Transcatheter versus surgical aortic valve replacement: a meta-analysis of comparative outcomes in low- and intermediate-risk patients with severe aortic stenosis
ORIGINAL RESEARCH ARTICLE
Transcatheter versus surgical aortic valve replacement: a meta-analysis of comparative outcomes in low- and intermediate-risk patients with severe aortic stenosis
Article Summary
- DOI: 10.24969/hvt.2024.519
- CARDIOVASCULAR DISEASES
- Published: 06/10/2024
- Received: 10/08/2024
- Revised: 15/09/2024
- Accepted: 16/09/2024
- Views: 7039
- Downloads: 2888
- Keywords: Aortic stenosis, valvular heart disease, transcatheter aortic valve replacement, surgical aortic valve replacement, mortality, stroke, myocardial infarction, prosthetic valve endocarditis
Address for Correspondence: Omar Sameer Hamodat, Faculty of medicine, University of Sharjah, University City Rd, University City, Sharjah, 27272, UAE Home address: Al Butina, Sharjah, 99999, UAE
Email: lionelomar109110@gmail.com; U21100421@sharjah.ac.ae Mobile: +971508661649/ +971569427920
ORCID: Saif Almuzainy –0009- 0000- 3090-8413; Omar Sameer Hamodat - 0009-0002-3358-8990;
Salma Nizar– 0009-000-2891-2528
Saif Almuzainy, Omar Sameer Hamodat, Salma Nizar
Faculty of Medicine, University of Sharjah, Sharjah, UAE
Abstract
Objective: Aortic stenosis is the most common valvular heart disease. This study aims to systematically analyze randomized clinical trials (RCTs) data comparing transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) in intermediate and low-risk patients with severe symptomatic aortic stenosis.
Methods: We conducted a meta-analysis of RCTs, performing an exhaustive search of major databases to identify studies comparing TAVR and SAVR in low- to intermediate-risk patients. We assessed mortality, stroke, length of hospital stay, and other perioperative outcomes.
Results: Nine RCTs with 8,884 patients (average age 77.76 years; 49.47% male) met the inclusion criteria. Baseline characteristics were comparable between TAVR and SAVR groups, with a low risk of bias. Pooled results showed a significant reduction in mortality for TAVR compared to SAVR (RR 0.75, 95% CI 0.61–0.92, p = 0.007, I² = 51%). TAVR significantly reduced stroke incidence (RR 0.66, 95% CI 0.49–0.89, p = 0.007, I² = 69%) and myocardial infarction (RR 0.60, 95% CI 0.37–0.96, p = 0.03, I² = 0%). No significant difference was found for prosthetic valve endocarditis (RR 1.06, 95% CI 0.55–2.06, p = 0.85, I² = 0%). Length of stay was significantly shorter for TAVR (MD -4.30 days, 95% CI -5.03 to -3.57, p = 0.00001, I² = 93%).
Conclusion: TAVR is a viable option for intermediate and low-risk patients with severe symptomatic aortic stenosis. Future research should focus on long-term outcomes and TAVR device durability, especially in younger, lower-risk populations.
Key words: Aortic stenosis, valvular heart disease, transcatheter aortic valve replacement, surgical aortic valve replacement, mortality, stroke, myocardial infarction, prosthetic valve endocarditis
Introduction
Aortic stenosis (AS) is the most widespread valvular heart disease globally and significantly contributes to morbidity and mortality worldwide; in particular, severe symptomatic AS is associated with poor prognosis and are more predisposed to complications as well as increased risk of sudden death (1). Previously, surgical aortic valve replacement (SAVR) was the definitive modality of choice for patients struggling with severe AS, improving their survival rate and quality of life (2). However, since the emergence of transcatheter aortic valve replacement (TAVR), a less-invasive therapeutic approach to treating AS patients and prolonging their lifespans, treatment options have expanded particularly for patients with comorbidities who are deemed unfit for surgical intervention (3).
While TAVR is the preferred modality for high surgical risk AS patients, the question remains whether it should be incorporated as an alternative to SAVR for intermediate and low-risk AS patients as well; newly emerging trials have demonstrated that TAVR is a safe and efficacious therapeutic option with promising short-to-intermediate outcomes (3, 4).
As TAVR has become the fundamental procedure for severe AS in elderly patients, especially the subset of patients deemed to be high or intermediate-risk by the Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM), the assessment of numerous aspects associated with its outcomes is crucial in finalizing whether or not TAVR is a viable therapeutic option for intermediate and low-risk severe symptomatic AS patients who would usually go for SAVR (5–9).
Within this framework, our meta-analysis aims to compare and contrast between TAVR and SAVR in terms of safety and efficacy, analyzing numerous cardiovascular complications, length of hospital stay as well as financial considerations to come to a definitive conclusion.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines during the preparation of this systematic review to report our methodology and results.
Inclusion criteria.
The following criteria were applied for inclusion in the study: (1) randomized clinical trials; (2) comparison of TAVR and SAVR; (3) the population consists of elderly patients (generally 65 years and older) with severe symptomatic aortic stenosis, ranging from low to intermediate surgical risk based on evaluations by a multidisciplinary heart team using a risk model from the Society of Thoracic Surgeons (STS) to estimate 30-day mortality risk (10).; (4) reporting of outcomes, such as all-cause mortality, stroke, prosthetic valve endocarditis, and length of hospital stay. We excluded nonrandomized studies, animal studies, non-English publications, case reports, case series, editorials, reviews and theses without original data.
Search Strategy
To identify all clinical trials comparing TAVR and SAVR in elderly patients with severe symptomatic aortic stenosis, ranging from low to intermediate surgical risk, we conducted a systematic literature search across several medical databases, including PubMed, Scopus, Ovid, CINAHL, and ProQuest, through July 2024. Our search strategy utilized specific keywords and Medical Subject Headings (MeSH) terms relevant to our study objectives. The search terms included “Transcatheter Aortic Valve Replacement,” "Transcatheter aortic valve implantation,” “Surgical Aortic valve replacement,” “Surgical aortic valve implantation,” “Cost-effectiveness,” “Health economics,” “complications,” “Stroke,” “Endocarditis,” and “Mortality.”
Selection of Studies
The screening process involved two independent reviewers and was conducted in two stages: first, the titles and abstracts of retrieved studies were assessed for relevance, followed by a detailed review of the full texts of studies that appeared potentially eligible. Any disagreements between reviewers were resolved through discussion.
Data Extraction
Three authors independently extracted data using an online data extraction form. The extracted data were categorized into the following areas: 1) Study Design and Characteristics, detailing the study type and key methodological aspects; 2) Baseline Characteristics of the Population, including demographic and clinical details such as age, sex, and comorbidities; 3) Quality Assessment, utilizing the Cochrane Risk of Bias (ROB 1) tool; and 4) Outcomes, including mortality rates at 30 days, 1 year, and 2 years, as well as the incidence of myocardial infarction (MI) and stroke, and the length of hospital stay. Clinical outcomes, including death, stroke, myocardial infarction, and endocarditis, were defined according to the Valve Academic Research Consortium-2 (VARC-2) criteria (11).
Quality assessment of the included Studies
Two authors independently evaluated the quality of the included RCTs using the Cochrane Risk of Bias (ROB 1) tool, assessing seven specific items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Any discrepancies in the assessments were resolved through discussion.
Dealing with Missing Data
In cases where the mean and standard deviation (SD) were not reported, we calculated these values using the median, interquartile range, and sample size, following the methodology outlined by Wan et al. (2014)(9).
Data Analysis and Synthesis
In this research, continuous data are shown as the average value along with the standard deviation, while categorical data are presented as counts and percentages. For the meta-analyses, risk ratios were used to analyze categorical data, and mean differences were used for continuous data. We combined data from variables reported in at least two studies. To do this, we used the Mantel-Haenszel method for categorical data and the inverse variance method for continuous data.
We checked for differences between studies by visually inspecting forest plots and calculating the Chi-square and I-square statistics. If there was a lot of variation (indicated by an I-square value above 50% or a Chi-square p-value less than 0.1), we conducted sensitivity analyses to explore the causes. In cases where significant differences were found between studies, we applied a random-effects model to account for variations in study methods and participant characteristics. If there was little to no variation, a fixed-effects model was used instead. We calculated risk estimates with 95% confidence intervals using the RevMan 5.3 software.
Publication bias
It was not possible to assess publication bias due to the relatively small number of included studies (<10) (12).
Results
Study selection
From 14,384 initial records, 4,875 duplicates were removed, leaving 9,509 for screening. After excluding 9,484 based on titles and abstracts, 25 full texts were assessed, and 16 were excluded for incorrect study design. Ultimately, 9 studies were included in the meta-analysis. The selection process is detailed in the PRISMA flow diagram (Fig. 1).
Study characteristics
A total of 9 studies were included in this systematic review. The key characteristics of these studies are summarized in Table 1. These characteristics include the study design, population, intervention details, comparator, and main findings.
The studies included 8,884 patients from 9 RCTs, averaging 77.76 years in age, with 49.47% being male. The baseline characteristics were comparable for TAVR and SAVR groups. Hypertension was present in 80.97% vs. 81.85%, diabetes in 26.94% vs. 27.33%, coronary artery disease in 35.91% vs. 36.21%, atrial fibrillation in 22.34% vs. 23.89%, previous stroke in 12.96% vs. 12.7%, and chronic obstructive pulmonary disease in 16.26% vs. 17.94%. Prior percutaneous coronary intervention or coronary bypass surgery occurred in 24.37% vs. 22.2% of patients. The mean STS scores were 3.02 for TAVR and 3.07 for SAVR, while the Log EuroSCOREs were 5.4 and 5.56, respectively. Additionally, NYHA (3/4) scores were 44.83% for TAVR and 44.38% for SAVR. Detailed demographics are presented in Table 2.
Risk of bias
Risk of bias assessment using the Cochrane risk of bias tool revealed a low to negligible risk of bias in the 9 included studies. The overall effect of bias on each study is shown in Figure 2.
Pooled results
A total of 16,602 patients (TAVR: 8,410; SAVR: 8,192) contributed to the analysis at 30 days, 1 year, and 2 years. The results demonstrated a statistically significant reduction in mortality for TAVR patients compared to SAVR, with a relative risk (RR) of 0.75 (95% CI 0.61–0.92, p = 0.007), as shown in Fig. 3. However, there was substantial heterogeneity among the included studies (I² = 51%). In a similar analysis, 16,106 patients (TAVR: 8,188; SAVR: 7,918) were evaluated for stroke outcomes at 30 days and 1 year. The findings indicated a significant reduction in stroke incidence for TAVR patients, with an RR of 0.66 (95% CI 0.49–0.89, p = 0.007), as depicted in Figure 4.
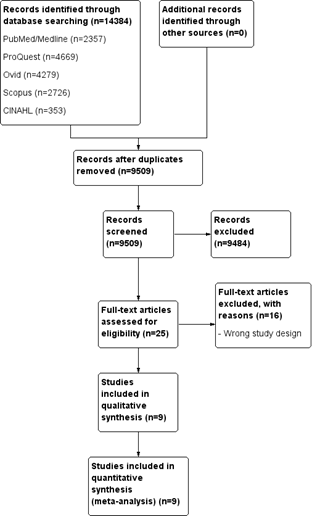
Figure 1. PRISMA flow diagram
|
Table 1. Study characteristics |
|||||
|
Study |
Study design |
Population |
Intervention |
Comparator |
Findings |
|
|
Randomized noninferiority |
Low-risk patients with severe, symptomatic AS |
TAVR (valve prostheses selected according to operator discretion) |
SAVR (valve prostheses selected according to operator discretion) |
TAVI in patients at low or intermediate surgical risk, had noninferior death from any cause or stroke at 1 year in comparison to SAVR |
|
|
Multinational, prospective, randomized study |
Severe AS, trileaflet aortic valve morphology, low predicted risk of death |
TAVR (CoreValve, Evolut R, or Evolut PRO, Medtronic) |
SAVR |
Low–surgical risk patients who underwent TAVR had durable benefits with regard to all-cause mortality and disabling stroke compared with SAVR. |
|
|
Randomized, multicenter, superiority |
Patients ≥70 years old with severe AS and no significant CAD |
TAVR (Medtronic CoreValve) |
SAVR |
No significant differences were found between the 2 procedures regarding death from any cause, stroke, or MI after 1 year. |
|
Leon/ PARTNER 2 (2016)(10)
|
Multicenter randomized clinical trial |
Patients with severe symptomatic AS at low surgical mortality risk |
TAVR (SAPIEN 3 valve) |
SAVR |
In intermediate-risk patients, TAVR was similar to SAVR with respect to the primary end point of death or disabling stroke. |
|
|
Multicenter, randomized |
Patients with severe AS and a low risk for death with surgery |
TAVR (SAPIEN 3 system), (Edwards Lifesciences) |
SAVR with a commercially available bioprosthetic valve |
At low surgical-risk, the rate of the composite of death, stroke, or rehospitalization at 1 year was significantly lower with TAVR than with surgery. |
|
|
Prospective multicenter international randomized |
Elderly (≥65 years) patients with severe AS and small aortic annulus |
TAVR (SAPIEN 3/Ultra, Evolut R/PRO/PRO+/FX, and Acurate neo/neo2 valves) |
SAVR |
Patients with AS low-to-intermediate-risk showed no evidence of TAVR superiority versus SAVR in valve hemodynamic outcomes and clinical outcomes. |
|
|
Randomized, multicenter, non-inferiority |
Operable patients with isolated AS, aged ≥75 years |
TAVR (Edwards Sapien) |
SAVR |
a-TAVR is associated with higher complications in low-risk patients and lower device success rates in comparison to SAVR |
|
SURTAV| (2022)(23)
|
Randomized, multicenter, non-inferiority |
Patients with symptomatic, severe AS at intermediate surgical risk |
TAVR (CoreValve (84%)
|
SAVR |
TAVR in symptomatic intermediate surgical risk patients is noninferior to surgery regarding death from any cause or disabling stroke at 24 months |
|
Table 1. Study characteristics (continued from page …) |
|||||
|
Study |
Study design |
Population |
Intervention |
Comparator |
Findings |
|
Toff (2022)(24)
|
Randomized clinical trial, multicenter |
Patients aged ≥70 years with severe, symptomatic AS and moderately increased operative risk |
TAVR using any valve with a CE mark |
SAVR |
TAVR is noninferior to surgery regarding all-cause mortality at 1 year among intermediate surgical risk patients aged 70 or above |
|
AS - aortic stenosis, CAD - coronary artery disease, CE mark - (indicating the valve meets all legal and safety requirements for sale throughout the European Economic Area), MI –myocardial infarction, TAVR- transcatheter aortic valve replacement, SAVR - surgical aortic valve replacement |
|||||
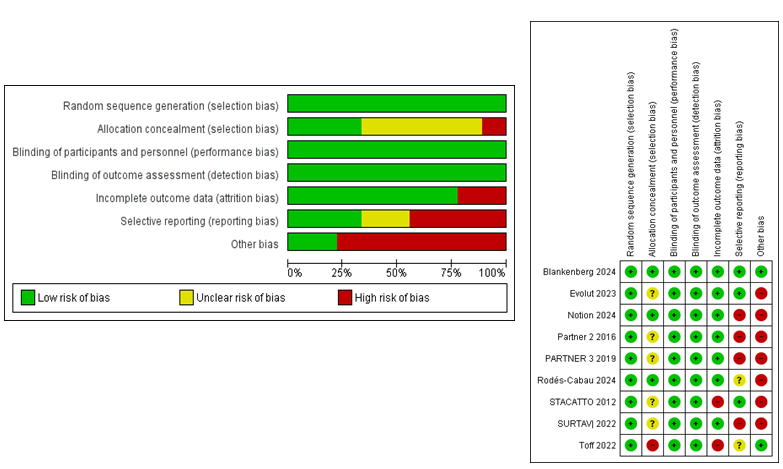
Figure 2. Risk of bias assessment
|
Table 2. Baseline clinical characteristics in TAVR versus SAVR groups |
|
||||||||||||||
|
Study |
Group |
Sample size |
Male, n (%) |
Age, years |
Mean STS score |
NYHA score (3/4), n(%) |
Log EuroSCORE |
AF, n(%) |
CAD, n(%) |
Stroke, n(%) |
Hypertension, n(%) |
Diabetes, n(%) |
COPD |
Prior PCI or CABG, n(%) |
|
|
Blankenberg (2024)(20)
|
TAVR |
701 |
390 (56) |
74.3 (4.6) |
1.8(0.9) |
321 (46.2) |
2.1 (1.4) |
201 (28.9) |
238 (34.3) |
42 (6.1) |
588 (84.7) |
588 (33.8) |
101 (14.5) |
|
|
|
SAVR |
713 |
400 (57.3) |
74.6 (4.2) |
1.9(1) |
318 (45.6) |
2.1 (1.8) |
191 (27.4) |
266 (38.2) |
42 (6) |
605 (87.2) |
605 (32.8) |
118 (16.9) |
|
||
|
Evolut (2023)(21)
|
TAVR |
730 |
464 (63.6) |
74.1 (5.8) |
2.0 (0.7) |
182 (24.9) |
|
112 (15.4) |
|
|
618 (84.8) |
229 (31.4) |
106 (15.1) |
121 (16.6 ) |
|
|
SAVR |
684 |
451 (65.9) |
73.7 (5.9) |
1.9 (0.7) |
193 (28.2) |
|
98 (14.4) |
|
|
564 (82.6) |
210 (30.7) |
118 (18 ) |
102 (14.9) |
||
|
Notion (2024)(14)
|
TAVR |
145 |
78 (53.8) |
79.2 (4.9) |
2.9 (1.6) |
70 (48.6) |
|
40 (27.8) |
8 (5.5) |
|
103 (71.0) |
26 (17.9) |
17 (11.7) |
11(7.6) |
|
|
SAVR |
135 |
71 (52.6) |
79.0 (4.7) |
3.1 (1.7) |
61 (45.5) |
|
34 (25.6) |
6 (4.4) |
|
103 (76.3) |
28 (20.7) |
16 (11.9 ) |
12 (8.9) |
||
|
PARTNER 2 (2016)(10)
|
TAVR |
1011 |
548 (54.2) |
81.5 (6. 7) |
5.8 (2.1) |
782 (77.3) |
|
313 (31.0) |
700 (69.2) |
325 (32.1) |
|
381 (37.7) |
321 (31.8) |
513 (50.7) |
|
|
SAVR |
1021 |
560 (54.8) |
81.7 (6.7) |
5.8 (1.9) |
776 (76.1) |
|
359 (35.2) |
679 (66.5) |
317 (31.0) |
|
349 (34.2) |
306 (30.0) |
440 (43.1) |
||
|
PARTNER 3 (2019)(13)
|
TAVR |
496 |
335 (67.5) |
73.3 (5.8) |
1.9 (0.7) |
155 (31.2) |
1.5 (1.2) |
78 (15.7) |
137 (27.7) |
17 (3.4) |
|
155 (31.2) |
25 (5.1) |
|
|
|
SAVR |
454 |
323 (71.1) |
73.6 (6.1) |
1.9 (0.6) |
108 (23.8) |
1.5 (0.9) |
85 (18.8) |
127 (28.0) |
23 (5.1) |
|
137 (30.2) |
28 (6.2) |
|
||
|
Rodés-Cabau (2024)(11) |
TAVR |
77 |
4 (5.2) |
75.9 (5.3) |
2.55 (1.1) |
23 (29.9) |
|
6 (7.8) |
17 (22.1) |
|
62 (80.5) |
23 (29.9) |
7 (9.1) |
17 (22.1) |
|
|
SAVR |
74 |
7 (9.5) |
75.1 (4.9) |
2.47 (1.2) |
24 (32.4) |
|
14 (18.9) |
14 (18.9) |
|
61 (82.4) |
22 (29.7) |
14 (18.9) |
14 (18.9) |
||
|
STACATTO (2012)(22) |
TAVR |
34 |
9 (26) |
80 (3.6) |
3.1 (1.5) |
|
9.4 (3.9) |
|
|
|
|
1 (2.9) |
1 (2.9) |
|
|
|
SAVR |
36 |
12 (33.3) |
82 (4.4) |
3.4 (1.2) |
|
10.3 (5.8) |
|
|
|
|
3 (8.3) |
1 (2.8 ) |
|
||
|
SURTAV| (2022)(23) |
TAVR |
864 |
498 (57.6) |
79.9(6.2) |
4.4 (1.5) |
520 (60.2) |
11.9 (7.6) |
243 (28.1) |
541 (62.6) |
151 (17.5) |
801 (92.7) |
296 (34.3) |
305 (35.4) |
320 (37.0) |
|
|
|
438 (55.0) |
79.7(6.1) |
4.5 (1.6) |
463 (58.2) |
11.6 (8.0) |
211 (26.5) |
511 (64.2) |
130 (16.3) |
719 (90.3) |
277 (34.8) |
267 (33.5) |
306 (38.4) |
|
|
Toff (2022)24 |
TAVR |
458 |
SAVR |
796 |
2.7 (1.1) |
184 (40.3) |
2.1 (1.2) |
110 (24) |
133 (30) |
26 (5.7) |
328 (72.1) |
107 (23.4) |
95 (20.7) |
56 (12.2) |
|
SAVR |
455 |
242 (53.2) |
81(4.5) |
2.7(1) |
204 (45.2) |
2.3 (1.3) |
110 (24.3) |
145 (33.3) |
23 (5.1) |
327 (72.3) |
111 (24.5) |
106 (23.3) |
41 (9) |
|
|
Data are n, mean (SD), or n (%). AF - atrial fibrillation, CABG - coronary artery bypass grafting, CAD - coronary artery disease, COPD - chronic obstructive pulmonary disease, PCI - percutaneous coronary intervention, STS - Society of Thoracic Surgeons, TAVR- transcatheter aortic valve replacement, SAVR - surgical aortic valve replacement |
||||||||||||||
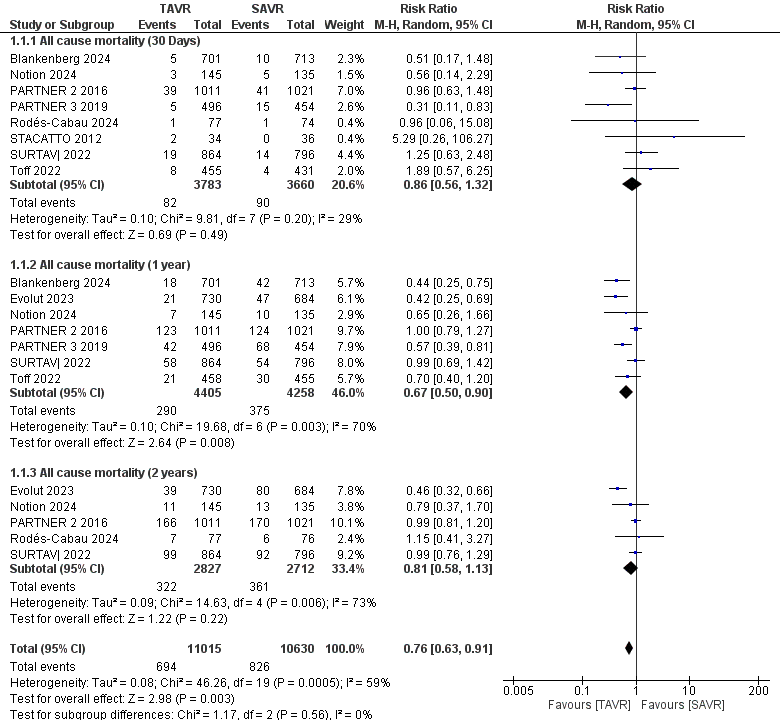
Figure 3. Forest plot for mortality showing individual and pooled relative risk for TAVR vs SAVR in patients at 30 days, 1 year, and 2 years. The pooled RR with 95% CI was measured using a random effects model. Each square and horizontal line represents the point estimate and 95% CI for each study's RR, respectively. The diamond signifies the pooled RR, with its center denoting the point estimate and its width representing the 95% CI
CI – confidence interval, RR – relative risk, TAVR- transcatheter aortic valve replacement, SAVR - surgical aortic valve replacement
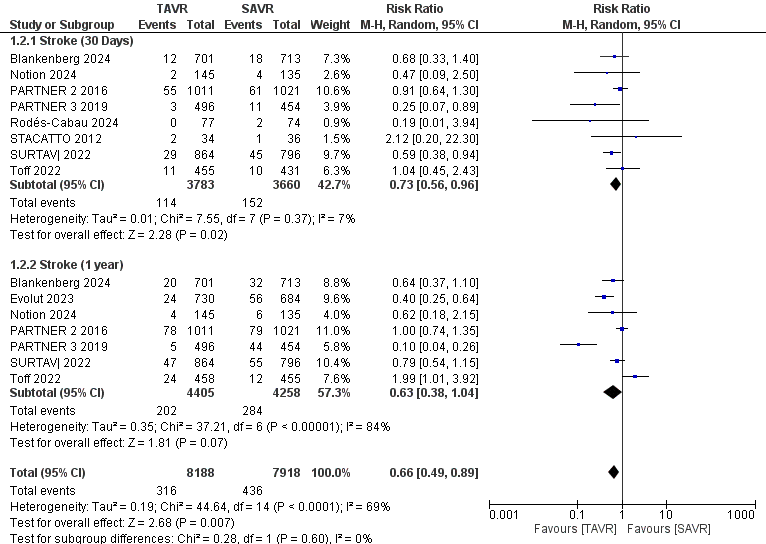
Figure 4. Forest plot for stroke showing individual and pooled relative risk for TAVR vs SAVR in patients with severe aortic valve stenosis at 30 days and 1 year
CI – confidence interval, RR – relative risk, TAVR- transcatheter aortic valve replacement, SAVR - surgical aortic valve replacement
This analysis also showed considerable heterogeneity (I² = 69%). For myocardial infarction outcomes, 5,607 patients (TAVR: 2,832; SAVR: 2,775) were assessed at 30 days. The results revealed a significant reduction in myocardial infarction for TAVR patients, with an RR of 0.60 (95% CI 0.37–0.96, p = 0.03), illustrated in Figure 5, with no heterogeneity detected among the studies (I² = 0%).
A separate analysis of prosthetic valve endocarditis included 8,275 patients (TAVR: 4,028; SAVR: 4,193) at 30 days and 1 year. The reduction in endocarditis was not statistically significant for TAVR compared to SAVR, with an RR of 1.06 (95% CI 0.55–2.06, p = 0.85), as shown in Figure 6, and no heterogeneity was observed (I² = 0%). Lastly, for the length of stay (LOS) analysis, 3,267 patients (TAVR: 1,733; SAVR: 1,534) were included. The analysis demonstrated a statistically significant reduction in LOS for TAVR patients, with a mean difference of -4.30 days (95% CI -5.03 to -3.57, p = 0.00001), as depicted in Figure 7. However, this analysis revealed significant heterogeneity among the studies (I² = 93%).

Figure 5. Forest plot for MI showing individual and pooled relative risk (RR) for TAVR vs SAVR in patients with severe aortic valve stenosis at 30 days
CI – confidence interval, MI – myocardial infarction, RR – relative risk, TAVR- transcatheter aortic valve replacement, SAVR - surgical aortic valve replacement
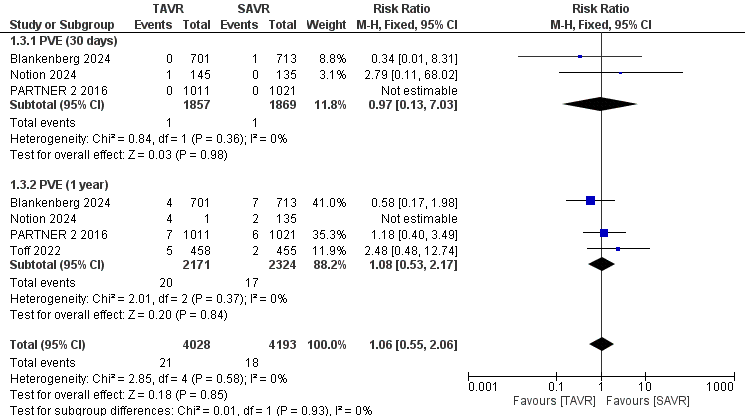
Figure 6. Forest plot for PVE showing individual and pooled relative risk for TAVR vs SAVR in patients with severe aortic valve stenosis at 30 days and 1 year
CI – confidence interval, RR – relative risk, PVE – prosthetic valve endocarditis, TAVR- transcatheter aortic valve replacement, SAVR - surgical aortic valve replacement
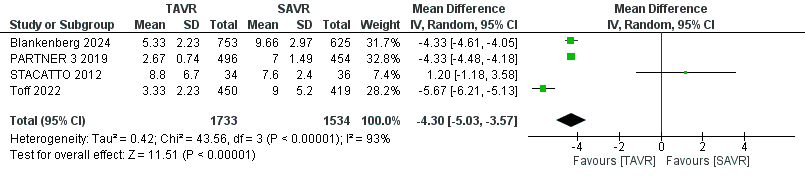
Figure 7. Forest plot for LOS showing individual and pooled relative risk (RR) for TAVR vs SAVR in patients with severe aortic valve stenosis
CI – confidence interval, LOS – length of hospital stay, RR – relative risk, TAVR- transcatheter aortic valve replacement, SAVR - surgical aortic valve replacement
Subgroup analysis
A subgroup analysis based on follow-up duration demonstrates differing outcomes between TAVR and SAVR across multiple RCTs. At the 30-day mark, the relative risk (RR) of all-cause mortality for TAVR compared to SAVR was 0.86 (95% CI 0.56–1.32, p=0.49), indicating no significant difference between the two groups. At 1 year, however, there was a significant reduction in relative risk with TAVR (RR 0.67, 95% CI 0.50–0.90, p = 0.008), favoring this intervention over SAVR. By the 2-year follow-up, the analysis showed no significant difference again, with an RR of 0.81 (95% CI 0.58–1.13, p=0.22). Thus, the only statistically significant finding favoring TAVR was observed at the 1-year mark, while no significant differences were detected at the 30-day and 2-year time points. The overall effect of the analysis, which combines all follow-up durations, indicates a significant reduction in all-cause mortality for patients undergoing TAVR compared to SAVR (RR 0.76, 95% CI 0.63–0.91, p=0.003), suggesting that TAVR is associated with a significantly lower risk of mortality compared to SAVR.
Six studies reported the prostheses type. A subgroup analysis based on the type of valve used in TAVR (self-expanding bioprosthetic valves vs. balloon expanded bioprosthetic valves) revealed that TAVR with self-expanding valves significantly reduced the risk of all-cause mortality compared to SAVR, with a relative risk (RR) of 0.61 (95% CI 0.42–0.89, p=0.010). In contrast, the use of balloon-expandable valves in TAVR did not show a statistically significant difference in mortality compared to SAVR (RR 0.82, 95% CI 0.56–1.19, p= 0.30). In the subgroup analysis comparing mortality between balloon-expandable and self-expanding valves, the incidence of mortality in the surgical group was higher when compared to the self-expanding valve group than when compared to the balloon-expandable valve group, as evidenced by data from PARTNER 3 and Evolut Low Risk studies (13, 21). This suggests that the reduced mortality benefit associated with self-expanding valves might be more pronounced compared to balloon-expandable valves. Overall, TAVR was associated with a significantly lower risk of all-cause mortality (RR 0.70, 95% CI 0.54–0.92, p = 0.010) compared to SAVR. Despite the moderate heterogeneity observed across studies, there was no significant difference between the subgroups based on valve type (p = 0.28), highlighting the consistent benefit of TAVR over SAVR.
Sensitivity Analysis
Confirming the robustness of our findings, exclusion sensitivity analyses in all-cause mortality did not reveal disproportionate effects of any single study on the composite pooled results for each individual endpoint. However, for stroke at 30 days, removing either the Blankenberg or Surtavi study (20, 23) rendered the results non-significant, highlighting the significant influence of these studies (). Conversely, for stroke at 1 year, the results became significant when the Toff study (24) was excluded, indicating that this study may have moderated the overall effect.
In the case of myocardial infarction (MI), the exclusion of any one of the Notion, Blankenberg, or Partner 2 studies (10, 14, 20) led to non-significant results, demonstrating the sensitivity of the pooled estimates to these particular studies.
Meanwhile, for length of stay (LOS) and prosthetic valve endocarditis (PVE), no single trial had a significant impact on the pooled estimates, underscoring the stability and reliability of these findings.
Discussion
Our meta-analysis analyzed over 8000 low to intermediate-risk patients with severe symptomatic AS, comparing TAVR and SAVR across various outcomes. The results generally favor TAVR, showing either lower or comparable mortality rates, supporting the view that TAVR’s less invasive nature reduces early postoperative mortality, as noted by Mack et al. (13).
Studies like NOTION (14) and by Søndergaard et al. (15) reported fewer strokes with TAVR, likely due to avoiding cardiopulmonary bypass and aortic cross-clamping, which are risk factors in SAVR. TAVR was also associated with fewer MIs. Gupta et al.(16) attributed this to TAVR’s minimally invasive nature, reducing myocardial stress and injury.
TAVR showed lower rates of prosthetic valve endocarditis. Butt et al. (17) found a significantly reduced incidence of infective endocarditis in TAVR patients, likely due to shorter procedural times and a less invasive approach. Additionally, TAVR patients had shorter hospital stays. Baron et al. (18) reported reduced intensive care unit stay and overall hospital stay durations, underscoring TAVR’s efficiency and quicker recovery.
However, TAVR incurs higher initial costs. Baron et al. (18) and Galper et al. (19) noted that TAVR is more expensive upfront than SAVR. Despite this, TAVR can lead to savings in hospitalization and physician fees. There were mixed results on total admission costs—Baron et al. (18) found TAVR slightly less expensive, while Galper et al. (19) indicated higher costs, highlighting the need for further studies to balance these factors.
TAVR’s benefits influence clinical practice and policy. Its quicker recovery and reduced early mortality make it a viable option for high-risk patients or those with comorbidities. TAVR’s less invasive nature results in shorter hospital stays and lower resource use, potentially easing the burden on healthcare systems. Nonetheless, higher initial costs challenge widespread adoption. Policymakers need to weigh these costs against the long-term benefits. Ongoing cost-effectiveness studies are crucial for shaping compensation policies and ensuring equitable access. Standardizing procedures and best practices can also enhance outcomes and reduce variability.
Future research should focus on long-term outcomes and device durability, especially in younger and lower-risk populations. Comparative studies of TAVR devices and techniques are essential for optimizing patient selection and outcomes. Including diverse patient populations in trials will improve generalizability. Addressing these research gaps will enhance our understanding of TAVR’s potential and improve patient care.
Study limitations
Despite our findings, there are limitations. Variability in patient populations and follow-up durations may affect generalizability. Differences in endpoint definitions and reporting complicate comparisons, highlighting the need for standardized definitions. The predominance of studies from high-income countries may limit applicability to lower-resource settings. Additionally, variations in procedural techniques, valve types, and operator expertise may contribute to outcome variability.
Conclusion
According to our meta-analysis, currently available data suggests that TAVR is a highly promising and viable therapeutic option for severe symptomatic AS patients with low and intermediate surgical risk, demonstrating a trend towards reduced postoperative mortality, quicker recovery and shorter hospital stays as well as a marked decrease in cardiovascular complications compared to its alternative modality, SAVR. The current evidence base is strong and supports widespread utilization of TAVR for intermediate and low-risk patients, yet limitations exist regarding variability in patient populations and follow-up durations, as well as variations in procedural techniques, valve types, and operator expertise, all of which may affect the generalizability of our results.
To definitively derive conclusions on TAVR versus SAVR, standardized endpoint definitions are needed. Finally, future research should emphasize long-term results and the durability of TAVR devices, especially for younger and lower-risk groups.
Ethics: As the study is the analysis of published literature data, no Ethics Committee approval is required.
Peer-review: External and Internal
Conflict of interest: None to declare
Authorship: S.A., O.H., S.N. equally contribute to the study and thus
fulfilled authorship criteria.
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use: Authors declared they do did not use A.I. assisted technologies while preparation of manuscript
References
| 1.Gashi M. Symptomatic severe aortic stenosis. In: S. Aronow W, editor. Aortic Stenosis - Recent Advances, New | ||||
| Perspectives and Applications. IntechOpen 2022. | ||||
| 2. Kalogeropoulos AS, Redwood SR, Allen CJ, Hurrell H, Chehab O, Rajani R, et al. A 20-year journey in transcatheter | ||||
| aortic valve implantation: Evolution to current eminence. Front Cardiovasc Med 2022; 9: 971762. | ||||
| 3. Otto CM, Prendergast B. Aortic-valve stenosis--from patients at risk to severe valve obstruction. N Engl J Med. 2014; https://doi.org/10.1056/NEJMra1313875 |
||||
| 371: 744-56. | ||||
| 4.Arnold SV, Zhang Y, Baron SJ, McAndrew TC, Alu MC, Kodali SK, et al. Impact of short-term complications on mortality | ||||
| and quality of life after transcatheter aortic valve replacement. JACC Cardiovasc Interv 2019; 12: 362-9. https://doi.org/10.1016/j.jcin.2019.04.032 |
||||
| 5. Sharma T, Krishnan AM, Lahoud R, Polomsky M, Dauerman HL. National Trends in TAVR and SAVR for Patients with | ||||
| Severe Isolated Aortic Stenosis. J Am Coll Cardiol 2022; 80:2054-6. doi: 10.1016/j.jacc.2022.08.787. | ||||
| 6. Baumgartner H. The 2017 ESC/EACTS guidelines on the management of valvular heart disease : What is new and what has changed compared to the 2012 guidelines? Wien Klin Wochenschr 2018; 130: 168-71. https://doi.org/10.1007/s00508-017-1297-5 |
||||
| 7. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2017; 135: e1159-95. https://doi.org/10.1161/CIR.0000000000000503 |
||||
| 8. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med 2019; 380: 1706-15. https://doi.org/10.1056/NEJMoa1816885 |
||||
| 9. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14:135. https://doi.org/10.1186/1471-2288-14-135 |
||||
| 10. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016; 374: 1609-20. https://doi.org/10.1056/NEJMoa1514616 |
||||
| 11.Rodés-Cabau J, Ribeiro HB, Mohammadi S, Serra V, Al-Atassi T, Iñiguez A, et al. Transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis and small aortic annulus: A randomized clinical trial. Circulation 2024; 149: 644-55. https://doi.org/10.1161/CIRCULATIONAHA.123.067326 |
||||
| 12. Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343: d4002. https://doi.org/10.1136/bmj.d4002 |
||||
| 13. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019; 380: 1695-705. https://doi.org/10.1056/NEJMoa1814052 |
||||
| 14. Thyregod HGH, Jørgensen TH, Ihlemann N, Steinbrüchel DA, Nissen H, Kjeldsen BJ, et al. Transcatheter or surgical aortic valve implantation: 10-year outcomes of the NOTION trial. Eur Heart J 2024; 45: 1116-24. https://doi.org/10.1093/eurheartj/ehae043 |
||||
| 15. Søndergaard L, Steinbrüchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, et al. Two-year outcomes in patients with severe aortic valve stenosis randomized to transcatheter versus surgical aortic valve replacement: The all-comers nordic aortic valve intervention randomized clinical trial. Circ Cardiovasc Interv 2016; 9: e003665 https://doi.org/10.1161/CIRCINTERVENTIONS.115.003665 |
||||
| 16.Gupta T, Khera S, Kolte D, Goel K, Kalra A, Villablanca PA, et al. Transcatheter versus surgical aortic valve replacement in patients with prior coronary artery bypass grafting: Trends in utilization and propensity-matched analysis of in-hospital outcomes. Circ Cardiovasc Interv 2018; 11: e006179. https://doi.org/10.1161/CIRCINTERVENTIONS.117.006179 |
||||
| 17. Butt JH, Ihlemann N, De Backer O, Søndergaard L, Havers-Borgersen E, Gislason GH, et al. Long-term risk of infective endocarditis after transcatheter aortic valve replacement. J Am Coll Cardiol 2019; 73: 1646-55. https://doi.org/10.1016/j.jacc.2018.12.078 |
||||
| 18.Baron SJ, Wang K, House JA, Magnuson EA, Reynolds MR, Makkar R, et al. Cost-Effectiveness of transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at intermediate risk. Circulation 2019; 139: 877-88. 19.Galper BZ, Chinnakondepalli KM, Wang K, Magnuson EA, Lu M, Thourani VH, et al. Economic Outcomes of Transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis and low surgical risk: Results from the PARTNER 3 Trial. Circulation 2023; 147: 1594-605. https://doi.org/10.1161/CIRCULATIONAHA.122.062481 |
||||
| 20.Blankenberg S, Seiffert M, Vonthein R, Baumgartner H, Bleiziffer S, Borger MA, et al. Transcatheter or surgical treatment of aortic-valve stenosis. N Engl J Med 2024; 390: 1572-83. https://doi.org/10.1056/NEJMoa2400685 |
||||
| 21.Forrest JK, Deeb GM, Yakubov SJ, Gada H, Mumtaz MA, Ramlawi B, et al. 3-Year outcomes after transcatheter or surgical aortic valve replacement in low-risk patients with aortic stenosis. J Am Coll Cardiol 2023; 81: 1663-74. https://doi.org/10.1016/j.jacc.2023.02.017 |
||||
| 22. Nielsen HHM, Klaaborg KE, Nissen H, Terp K, Mortensen PE, Kjeldsen BJ, et al. A prospective, randomised trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: the STACCATO trial. EuroIntervention 2012; 8: 383-9. https://doi.org/10.4244/EIJV8I3A58 |
||||
| 23. Van Mieghem NM, Deeb GM, Søndergaard L, Grube E, Windecker S, Gada H, et al. Self-expanding transcatheter vs surgical aortic valve replacement in intermediate-risk patients: 5-year outcomes of the SURTAVI randomized clinical trial. JAMA Cardiol 2022; 7: 1000-8. https://doi.org/10.1001/jamacardio.2022.2695 |
||||
| 24. UK TAVI Trial Investigators, Toff WD, Hildick-Smith D, Kovac J, Mullen MJ, Wendler O, et al. Effect of transcatheter aortic valve implantation vs surgical aortic valve replacement on all-cause mortality in patients with aortic stenosis: A randomized clinical trial. JAMA 2022; 327: 1875-87. https://doi.org/10.1001/jama.2022.5776 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

