.22543/7674.72.P148155 https://doi.org/10.22543/7674.72.P148155 |
||||
| 2. Yehuda A, Charron P, Imazio M, Badano L, Baron-Esquivias G, Bogaert J, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. Pol Heart J 2015; 73: 1028-91. doi: 10.1093/eurheartj/ehv318 https://doi.org/10.1093/eurheartj/ehv318 |
||||
| 3. Stergioula A, Kokkali S, Pantelis E. Multimodality treatment of primary cardiac angiosarcoma: a systematic literature review. Cancer TreaT Rev 2023; 102617. doi: 10.1016/j.ctrv.2023.102617 https://doi.org/10.1016/j.ctrv.2023.102617 |
||||
| 4. Yamani N, Abbasi A, Almas T, Farouk M, Unzek S. Diagnosis, treatment, and management of pericardial effusion-review. Ann Med Surg 2022; 80: 104142. doi: 10.1016/j.amsu.2022.104142 https://doi.org/10.1016/j.amsu.2022.104142 |
||||
| 5. King T, Day W, Dore M, Joseph A. Primary cardiac angiosarcoma: pericardial erosion and disseminated metastasis in a young active duty man. Ann Int Med Clin Cas 2022; 1: e220430. doi: 10.7326/aimcc.2022.0430 https://doi.org/10.7326/aimcc.2022.0430 |
||||
| 6. Udit Y, Ankit M. Primary pericardial angiosarcoma: case report and review of treatment options. Ecancer Med Sci 2020; 14: 1056. doi: 10.3332/ecancer.2020.1056 https://doi.org/10.3332/ecancer.2020.1056 |
||||
| 7. Kumari N, Bhandari S, Ishfaq A, Butt RRS, Ekhator Ch, Karski A. Primary cardiac angiosarcoma: a review. Cureus 2023; 15; e41947. doi: 10.7759/cureus.41947 https://doi.org/10.7759/cureus.41947 |
||||
| 8. Chen Y, Li Y, Zhang N, Shang J, Li X, Liu J, et al. Clinical and imaging features of primary cardiac angiosarcoma. Diagnostics 2020; 10: 776. doi: 10.3390/diagnostics10100776 https://doi.org/10.3390/diagnostics10100776 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

