Annular constrictive pericarditis causing hour-glass contracture of the right ventricle
CASE REPORT
Annular constrictive pericarditis causing hour-glass contracture of the right ventricle
Article Summary
- DOI: 10.24969/hvt.2024.525
- CARDIOVASCULAR DISEASES
- Published: 10/11/2024
- Received: 30/07/2024
- Revised: 29/09/2024
- Accepted: 03/11/2024
- Views: 3558
- Downloads: 2456
- Keywords: Annular constrictive pericarditis, median sternotomy, off pump total pericardiectomy, hour-glass contracture of right ventricle
Address for Correspondence: Utkarsh Sanghavi, Department of Cardiovascular & Thoracic Surgery, U. N. Mehta Institute of Cardiology and Research Center, Civil Hospital Campus, Asarwa, Ahmedabad, Gujarat, 380016, India
Email: utkarshsanghavi7@gmail.com Phone: +93 734 944 8328
Bhargav Patel1, Jenil Bhatt1, Utkarsh Sanghavi2
Department of Cardiovascular & Thoracic Surgery, U. N. Mehta Institute of Cardiology and Research Center, Civil Hospital Campus, Asarwa, Ahmedabad, Gujarat, 380016, India
Abstract
Objectives: Constrictive pericarditis (CP) is characterized by the encasement of the heart by a rigid non-pliable pericardium due to dense fibrosis and adhesions. We hereby describe a rare case of annular constrictive pericarditis (ACP) causing localized constriction of right ventricle leading to its hour-glass contracture, which to our knowledge is the second case managed successfully for ACP.
Case presentation: A 13-year old boy presented with complaints of dyspnea on exertion NYHA class III his daily activities. On examination, his respiratory rate was 28 cycles per minute, jugular venous pressure was raised by 15 cm from sternal angle and had a prominent y descent and with a positive Kussmaul’s sign. Hence, a clinical diagnosis of constrictive pericarditis was suspected. After routine blood investigations, chest X-ray, computed tomography (CT) scan and informed consent, total pericardiectomy was done. The gross and microscopic features were compatible with tuberculous pericarditis. Patient was discharged on post-operative day 5.
Conclusions: ACP is a rare form of CP, which should always be suspected in patients presented with predominant right heart failure symptoms and total pericardiectomy should be performed at the earliest.
Graphical abstract

Keywords: Annular constrictive pericarditis, median sternotomy, off pump total pericardiectomy, hour-glass contracture of right ventricle
Introduction
Constrictive pericarditis (CP) causes thickening of pericardium leading to encasement of the heart inside rigid and non-pliable pericardium due to dense and adhesions and fibrosis. The inflamed/scarred pericardium limits diastolic filling which leads to impaired diastolic function and ultimately to heart failure, which manifests as systemic congestion (1).
Though the true prevalence of CP is unknown, it is seen in 0.2–0.4% of patients after cardiac surgery (2) and can also occur due to inflammation, a variety of etiologies or after pericardial trauma. The most common etiology is idiopathic in developed countries but in developing countries like India tuberculosis is still a common cause.
Chronic constrictive pericarditis was first described by Lower in 1669 (3), while Sauerbruch performed first successful pericardiectomy in 1913 (4). Chronic CP can also cause localized constriction due to pericardial bands, which causes external compression of atrioventricular groove and leads to left or right ventricular inflow obstruction. Previous studies suggest that with intervention, symptoms progresses and early death can occur whereas with surgery the survivors have shown vast improvement in their symptoms and quality of life (5). Though the impact of pericardial calcification on perioperative mortality in CP is unclear, Ling et al. (6) in their study had higher perioperative mortality in patients with calcification whereas in a study by Gimlette (7) calcification was associated with poor postoperative outcome.
Here we report a case of annular constrictive pericarditis causing hour-glass contracture of right ventricle (RV) requiring surgical resection.
Case report
A 13-year old boy presented to our clinic with complaints of dyspnea on exertion New York Heart Association class III his daily activities. He also had a history of bilateral pedal edema and recurrent abdominal distension since three years, which used to be relieved with medications. Patient had a family history of tuberculosis (TB) and therefore TB-PCR (TB -polymerase chain reaction) test was done, which was negative. On examination, his respiratory rate was 28 cycles per minute, jugular venous pressure was raised by 15 cm from sternal angle and had a prominent y descent and with a positive Kussmaul’s sign. Hence, a clinical diagnosis of constrictive pericarditis was suspected. Laboratory investigations (complete blood count, liver function test, renal function test, coagulation profile) were within normal limits. Chest Xray showed constriction over RV (Fig. 1). Further evaluation with 2D echocardiography showed septal bounce, normal left ventricular function, mild right ventricular dysfunction and dilated inferior vena cava (non-collapsible).
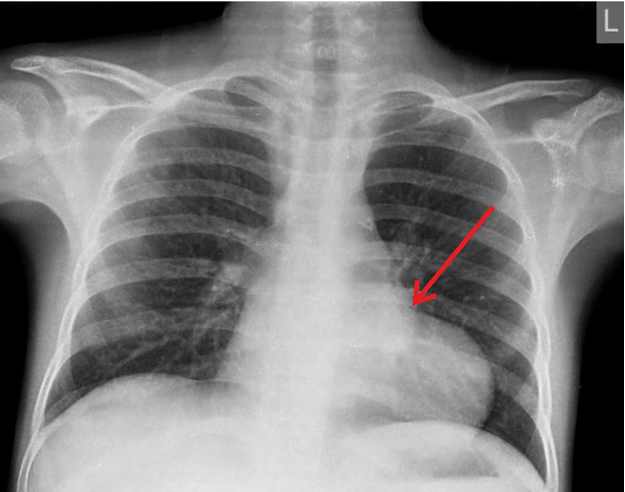
Figure 1. Pre-operative chest roentgenogram showing constriction over right ventricle - arrow shows localized constriction
Contrast enhanced CT of chest showed loculated pericardial collection (23 x 36 x 58 mm) with generalized thickening of pericardium (thickness varying from 6mm to 13mm) with excessive thickening locally over RV causing localized constriction of RV and dilated inferior vena cava and hepatic veins (Fig. 2). Hence, the diagnosis of chronic constrictive pericarditis with a localized thickening was made.
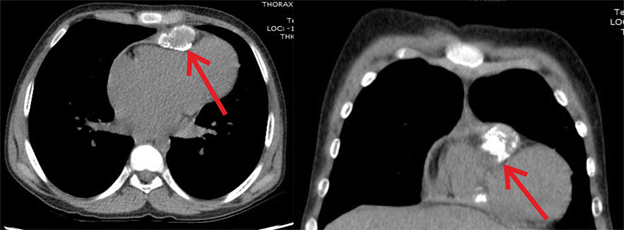
Figure 2. Contrast enhanced computed tomography scan showing loculated pericardial collection with calcium extending into myocardium of right ventricle causing localized constriction (arrow) leading to hour glass contracture of right ventricle
After routine laboratory investigations (complete blood count, renal function tests, liver function tests and coagulation profile) which were within normal limits, discussion with cardiologist, cardiac anesthesiologist and pulmonologist and obtaining written informed consent, patient was taken for surgery. Median sternotomy was done. The pericardium was thick, heavily calcified, densely adhered to myocardium with calcium speckles invading the myocardium. The pericardium was very densely adhered locally all round the RV causing localized constriction leading to hour-glass contracture of the RV. Intra-operatively, even a small cavity of caseous material over anterior aspect of RV was also seen. Off pump total pericardiectomy (phrenic to phrenic) was performed. While releasing the pericardium over left ventricle one of the diagonal branches was injured, which was repaired with 8-0 polypropylene suture.
Post-operatively patient was hemodynamically stable and was discharged on post-operative day 5. As the patient had a family history of tuberculosis, with intra-operative presence of caseous material and also belonged to developed country like India where the most common cause of CP is tuberculosis, patient was empirically started on anti-tuberculosis treatment on post-operative day 4. The final histopathology report revealed necrotizing granulomas compatible with tuberculous pericarditis. At 36-months follow up, patient was asymptomatic and echocardiography showed normal biventricular function, normal valves and no septal bounce.
Discussion
Pericarditis can be acute or chronic. Acute pericarditis accounts for 5% of admissions for non-cardiac chest pain and occur as a manifestation of underlying systemic condition or as an isolated entity. Constrictive pericarditis can be idiopathic or can be a sequel of an acute process. Common causes of chronic CP include idiopathic, post radiation therapy, tuberculosis, viral infections or cardiac surgery (8).
Bertog et al. (5) in their study, demonstrated that the long- term survival was related to the etiology of CP. Their results showed that survival was excellent in cases of idiopathic and miscellaneous causes and a little lower in post-surgical patients and further lower in patients with post-radiation therapy. The other independent predictors of overall survivors were age, pulmonary artery pressure, creatinine levels, serum sodium levels and left ventricular systolic dysfunction whereas pericardial calcification alone was not an independent predictor of survival. These parameters can be used to assess the outcomes following pericardiectomy for chronic CP (5).
Generalized constriction is usually more common in chronic CP but in rare cases localized CP can occur (9). The clinical presentation in localized form depends on the location of constriction, which may vary from obstruction of right ventricular outflow tract, to coronary obstruction, or pulmonary stenosis (10). Annular CP was first described by Paul and his colleagues in 1948 and is extremely rare. Mounsey later described the details of localized CP (10). During pericardiectomy there is a risk of injury to coronary arteries, cardiac veins and to myocardium which increases the risk of operative morbidity and mortality (10). First was reported by Matsuno et al. (10) in 64-year old male and to our knowledge this is the second case of successful surgical management of annular CP.
Diagnosis of CP is usually made using transthoracic echocardiography (TTE) which is the main diagnostic tool but other modalities like CT, magnetic resonance imaging and cardiac catheterization can be used to obtain more details or when TTE is not diagnostic. Total pericardiectomy (phrenic to phrenic and innominate vein to diaphragm) is the gold standard treatment for chronic CP. Partial pericardiectomy is technically easier but is known to cause higher chances of re-constriction either generalized or localized (11). Total pericardiectomy can be done with or without the use of cardio-pulmonary bypass and via thoracotomy or via median sternotomy. The risk of perioperative hemorrhage and other postoperative complications increases with the use of cardio-pulmonary bypass but the extent of pericardiectomy is more important than the type of approach for better outcomes (12).
Conclusion
CP is a potentially curable with pericardiectomy, which is performed with a low risk in experienced centers. Annular or localized constrictive pericarditis though a rare form of CP should always be suspected by clinicians especially in patients presenting with symptoms of right heart failure. In annular CP, total pericardiectomy is indicated at the earliest and can be performed without the use of cardio-pulmonary bypass
Ethics: The patient consented to the submission and publication of this report.
As per our Institute protocol we don’t have to approve the (Image and Case Report) study before publication.
All procedures were in accordance of the ethical standards of our institutional committee and an informed consent was obtained from a patient for all procedures.
Peer-review: External and internal
Conflict of interest: None to declare
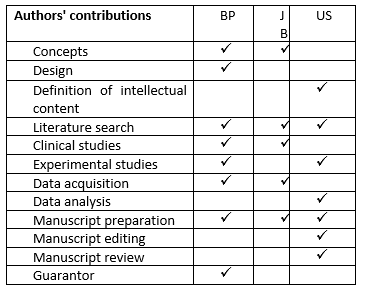
Authorship: All authors equally contributed to case management, manuscript preparation and fulfilled the authorship criteria (See table below).
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use: Authors declared they did not use A.I.- assisted technologies in preparation of manuscript
Availability of data and material: Not applied
References
| 1. Myers RB, Spodick DH. Constrictive pericarditis: clinical and pathophysiologic characteristics. Am Heart J 1999; 138: 219-32. https://doi.org/10.1016/S0002-8703(99)70105-5 PMid:10426832 |
||||
| 2. Miranda WR, Oh JK. Constrictive pericarditis: a practical clinical approach. Progress Cardiovasc Dis 2017; 59:369-79. https://doi.org/10.1016/j.pcad.2016.12.008 PMid:28062267 |
||||
| 3. McCAughan BC, Schaff HV, Piehler JM, Danielson GK, McGoon DC. Early and late results of pericardiectomy for constrictive pericarditis. J Thorac Cardiovasc Surg 89: 340- 50. https://doi.org/10.1016/S0022-5223(19)38783-5 PMid:3974269 |
||||
| 4. Sauerbruch F. Die Chirurgie der Brustorgane. 2nd ed. Springer; Berlin: 1925. pp 286-304. | ||||
| 5. Bertog SC, Thambidorai SK, Parakh K, Schoenhagen P, Ozduran V, Houghtaling PL, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol 2004; 43: 1445-52. https://doi.org/10.1016/j.jacc.2003.11.048 PMid:15093882 |
||||
| 6. Ling LH, Oh JK, Breen JF, Danielsen GK, Mahoney DW, Seward JB, Tajik AJ.. Calcific constrictive pericarditis: is it still with us? Ann Intern Med 2000; 132: 444-50. https://doi.org/10.7326/0003-4819-132-6-200003210-00004 PMid:10733443 |
||||
| 7. Gimlette TM. Constrictive pericarditis. Br Heart J 1959; 21: 9-16. https://doi.org/10.1136/hrt.21.1.9 PMid:13618457 PMCid:PMC517959 |
||||
| 8. Gopaldas RR, Dao TK, Caron NR, Markley JG. Predictors of in-hospital complications after pericardiectomy: a nationwide outcomes study. J Thorac Cardiovasc Surg 2013; 145: 1227-33. https://doi.org/10.1016/j.jtcvs.2012.03.072 PMid:22578895 |
||||
| 9. Bishara F, Muneer K, Sajeev CG. Chronic constrictive pericarditis with right ventricular outflow tract obstruction. IHJ CardiovascCase Rep 2017; 1: 48-50. https://doi.org/10.1016/j.ihjccr.2016.09.004 |
||||
| 10. Matsuno Y, Shimabukuro K, Ishida N, Takemura H. Off-pump complete pericardiectomy for an unusual case of annular constrictive pericarditis. Ann Thorac Surg 2012; 94: e45-7. https://doi.org/10.1016/j.athoracsur.2012.01.080 PMid:22818335 |
||||
| 11. Culliford AT, Lipton M, Spencer FC. Operation for chronic constrictivepericarditis: do the surgical approach and degree of pericardial resectioninfluence the outcome significantly? Ann Thorac Surg 1979; 29: 146-52. https://doi.org/10.1016/S0003-4975(10)61653-0 PMid:7356365 |
||||
| 12. DeValeria PA, Baumgartner WA, Casale AS, Greene PS, Cameron DE, et al. Current indications, risks, and outcome after pericardiectomy. Ann Thorac Surg 1991; 52: 219-24. https://doi.org/10.1016/0003-4975(91)91339-W PMid:1863142 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

