Perioperative renal oximetry via near-infrared spectroscopy for prediction of acute kidney injury in infants undergoing congenital heart surgery: An observational study
ORIGINAL RESEARCH ARTICLE
Perioperative renal oximetry via near-infrared spectroscopy for prediction of acute kidney injury in infants undergoing congenital heart surgery: An observational study
Article Summary
- DOI: 10.24969/hvt.2024.526
- CARDIOVASCULAR DISEASES
- Published: 21/11/2024
- Received: 30/08/2024
- Revised: 25/10/2024
- Accepted: 26/10/2024
- Views: 4686
- Downloads: 2537
- Keywords: Acute kidney injury, cardiopulmonary bypass, creatinine, renal oximetry, near-infrared spectroscopy, cardiac surgery procedures, infant
Address for Correspondence: Abhishek Verma, Department of Cardiac Anesthesia, U. N. Mehta Institute of Cardiology and Research Center, Civil Hospital Campus, Asarwa, Ahmedabad, Gujarat, India, 380016
Email: abhishek.verma.eye@gmail.com Phone: +939453198823
Abhishek Verma1a*, Jigisha Pujara1a, Vivek Kaul1a, Karan Kaushik1a, Shubendu Bajpai1b, Bharat Makwana1a
1aDepartment of Cardiac Anesthesia and Critical Care, U. N. Mehta Institute of Cardiology and 1bResearch Center, Civil Hospital Campus, Asarwa, Ahmedabad, Gujarat, India
Abstract
Objective: Acute kidney injury (AKI) is a frequent complication after pediatric cardiac surgery with cardiopulmonary bypass (CPB). Serum creatinine and biomarkers cannot be continuously monitored. Near- infrared spectroscopy (NIRS) allows continuous assessment of regional tissue oximetry (rSO2) and reflects renal rSO2 when placed on flank overlying kidney. The aim of our study was to establish whether there is a relation between rSO2 values measured by NIRS in early AKI in infants following cardiac surgery.
Methods: Renal NIRS was monitored continuously post-operatively for first 24 hours in sixty infants undergoing cardiac surgery. Patients were divided in AKI (n=21) and non-AKI (n=39) groups. Mean blood pressure, oxygen saturation, central venous oxygen saturation, serum lactate, serum uric acid, blood urea, fluid intake and urine output were measured at various time points. Poor outcome was defined as length of intensive care unit and hospital stays and raised mortality.
Results: 21 patients developed AKI (35%) by AKIN criteria. AKI group patients had significantly prolonged CPB time (p=0.03) and aortic cross-clamp time (p<0.001) compared to non-AKI group patients. There was significant increase in creatinine at 24 hours (p<0.001) in AKI group compared to non-AKI group. AKI patients had significantly lower mean renal rSo2 post-operatively at 8 hours (76.95 (8.65) vs 81.23 (10.44), p=0.044) and 12 hours (73.95 (9.41 vs 81.49 (8.1), p=0.006). Poor outcome parameters (long ICU and hospital stay, death) were significantly more frequent in AKI group than in non-AKI group (all p<0.05).
Conclusion: Continuous monitoring of renal rSO2 can predict subclinical AKI in early phase, allowing early therapeutic intervention before further progression of AKI.
Key words: Acute kidney injury, cardiopulmonary bypass, creatinine, renal oximetry, near-infrared spectroscopy, cardiac surgery procedures, infant
Introduction
Acute kidney injury (AKI) is a common complication in pediatric cardiac surgery, with an incidence of 20-86% (1). It is associated with higher morbidity and mortality (2). Small increases in serum creatinine (sCr) levels reflect renal damage and are able to predict poor outcomes (3). Nevertheless, given that a detectable increase in the levels of sCr does not occur immediately after onset of AKI and sCr levels alone are of limited value for the early identification of the risk of AKI (4).
Identifying risk factors, earlier diagnosis, and prevention of AKI after cardiac surgery in infancy are utmost importance. AKI classifications such as the Pediatric Renal-Injury-Failure-Loss-End (pRIFLE) stage and the Acute Kidney Injury Network (AKIN) criteria based on creatinine clearance and oliguria for renal dysfunction (5, 6). Although changes in these parameters may reflect renal injury but they are relatively insensitive and late markers of AKI.
Recently, markers of early renal injury, such as neutrophil gelatinase-associated lipocalcin, cystatin C, kidney injury molecule, and interleukin-8 have been shown to facilitate earlier diagnosis of AKI (7). Although these biomarkers show some benefits for early detection of AKI but they cannot be measured continuously to monitor renal function in real-time, which is needed to optimize the renal condition.
Graphical abstract
Near-infrared spectroscopy (NIRS) is a new non-invasive technique, which evaluates real-time regional oximetry (rSO2) by measuring the relative concentrations of oxygenated and deoxygenated hemoglobin within a local tissue area (7). Previous studies have suggested that lower rSO2 values can predict the development of organ dysfunction in the brain or other organs after surgery (8).
Renal rSO2 measured by NIRS can reflect renal desaturation or hypoxia, which may contribute to AKI.
Our primary aim was to establish whether there is a relation between early acute kidney injury with mean renal saturation measured by NIRS. The secondary aim was to depict the impact of AKI on hospital stay, intensive care unit stay, and mortality.
Methods
Study design and population
This was a prospective, observational study lasted for 2 years (October 2018 – October 2020) and conducted on 60 infants, undergoing elective surgery for complex congenital heart disease with cardiopulmonary bypass (CPB).
Exclusion criteria were age > 12 months (NIRS values depends upon skin thickness depth of measuring area and that approximately same in infants), use of nephrotoxic drugs within 48 hours of surgery, underlying renal dysfunction, gestational age < 34 weeks at the time of surgery. Patients were divided in 2 groups: those who developed AKI – 21 patients and non-AKI – 39 patients.
An approval of Institutional Ethical Committee (UNMICRC/C.ANESTHE/2018/24) was obtained and a written, informed consent was taken from infants’ parents.
Baseline variables
Demographic, anthropometric and clinical information including age at operation, preoperative as weight, body surface area, Risk Adjustment for Congenital Heart Surgery (RACHS-1) classification (9); intraoperative - duration of CPB, aortic cross-clamp (ACC) time, hemodynamic parameters as mean arterial pressure (MAP), oxygen saturation; arterial blood gas variables as central venous oxygen saturation (ScvO2), lactate level and partial oxygen pressure (pO2); vasoactive inotropic score (VIS), serum creatinine, urea levels, uric acid levels and urine analysis; length of intensive care unit (ICU) stay and hospital stay, mortality were recorded.
Perioperative variables
Risk adjustment for congenital heart surgery (RACHS-1) scoring system was used to categorize congenital cardiac surgery. This scale used to evaluate and stratify anatomic diversity into six categories based on type of performed surgery, age at operation and similar in-hospital mortality (9).
Anesthesia was induced using midazolam (0.1 mg/kg), fentanyl (2-5 µg/kg), ketamine (2-3 mg/kg) and vecuronium (0.1 mg/kg). Right side internal jugular central venous cannula along-with radial/femoral arterial cannulation were done after induction. Anesthesia was maintained with sevoflurane and intermittent boluses of fentanyl and vecuronium.
NIRS for evaluation of renal oximetry
The patient received routine standard care in the postoperative (post-op) period according to institutional protocol. After shifting the patient from operation theater to post-op ICU renal saturation was monitored with NIRS (INVOS 5100C; Somanetics Co., Troy, MI) throughout 24 hours. The pediatric sensor was placed over the right flank at vertebral level of T10 - T12 for renal oximetry and rSO2 was recorded. The mean renal saturation in 2 hours, 4 hours, 8 hours, 12 hours, and 24 hours was collected in postoperative recovery. This study measure NIRS only in postoperative period because intracardiac shunting present preoperatively could cause a shift in baseline saturation. Till date, the threshold of renal saturation is lacking. According to the critical value of ~65 % in cerebral NIRS, somatic values are 10-20 points higher (10). Fluid was given according to body surface area as per protocol. Nurses were unaware of the collection of data for the study.
The blood sample was collected from the central vein in the postoperative period at 4 hours, 8 hours, 12 hours, and 24 hours.
Definitions
AKI was defined using criteria proposed by the Acute Kidney Injury Network (AKIN) and kidney disease improving global outcome (KDIGO) group and recently validated in a study of infants with congenital heart disease (6, 11).
The patient was considered having AKI if they met AKIN stage 1 criteria (defined as increase of serum creatinine by either 0.3 mg/dl or more or a 50 % increase from preoperative baseline).
Statistical analysis
Sample size: minimum 51 infants were required for 80% power of the study. We recruited 60 patients as total study subjects considering drop-outs.
Statistical analysis was done with the help of SPSS 26.0 version of statistical software. Quantitative data are represented as mean with standard deviation whereas qualitative data are represented as number with percentage. Chi-square test was used for qualitative comparative data. Independent and dependent t test was used for quantitative data compared based on AKI. A p<0.05 value was considered as a statistically significant.
Results
This study includes 60 infants and out of these 21 (31%) develop AKI in the post-op period. Comparisons of demographic, anthropometric, clinical and perioperative variables, outcomes between AKI and NAKI groups are shown in Table 1. There was no differences in weight, body surface area or RACHS score and preoperative creatinine between groups, however the infants who developed AKI were older that NAKI children (p=0.0004). Both groups did not differ by mean MAP and oxygen saturation. On the other hand, ScvO2 and pO2 were significantly lower in AKI group as compared to NAKI groups (p=0.014 and p<0.0001, respectively), while there were no differences in lactate levels.
Analysis of intraoperative variables demonstrated that infants with AKI had markedly higher VIS, prolonged ACC and CPB times (p<0.0001, p=0.0001 and p=0.03, respectively).
In the postoperative period patients with AKI had a high vasoactive inotropic score, high postoperative serum creatinine level and low mean renal NIRS (Table 1 and Fig. 1, Fig. 3).
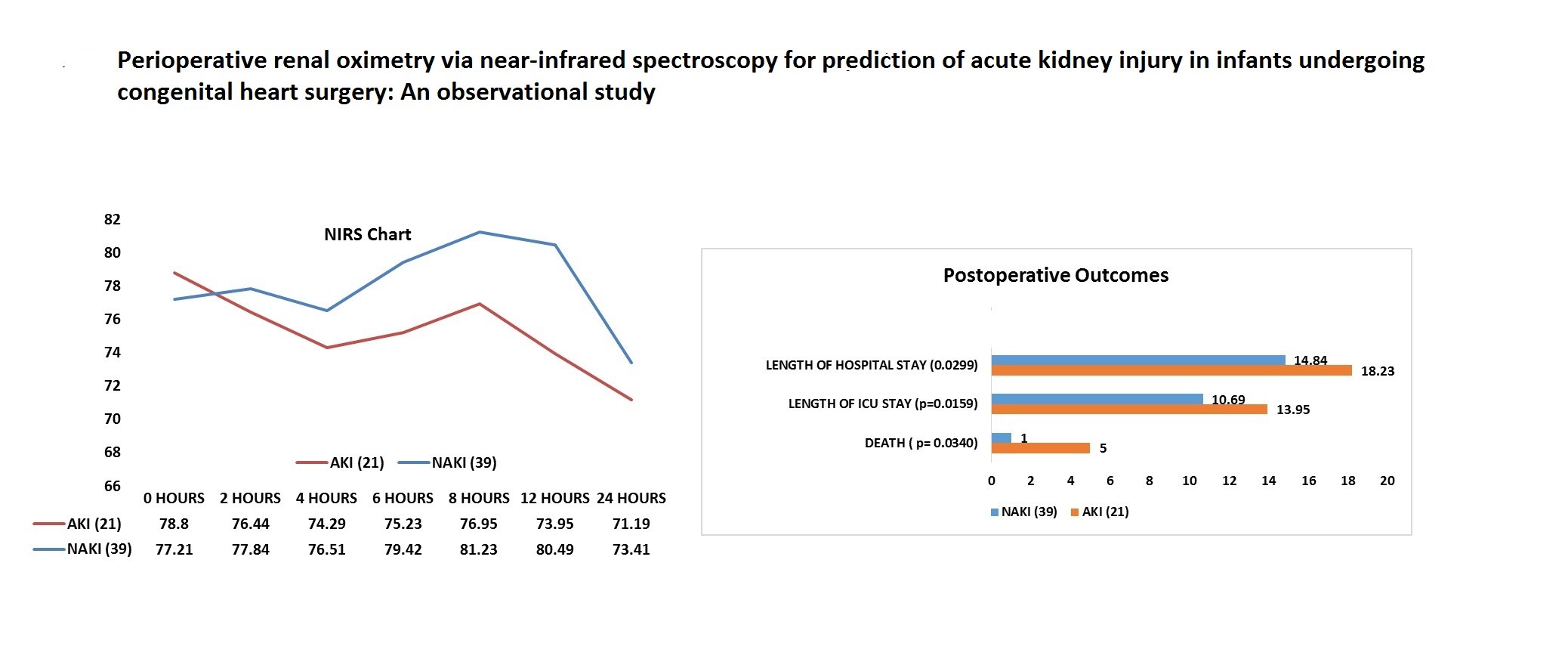
Differences between groups in serum creatinine level at 4th hour, 8th hour, and 12th hour were not statistically significant, but at 24th hour creatinine levels were significantly higher in AKI group (p<0.001) (Table 1, Fig. 1).
This study found that urine output (Fig. 2) was not statistically significant in any period of time taken in study.
Mean renal NIRS showed a significant differences (p<0.001) between groups at 8th hour and 12th hour (Table 2. Fig. 3). Renal desaturation was higher and persisted in AKI as compared to NAKI group. Patients with early low mean renal NIRS later were found to have raised creatinine level.
This study also showed that development of AKI in post-op period increase morbidity and mortality of patients. Our data suggest AKI group had longer ICU and hospital stay times (p=0.0159 and p=0.0299, respectively) than NAKI group. It was seen that death (6 patients died) was more often in patients with AKI than NAKI group (23.8 vs 2.56%, p=0.034) (Table 2, Fig.4).
|
Table 1. Demographic, clinical and perioperative variables |
|||
|
Variables |
AKI group (n=21) |
NAKI group (n=39) |
p |
|
Age, days |
88.05 (36.25) |
58.97 (35.71) |
0.0004 |
|
Weight, kg |
3.280 ( 0.82) |
3.660 (0.93) |
0.1216 |
|
BSA, m2 |
0.220 ( 0.04) |
0.230 (0.05) |
0.433 |
|
Creatinine, mg/dl |
0.47 ( 0.07) |
0.49 (0.11) |
0.509 |
|
Blood urea, mg/dl |
14.50 ( 4.35) |
23.75 (12.90) |
0.002 |
|
Uric acid, mg/dL |
6.13 ( 3.57) |
5.27 (1.28) |
0.183 |
|
RACHS-1 scale |
3.710 ( 0.47) |
3.290 ( 0.92) |
0.0555 |
|
Hemodynamic variables |
|||
|
MAP, mm Hg |
48.10 ( 6.21) |
49.49 ( 6.86) |
0.442 |
|
Spo2, % |
78.91 ( 13.52) |
78.92 ( 12.46) |
0.996 |
|
ABG variables |
|||
|
Scvo2, % |
34.05 ( 7.94) |
41.67 (12.52) |
0.014 |
|
Lactate, mmoL/L |
1.39 ( 0.40) |
2.59 (2.93) |
0.0683 |
|
pO2, mmHg |
47.77 ( 16.77) |
61.43 (10.58) |
<0.0001 |
|
Intraoperative and postoperative variables |
|||
|
VIS |
17.38 ( 1.69) |
13.13 (3.22) |
<0.0001 |
|
ACC time, min |
84.48 ( 19.96) |
76.31 (46.55) |
0.0001 |
|
CPB time, min |
109.95 ( 54.72) |
107.90 (32.45) |
0.03 |
|
Serum creatinine, mg/dL |
|||
|
4hr |
0.59 (0.49) |
0.43 (0.50) |
0.24 |
|
8hr |
0.81 (0.40) |
0.64 (0.48) |
0.18 |
|
12hr |
1.0 (0.20) |
0.92 (0.50) |
0.2387 |
|
24 hr |
1.02 (0.22) |
0.56(0.48) |
<0.0001 |
|
Urine output, ml/kg/hr |
|||
|
4th hour |
3.89 (0.82) |
3.64 (0.88) |
0.293 |
|
8th hour |
3.29 (0.91) |
2.99 (0.82) |
0.192 |
|
12th hour |
3.61 0.85) |
3.16 (0.85) |
0.037 |
|
24th hour |
1.5 ( 0.34) |
1.33 (0.38) |
0.09 |
|
Data are presented as mean (SD) AKI –acute kidney injury, ABG - arterial blood gas analysis, NAKI – non-AKI, pO2 - partial oxygen pressure, ScvO2 - central venous oxygen saturation, SpO2 – oxygen saturation, VIS – vasoactive inotropic score |
|||
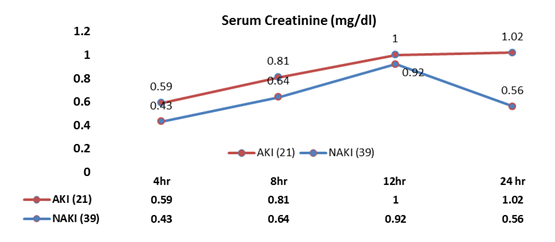
Figure 1. Levels of serum creatinine at interval of 4,8,12,24 hour
AKI –acute kidney injury, NAKI – non-AKI

Figure 2. Urine output at interval of 4,8,12 and 24 hour
AKI –acute kidney injury, NAKI – non-AKI
|
Table 2. Renal saturation and outcomes in infants undergoing cardiac surgery |
|||
|
Variables |
AKI group (n-21) |
NAKI group (n=39) |
p |
|
Renal saturation (rSO2), % |
|||
|
4th hour |
74.29 ( 10.2) |
76.51 ( 9.84) |
0.4139 |
|
8th hour |
76.95 ( 8.65) |
81.23 ( 10.44) |
0.0448 |
|
12th hour |
73.95 ( 9.41) |
80.49 ( 8.10) |
0.0066 |
|
24th hour |
71.19 ( 8.11) |
73.41( 8.77) |
0.3413 |
|
Outcomes |
|||
|
ICU stay, days |
13.95(4.10) |
10.69(5.20) |
0.0159 |
|
hospital stay, days |
18.23(5.22) |
14.34(5.39) |
0.0299 |
|
Death, n(%) |
5 (23.8) |
1 (2.56) |
0.0340 |
|
Data are presented as Mean ( S.D and n(%) AKI –acute kidney injury, ICU – intensive care unit, NAKI – non-AKI, rSO2 - renal saturation |
|||
Discussion
In the current study, postoperative AKI occurred in 21 patients.
Our study demonstrated that infants with AKI were of older age, had lower partial oxygen pressure and central venous oxygen saturation, did not differ preoperatively by serum creatinine level, had longer CPB and ACC times and need more inotropic support. After operation analysis of dynamic changes in serum creatinine level, urine output and renal saturation revealed significant changes in creatinine level at 24th hour after surgery. Renal saturation showed changes in values earlier at 8th hour after surgery. AKI was associated with longer ICU and hospital stay and higher mortality.
Although the pathogenesis of cardiac surgery-associated AKI is multi-factorial, it was believed that renal ischemia caused by hypo-perfusion was the most important factor (12, 13).
In this study, only infants were included. Early detection of deterioration in renal function and perfusion is important for the effective management of patients with AKI after cardiac surgery. In the present study, slow decreases in renal rSO2 were observed after CPB in the post-op period and mostly those patients were later diagnosed as AKI .
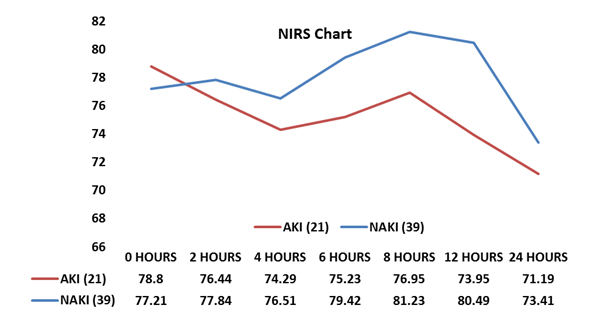
Figure 3. Mean NIRS (rSO2) at interval of baseline, 2, 4,8,12 and 24 hour
AKI –acute kidney injury, NAKI – non-AKI, NIRS - near-infrared spectroscopy, rSO2 renal real-time regional oximetry
Figure 4. Post-operative outcomes
AKI –acute kidney injury, NAKI – non-AKI
It has been observed that despite the use of optimal support and better strategy, the AKI rate in pediatric cardiac surgery still remains high. To diagnose AKI early in addition to clinical examination, various strategies are used in pRIFLE, AKIN, and KIDGO classification (5, 6, 11). All these are based on urine output and serum creatinine, which signals when 25-50% functional loss already done in kidney as proved in many previous studies.
So in our study raise in serum creatinine level occurred at more than 24 hours and reflected decline in filtration rate of kidney but not as an early marker.
Urine output is an independent predictor of AKI according to AKIN criteria; however urine output in our patients did not show significant differences. Due to use of diuretics in the pre-op and post-op period their values can change and unreliable.
Our observational study analyzed different parameter as renal saturation to predict AKI in early post-op period. We concluded that continuous rNIRS monitoring in infants after cardiac surgery may be a valuable and importantly, an early parameter for predicting AKI, and may allow early therapeutic intervention before further progression of AKI.
Of great practical value are its non- invasive and continuous character and the potential to deliver instantaneous values. In our study, rNIRS was superior to conventional biomarker of renal injury for early detection of developing AKI, although a combined model that incorporate biomarker and renal NIRS may be expected to have increased sensitivity and specificity
Raise in serum creatinine also confirms the diagnosis of AKI but its level may vary in infants during cardiac surgery due to hemodilution in CPB. Serum creatinine is the most commonly used test for diagnosis of AKI in postoperative period but excessive use of diuretics after cardiac surgery may alter serum creatinine level in infant’s circulation. Most importantly its level in body increases in AKI but late, so intervention and strategy for management of AKI is delayed and may compromise patient hemodynamics and increase morbidity.
We have assessed the early predictor of AKI after cardiac surgery. In this study, only infants were included as their skin thickness depth of kidney from electrode is almost identical. Early detection of deterioration in renal function and perfusion is important for the effective management of patients with AKI after cardiac surgery. In the present study, decreases in renal rSO2 were observed in the post-op period and that’s mean is measured at different intervals. Renal rSO2 decreases more rapidly in patients with AKI group than NAKI group.
In addition, sustained low renal rSO2 values significantly predicted the risk of AKI after surgery.
Intraoperative cerebral oxygen desaturation is associated with poor neurologic outcomes such as postoperative cognitive dysfunction and stroke (10). The use of NIRS to measure low renal oximetry has been reported to correlate with renal dysfunction in infants undergoing cardiac surgery (13). In that previous study, low renal oximetry values were observed after surgery, which preceded the peaks of creatinine level by 48 hours
CPB per se and hemodynamic compromise during the intra- and postoperative course could be responsible for postoperative AKI.
Various risk factors for the development of AKI have been noted, among them, bypass time, ischemia time, vasoactive inotropic score, and RACHS-1 score; however, sufficiently sensitive and specific early markers of renal injury have not been consistently defined. Somatic NIRS is thought to reflect tissue perfusion. According to our knowledge Validated critical baselines for renal rSO2 are lacking and do not discern between cyanotic and acyanotic cardiac lesions. Moreover, the definition of pathological NIRS values is not consistent over studies, ranging from 50% to 80%, and do not account for the magnitude and duration of desaturations. According to the threshold of 55% - 65% accepted in cerebral NIRS studies (10), 10 to 20 points higher in somatic than cerebral values in healthy infants. One study by Colasacco et al. (14) included NIRS measurements intraoperatively, but no correlation with AKI was reported. In each of these studies, different cutoff values for renal rSO2 were used to define critical desaturation episodes, including criteria for relevant time periods of desaturations, which impede comparisons between studies. In contrast to these studies, we could show a significant difference in renal desaturation, by our criteria, between infants with AKI and those without AKI developing at the earliest time point. Our study shows AKI is likely to develop in patients with low mean rSO2 patient in the post-op period. It was observed that the mean NIRS value after the surgery when measured at 4th hour was statistically insignificant (p=0.39) but at intervals of 8, 12 and 24 hour rNIRS values were statistically significantly different between AKI and non-AKI groups (p<0.001). So, here it was clearly shown if mean rNIRS remain <80% then chance of development of AKI increased significantly.
As CPB alters oxygen delivery, this difference was most distinct after stopping CPB; however, it was consistent over 24 hours postoperatively. Serum creatinine obviously will not change until 25%–50% of renal function is lost, and the effect of fluid dilution may conceal the real change in serum creatinine. Therefore, the rise in serum creatinine in neonatal patients is always delayed until 36–48 hours after surgery. Patients in an early phase of AKI might not be discerned and would miss timely intervention.
Bonsante et al. (15) showed patients with low renal NIRS on day 1 with peak serum creatinine from day 2 to day 7 after cardiac surgery were associated with AKI.
In our study, the increase in serum creatinine at 24th hour in post-op ICU was statically significant (p <0.05), which is comparable with the study results by Owen et al. (13).
Some neonates present with non-oliguric AKI, especially preterm neonates due to the higher proportion of body water (16).
Thus, urine output (UO) less than 0.5 ml/kg/hr is not sufficient, and some researchers refuse to accept urine output as a reliable indicator of AKI. In our study, urine output was not significantly different between AKI and NAKI groups at any time.
Our results on association of AKI with higher ICU and hospital stays, VIS and increase in mortality are in accordance with previous studies (16-18) that demonstrated association of AKI with longer hospital stay, high VIS and mortality (16-18).
Study limitations
There was a possibility that the NIRS sensor used in our study could not measure the regional tissue oxygen saturation of the true renal area (renal cortex and medulla) the measurement might be contaminated by rSO2 of the near renal area (perinephric and perinephric fat tissue surrounding the kidney).
Fluctuation in rNIRS can be easily seen during movement and position of patient also during bedding sometime electrode may lose contact which may distort your mean result but trend of rNIRS is also help in deciding vulnerable patients.
Several postoperative clinical factors contribute to the development of AKI, which were not considered in this study.
There are age-related changes (due to the maturation of organs continuing even after birth) that affect serum creatinine levels in neonates and infants.
Conclusion
Here we concluded that, low renal NIRS can have a promising role in prediction of AKI and can be used as a prognostic tool in pediatric cardiac surgery. Its specificity increase if we go with multimarker approach by taking other parameters like lactate, serum creatinine, blood urea, serum uric acid, urine output, along with clinical assessment.
Trend of rNIRS and time spend by rNIRS below threshold value is more superior so depends upon that case can be managed before the irreversible injury. Further growth in this area still lacking and up to date there is no guideline for cyanotic and acyanotic disease are present.
Ethics: The patients` parents consented to the submission and publication of this report. Informed consent was obtained from all infants` parents or guardians involved in this study at the time of treatment initiation.
The study is approved by Institutional Ethical Committee (UNMICRC/C.ANESTHE/2018/24).
All procedures were in kept in accordance with the ethical standards of our institutional committee and international standards on human studies
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: A.V., J.P., V.K., K.K., S.B., and B.M. equally contributed to manuscript preparation and fulfilled the authorship criteria.
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use: Authors declared they did not use A.I.- assisted technologies in preparation of manuscript
Availability of data and material: Not applied
References
| 1. Chew ST, Mar WM, Ti LK. Association of ethnicity and acute kidney injury after cardiac surgery in a South East Asian population. Br J Anaesth 2012; 110: 397-401. https://doi.org/10.1093/bja/aes415 PMid:23171723 |
||||
| 2. Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009; 119: 2444-53. https://doi.org/10.1161/CIRCULATIONAHA.108.800011 PMid:19398670 |
||||
| 3. Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009;119: 495-502. https://doi.org/10.1161/CIRCULATIONAHA.108.786913 PMid:19153273 |
||||
| 4.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol 2004; 15:1597-605. https://doi.org/10.1097/01.ASN.0000130340.93930.DD PMid:15153571 |
||||
| 5.Volpin L, Sugo EK, Consulin JC, Tavares TLG, Aragon DC, Carlotti APSPS. Epidemiology and outcome of acute kidney injury according to pediatric Risk, Injury, Failure, Loss and End-stage renal and kidney disease: improving global outcomes criteria in critically ill children- A prospective study. Pediatr Crit Care Med 2016; 17: e229-38. https://doi.org/10.1097/PCC.0000000000000685 PMid:26890198 |
||||
| 6.Wyckoff T, Augoustides JG. Advances in acute kidney injury associated with cardiac surgery: the unfolding revolution in early detection. J Cardiothorac Vasc Anesth 2012; 26: 340-5. https://doi.org/10.1053/j.jvca.2012.01.001 PMid:22405191 |
||||
| 7.Martensson J, Martling C R, Bell M. Novel biomarkers of acute kidney injury and failure: clinical applicability. Br J Anaesth 2012; 109: 843-50. https://doi.org/10.1093/bja/aes357 PMid:23048068 |
||||
| 8. Moerman A, Vandenplas G, Bové T, Wouters PF, De Hert SG. Relation between mixed venous oxygen saturation and cerebral oxygen saturation measured by absolute and relative near-infrared spectroscopy during off-pump coronary artery bypass grafting. British J Anaesth 2013;110: 258-65. https://doi.org/10.1093/bja/aes375 PMid:23103778 |
||||
| 9. Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Lezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 2002; 123: 110-8. https://doi.org/10.1067/mtc.2002.119064 PMid:11782764 |
||||
| 10.Murkin J M, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth 2009; 103 Suppl 1: i3-13. https://doi.org/10.1093/bja/aep299 PMid:20007987 |
||||
| 11. Jackson AH, Salerno P, Shaw M, Dhital K. Absent renal blood flow diagnosed by transesophageal echocardiography during cardiopulmonary bypass. J Cardiothor Vasc Anaesth 2012; 26: 1080-2. https://doi.org/10.1053/j.jvca.2011.07.028 PMid:21993401 |
||||
| 12. Rajagopal SK, Emani SM, Roy N, Westgate L, Bacha EA. Acute kidney injury and regional abdominal perfusion during neonatal aortic arch reconstruction. J Thorac Cardiovasc Surg 2010; 140: 453-8. https://doi.org/10.1016/j.jtcvs.2010.03.034 PMid:20447657 |
||||
| 13. Owens GE, King K, Gurney JG, Charpie JR. Low renal oximetry correlates with acute kidney injury after infant cardiac surgery. Pediatr Cardiol 2011; 32: 183-8. https://doi.org/10.1007/s00246-010-9839-x PMid:21085945 |
||||
| 14. Colasacco C, Worthen M, Peterson B, Lamberti J, Spear R. Near-infrared spectroscopy monitoring to predict postoperative renal insufficiency following repair of congenital heart disease. World J Pediat Congenit Heart Surg 2011; 2: 536-40. https://doi.org/10.1177/2150135111411932 PMid:23804464 |
||||
| 15. Bonsante F, Ramful D, Binquet C, Samperiz S, Daniel S, Gouyon JB, et al. Low renal oxygen saturation at near-infrared spectroscopy on the first day of life is associated with developing acute kidney injury in very preterm infants. Neonatol 2019; 115: 198-204. https://doi.org/10.1159/000494462 PMid:30645992 |
||||
| 16.Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med 2011;39: 1493-9. https://doi.org/10.1097/CCM.0b013e31821201d3 PMid:21336114 PMCid:PMC3286600 |
||||
| 17. Hazle MA, Gajarski RJ, Aiyagari R, Yu S, Abraham A, Donohue J, et al. Urinary biomarkers and renal near-infrared spectroscopy predict intensive care unit outcomes after cardiac surgery in infants younger than 6 months of age. J Thorac Cardiovasc Surg. 2013; 146: 861-7. https://doi.org/10.1016/j.jtcvs.2012.12.012 PMid:23317940 PMCid:PMC3653979 |
||||
| 18. Govender P, Tosh W, Burt C, Falter F. Evaluation of increase in intraoperative lactate level as a predictor of outcome in adults after cardiac surgery. J Cardiothor Vasc Anesth 2020; 34:877-84. https://doi.org/10.1053/j.jvca.2019.10.039 PMid:31787432 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

