Histophysiology of the lungs and oxygen transport function of the blood with an exclusively fatty diet
ORIGINAL RESEARCH ARTICLE
Histophysiology of the lungs and oxygen transport function of the blood with an exclusively fatty diet
Article Summary
- DOI: 10.24969/hvt.2024.537
- CARDIOVASCULAR DISEASES
- Published: 29/12/2024
- Received: 13/10/2024
- Revised: 14/11/2024
- Accepted: 15/11/2024
- Views: 3847
- Downloads: 2327
- Keywords: High-fat diet, rats, airway remodeling, acid-base equilibrium, pulmonary gas exchange, lactic acid
Author for Correspondence: Elmira M. Mamytova, Department of Neurology and Clinical Genetics named after A.M. Murzaliev Kyrgyz State Medical Academy named after I.K. Akhunbaev, Bishkek, Kyrgyzstan
Email: elmiramamytova@yahoo.com Mobile: +996 551 32 53 14
Aycholpon T. Israilova1a, Elmira M. Mamytova1a, Yusuf Kh-M. Shidakov2, Ayna Dj.Mamytova3, Daniil A.O1b,
Bolotbek Alymjan ulu2, Aigul T. Altynbekova4, Rustam R. Tuhvatshin1c
1aDepartment of Neurology and Clinical Genetics named after A.M. Murzaliev, 1bFaculty of Medicine, and 1cDepartment of Pathological Physiology, Kyrgyz State Medical Academy named after I.K. Akhunbaev, Bishkek, Kyrgyz Republic
2Laboratory of Modeling of Pathological Processes, Medical Faculty. Kyrgyz-Russian Slavic University named after B.N. Yeltsin, Bishkek, Kyrgyz Republic
3Department of Internal Diseases of the Kyrgyz State Medical Institute for Retraining and Advanced Training named after S.B., Daniyarov Bishkek, Kyrgyz Republic
4NAO "National Center for Child Rehabilitation", Astana, Republic of Kazakhstan
Abstract
Objective: To study of the histophysiology of the lungs and the oxygen transport function of blood in rats fed by an extreme fatty diet for 30 days.
Methods: The study design is a randomized experimental study. The work was carried out on white, mongrel male rats weighing 200-250g. During the 30 days period of time, 10 animals were fed exclusively with animal fat. Then the animals were slaughtered under general anesthesia, after preliminary blood sampling for subsequent determination of the lactate level and oxygen transport function of the blood. Histological preparations of the lungs were stained with hematoxylin-eosin and Van Gieson. Histological analysis of lung components was performed using the application for measuring microscopic objects Tor View. For statistical analysis, the Student's criterion was applied for independent and paired samples in the SPSS 22.0 program.
Results: An extreme fat diet consisting of 100% animal fat led to an increase in serum lactate level (0.79 (0.78)) vs 1.92 (0.28)) (p<0.001). Oxygen transport function decreased in animals of the dietary intervention group. The ratio of the rate of oxygen transport by arterial blood (VaVO2) to the rate of its consumption by tissues (VO2) in the main group decreased by 3 times (4.44 (0.47)) due to a pronounced decrease in the rate of oxygen transport by arterial blood (4.1042 (0.091)) vs. 64.4060 (0.14)) (p<0.001). There was a 2-fold shift of the buffer bases towards its decrease in the animals of the main group as compared to control (4.9764 (0.81) versus 8.19 (0.22)) (p<0.001). Remodeling of the histophysiology of the bronchial tree and branches of the pulmonary artery was characterized by pronounced heterogeneity, depending on the level of division (large, medium, bronchioles) of the bronchi and the associated pulmonary and bronchial arteries. At the level of the alveolar tree, areas of atelectasis with blood deposition and emphysema of the acinar apparatus of the lungs were observed. And at the level of the microcirculatory bed intravascular, vascular, and circulatory changes were noted.
Conclusions: A 30-day feeding of male rats exclusively with sheep fat led to an increase in lactic acid concentration, a decrease in the rate of oxygen transport by arterial blood and a deficiency of blood buffer bases, which were combined with microcirculatory changes, and at the alveolar level with foci of atelectasis with blood deposition, emphysema and inflammatory reaction.
Key words: High-fat diet, rats, airway remodeling, acid-base equilibrium, pulmonary gas exchange, lactic acid
Graphical abstract
Introduction
The incidence of nutrition-related diseases in the world is steadily increasing. This causes a flurry of scientific research on the effect of diet as a key factor of exposome on the structure and function of the central nervous system (1), cardiovascular (2-4), digestive (5), urinary systems and relatively limited respiratory system (6).
There are a number of studies on the effect of high-fat diets on metabolism in the body (7), brain (8), kidneys (9), intestines (10), liver (11), heart (12, 13), and pancreas (14) of rats.
An analysis of scientific evidence showed that the effects of a high-fat diet on various organs depended on the type of fat. For example, fat in the diet of experimental animals had both a protective and pathogenic effect on various organs. Prolonged consumption of large amounts of animal fats can increase cholesterol levels, clog arteries and increase the risk of stroke and other forms of heart disease, not to mention obesity, diabetes, cancer and many other conditions (13). And a higher intake of vegetable fats, polyunsaturated fats and vegetable oil was associated with a reduction in these risks. It should be noted, that the effects of high-fat diets depend not only on the type of fat (it is fat of animal or vegetable origin), but also on the duration of the diet itself and on the object of study (healthy animals or animals with simulated pathological conditions under experimental conditions), the age of the animal and the proportion of fat in the diet (35%, 50%, 70%).
As for the effect of a high-fat diet on the histomorphology of the lungs, there is one work devoted to the study of changes in the lungs during long-term observation (4 months before pregnancy and 21 days before childbirth) of female rats on a diet high in animal fats.
The lung pattern was characterized by foci of inflammation, interstitial fibrosis, hyperplasia of type II pneumocytes, many of which become foamy, eosinophilic, thickening of the septa and a decrease in the airiness of the alveoli (15).
Along with this, we did not find information about the effect of an isolated (with 100% animal fat content) fatty diet on the lungs during a short period of dietary intervention (30 days) neither in English-speaking nor in Russian-language sources of biomedical literature. The results presented in this paper are a fragment of a study in which it is also planned to study the second stage of restoration (reverse development) those changes in the lungs that occurred under the influence of an unbalanced diet.
The purpose of the study: To study of the histophysiology of the lungs and the oxygen transport function of blood in rats fed by an extreme fatty diet for 30 days.
Methods
Stud design and population
Study design: experimental randomized study.
Study population
Twenty 7-month-old healthy and sexually mature male Wistar rats with weight 200-250 gr were included in our study. Rats younger than 7 months and females were excluded. Twenty male rats were randomly divided by number into two groups (n=10 rats per group): control (standard food) and experimental (high fat diet).
The study was conducted on the basis of the Laboratory of Experimental Modeling of pathological processes of the Kyrgyz-Russian Slavic University (KRSU) in accordance with the rules of research and laboratory practice approved by the order of the Ministry of Health and Social Development of the Russian Federation No. 708 dated August 23, 2010 "On Approval of the Rules of Laboratory Practice". The animals were cared for and fed by the staff of the vivarium in accordance with international standards for conducting experimental work on animals used for scientific purposes (Consensus Guidelines on Animal Ethics and Welfare 2010).
The protocol of the study was approved by the local Ethics Committee of the Kyrgyz State Medical Academy named after I.K. Akhunbayev.
The procedure for conducting experimental procedures was drawn up in accordance with the research protocol and is shown in Figure 1.
Intervention
Diet
The isolated fat diet consisted of fat, while the standard feed contained second-grade wheat flour, oat flakes, cow's milk, table salt, herbs and meat - young pork. The animals had free access to water and food for 30 days. The high-fat diet consisted exclusively of fat, where the proportion of fat was 97%, the proportion of fat in the standard diet was 15% (Table 1). Tail fat consisted of fats (saturated, monounsaturated, polyunsaturated) - 97%; water – 0.3%; vitamins D, E, B4 – 2%; macroelements (phosphorus, sulfur, chlorine, sodium, selenium) – 0.5%; cholesterol - 0.2%.
|
Table 1. The composition of the extreme fat and standard diet |
|||
|
High fat diet |
Normal rat chow diet |
||
|
Nutrients |
% /100 gr |
Nutrients |
%\100gr |
|
Carbohydrate |
0 |
Carbohydrate |
49 |
|
Protein |
0 |
Protein |
21 |
|
Fat |
97 |
Fat |
15 |
|
Total energy (kcal\100g) |
902 |
Total energy (kcal\100g) |
312 |
Lactate levels and oxygen trasport
After slaughtering animals under general anesthesia (chloroform), the lactate level in blood samples was determined to clarify the degree of influence of an isolated fat diet on the oxygen transport function of the lungs and on the metabolic profile. Lactate (CL) and HB (hemoglobin) levels from the carotid artery were used to calculate the shift of buffer bases (BE), the rate of oxygen transport by arterial blood (VaVO2), the rate of oxygen consumption by body tissues (VO2) and the ratio between them VaVO2\VO2- (K) according to the regression formula. After determining the concentration of CL, the following regression formula was applied: CL = 13.8 x K-1.33 x BE =2.86 (3.66-CL); V2=4.88(1-1.47-3/2).
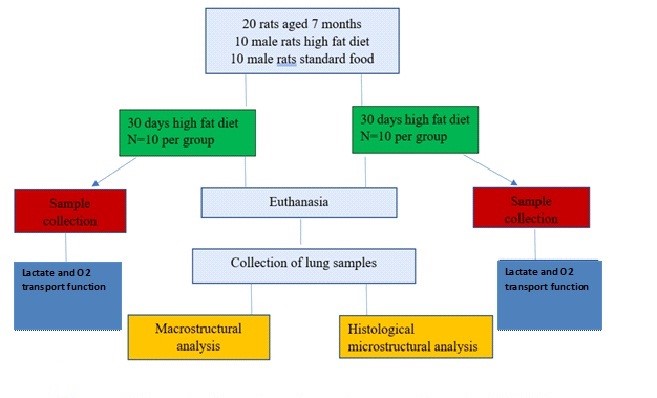
Figure 1. The order of experimental procedure according to ARRIVE protocol
Histological preparation of tissue samples
The laboratory rats were sacrificed for histomorphological analysis of the lungs. The animals were equally prepared for each moment of expiration. After slaughtering the animals, lung fragments were fixed in a 10% neutral formalin solution. Histological sections 6-7 microns thick were stained with hematoxylin-eosin and Van Gieson, which were studied under an Olympus x40 color microscope with simultaneous logging and microphotography using a digital camera coupled with the optical system of the microscope and a computer.
Histomorphological analysis
Histological analysis of lung components was performed using the application for measuring microscopic objects Tor View.
Statistical analysis
Statistical analysis was carried out using the SPSS 22.0 program. The continuous variables were designated as the mean and standard deviation. An independent two-sample t-test was performed to compare the indicators of oxygen transport function of blood, lactate and the shift of buffer bases between the experimental and control groups. The value of p≤0.05 was considered statically significant.
Results
Lactate levels and oxygen transport function of the blood data and comparison results
As Table 2 shows, an extreme fat diet consisting of 100% animal fat led to an increase in serum lactate levels (1.92 (0.28)) in animals of the experimental group, the values of which exceeded the values in animals of the control group (0.79 (0.78)) by 2.4 times.
Oxygen transport function in animals of the dietary intervention group was also impaired. So, in particular, there was a significant decrease (15 times) in VaVO2 in animals of the experimental group (4.10 (0.09)) compared with the control group (64.41 (0.14); and VO2 in animals of the experimental group changed moderately (4.06 (0.12), this indicates the reversibility of structural morphological changes in the lung parenchyma. K correspondingly decreased by 3 times due to a significant decrease in the rate of oxygen transport by arterial blood in animals of the dietary intervention group (4.44(0.47)), compared with the control group. There was a shift of the BE towards its decrease by 2 times in the animals of the experimental group (4.98 (0.81)), and the same parameter of the control group was 8.19 (0.22), (p<0.001).
|
Table 2. The effect of an extreme fat diet on lactate levels and oxygen transport function of the blood |
|||
|
Parameters |
The main group (n=10) |
The control group (n=10) |
p |
|
Lactate, mmol/l |
1.92 (0.28) |
0.79 (0.78) |
<0.001 |
|
VaVO2 |
4.10 (0.091) |
64.41(0.14) |
<0.001 |
|
VO2 |
4.06 (0.12) |
4.64 (0.06) |
<0.001 |
|
К |
4.44 (0.47) |
13.76 (0.33) |
<0.001 |
|
ВЕ |
4.98 (0.81) |
8.19 (0.22) |
<0.001 |
|
BE - shift of buffer bases, K - ratio of the rate of oxygen transport by arterial blood to the rate of oxygen consumption, VaVO2 - the rate of O2 transport by arterial blood, VO2 – tissue oxygen consumption |
|||
Morphological analysis
Effect of fat diet on histological characteristics of the lung
Changes at the level of large bronchi are characterized by a violation of microhemo- and lymphocirculation in the mucous and submucosal layers of the bronchial wall. As a result, hyperemia of the mucous membrane is observed in some areas of the wall of large bronchi, which leads to its thickening, increased folding of the epithelium, and partial loss of cilia. In other areas, the blood filling of the bronchial wall is reduced, where thinning of the mucous and submucosa is noted, the folds of the epithelium are straightened. The expanded bronchus acquires a cylindrical shape (Fig.1).
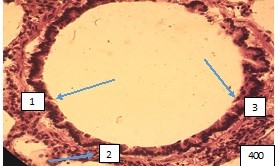
Figure 1. Large intrapulmonary bronchus of cylindrical shape. (1).The folds of the mucous membrane are straightened, stretched and tightly attached to the surrounding layers of the bronchial wall. It is not possible to distinguish the cell populations of the multi-row prismatic ciliated epithelium of the mucosa. (2). Bundles of smooth muscle cells are relaxed and elongated along the circumference of the bronchial wall. (3). The collagen stroma around the bronchus is coarsened and edematous. Paraffın fıllıng, hematoxylin -eosin staining, 400Х.
Therefore, the lumen of the large bronchi under a light microscope has a different shape and size. In the narrowed areas, swelling of smooth muscle cells and fibrous layers of the bronchial wall is observed. The collagen fibers around the bronchi look rough and swollen.
Paired with large bronchi, the branches of the pulmonary artery of the elastomuscular type are characterized by remodeling of all layers of the vascular wall. The edematous wall, consisting of polygonal endotheliocytes, rarely protrudes into the lumen of the vessel as a result of the proliferation of the longitudinal muscle layer (Fig.2).
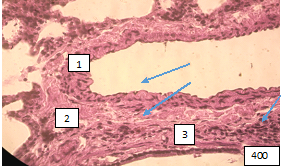
Figure (microphoto) 2. (1)A branch of the elastic pulmonary artery paired with a large bronchus in a state of dystonia. (2) Edema of the vascular wall, a focus of proliferation of the longitudinal muscle layer, (3) coarsening of loose fibrous tissue adventitia, fused collagen stroma. Staining with hematoxylin-eosin. Filling in paraffin X 400.
There is a coarsening of the loose fibrous connective tissue of adventitia and its fusion with the collagen stroma. Against this background, there are areas of lymphocytic infiltration (Fig.3).
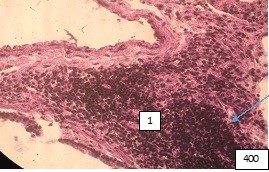
Figure (microphoto)3. (1) A branch of the pulmonary artery, paired with a large bronchus, surrounded by paravasal lymphocytic infiltration. Staining with hematoxylin-eosin. Filling in paraffin X 400
Changes at the level of the middle bronchi are expressed by areas of thinning of the wall, expansion of the lumen, as observed in cylindrical bronchiectasis of dystrophic genesis. In these cases, there is a violation of blood flow in the bronchial vessels. Along with this, on some preparations there is a picture of an inflammatory reaction with infiltration of the mucous membrane by macrophages and lymphocytes and accumulation of a small amount of mucus in the lumen. There is a slight swelling of the collagen and partially reticular stroma around the bronchi and accompanying vessels (Fig.4).
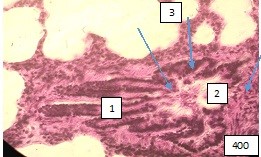
Figure (microphoto) 4. (1) Medium bronchus with pronounced wall deformation and polymorphocellular infiltration of the mucous membrane (2)The bronchial lumen contains mucus with an admixture of cellular elements (3) Edema of the surrounding stroma. Staining with hematoxylin-eosin. Filling in paraffin X 400.
The veins paired with these bronchi look atonic with stagnation of separated blood in the lumen and adhesion of the blood cells elements to the endothelium (Fig. 5).
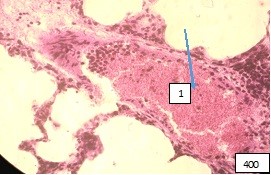
Figure (microphoto) 5. (1) Stagnation of separated blood in the pulmonary vein, paired with the middle bronchus. Staining with hematoxylin-eosin. Filling in paraffin X 400.
Bronchi with well-defined muscle septum-bundles, the contraction of which leads to almost complete closure of the lumen, attract attention. In these cases, the mucous membrane becomes sharply folded. Epithelial cells are grouped at the tops for folds. A picture of epithelial hyperplasia is created (Fig.6).
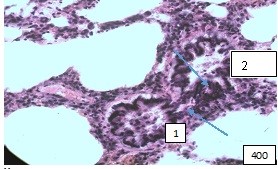
Figure (microphoto) 6. (1) Contraction of the bronchial muscle plate divided its lumen into two parts. Staining with hematoxylin-eosin. Filling in paraffin X 400
In the blood vessels of this level, thrombosis separation and stasis of blood are observed in the venular part
of the microcirculatory bed (Fig. 7, 8).
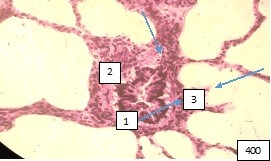
Figure 7 (microphoto). (1) Pronounced edema of the wall, (2) mucosal detachment, (3) peribronchial polymorphocellular infiltration. Staining with hematoxylin-eosin. Filling in paraffin X 400
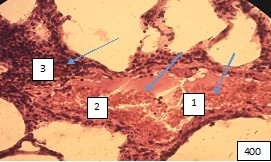
Figure (microphoto) 8. (1) Blood separation and (2) thrombosis, (3) paravasal inflammatory reaction. Staining with hematoxylin-eosin. Filling in paraffin X 400.
The most vulnerable link in the bronchial tree turned out to be terminal bronchioles, whose changes in some cases correspond to the picture of panbronchitis, when the entire wall and peribronchial environment are infiltrated by lymphocytes, macrophages. The lumen of the terminal bronchioles in this case is filled with exudative fluid. Of particular interest are terminal bronchioles with a wide lumen, distal to which there is emphysema of acinuses and specimens with an extremely narrow lumen in combination with distelectasis of the acinar apparatus (Fig. 9).
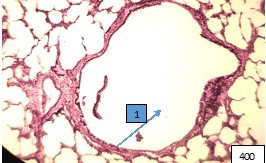
Figure (microphoto) 9. (1) Sharply expanded terminal bronchiole in the area of pulmonary emphysema. Staining with hemotoxillin-eosin.Filling in paraffin X 400
Against the background of the picture of the lung parenchyma of appropriate adaptation, there are foci of dystectasis, emphysema and inflammation (Fig. 10-12).
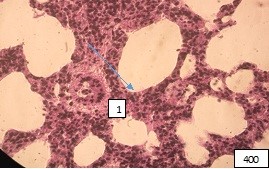
Figure 10 (microphoto): (1) The site of lung atelectasis, against which there is a sharply spasmodic arteriole with detached endotheliocytes. Staining with hematoxylin-eosin. Filling in paraffin X400

Figure 11 (microphoto): The site of emphysema of the acinar apparatus of the lungs. Staining with hematoxylin-eosin.Filling in paraffin X400
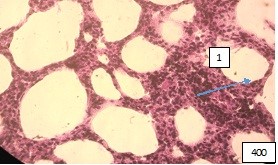
Figure 12 (microphoto). (1) A focus resembling lipid interstitial pneumonia. Staining with hemotoxillin-eosin. Filling in 12paraffin x400
Discussion
The lungs are the first organ where the metabolism of chylomicrons begins, coming from venous blood through the pulmonary artery system.
Under natural conditions, chylomicrons in the lungs are subjected to lipopectic effects, and thus the flow of fat from the small to the large circulatory system is regulated.
As is known, after eating fatty foods for a certain time, opalescence of blood plasma is observed due to the high concentration of chylomicrons in it or alimentary hyperlipidemia. In peripheral blood, chylomicrons are cleaved into non-esterified fatty acids (NEFA) under the action of lipoprotein lipase. NEFA is adsorbed by albumin and B-lipoproteins and transported to organs and tissues. The opalescence of blood plasma stops (16).
Further, some fatty acids in the liver are oxidized to form CO2 and adenosine triphosphate (ATP), and serve as the main substrate of energy metabolism in the liver. When fatty acids are oxidized, acetyl-coenzyme A (CoA) is formed, which turns into ketone bodies, then they form ATP and CO2 and H2O in tissues, after undergoing oxidation in the citric acid cycle. Ketone bodies eventually serve to a certain extent as a source of energy for the brain, skeletal and cardiac muscles. In the liver, acetyl-CoA is a material for the biosynthesis of cholesterol and bile acids. Fatty acids in the liver also serve for the biosynthesis of lipoproteins and free fatty acids in blood plasma (17).
Lipoprotenins transfer lipids to adipose tissue, where they are deposited as triglycerides, and free fatty acids are used by the heart and skeletal muscles as an energy source (18).
So the content of chylomicrons in the blood plasma returns to normal, it brightens up. Temporary alimentary induced hyperlipidemia is leveled and the level of lipids in the blood plasma returns to its initial level. The structural organization and functional specialization of the lungs are not disrupted. The oxygenation of hemoglobin in the lungs, the transport of oxygen by arterial blood and its utilization by tissues do not change (19).
Based on our material, the remodeling of the histophysiology of the structural components of the lungs was characterized by pronounced heterogeneity.
Changes at the level of small bronchi were more pronounced than at the level of large and medium bronchi. First of all, bronchioles were characterized by pronounced swelling of the wall, that is why it was not possible to detect their lumen. Which in turn led to a sharp decrease in the filling of the alveoli with air.
On histological preparations, there was an affection of the patency of terminal and respiratory bronchioles, due to infiltration of their walls; an abundance of lymph nodes and dust cells; an increased in the number of alveolar macrophages, a combination of hypertrophy and affection of the alveolar lining by glyaline membranes.
As it is known, the lungs take an active part in the metabolism of not only fats, but also proteins and carbohydrates (14). With a fat diet, it is quite possible to disrupt the synthesis and increase the catabolism of proteins that make up collagen and elastin of the lung stroma. Stagnation of blood in the wall of terminal and respiratory bronchioles narrowed their lumen, which led to distelectasis.
The pattern of remodeling of the alveolar tree with an exclusively fatty diet is no less mosaic than that of the bronchial tree.
As the picture on histological preparations shows, the disturbance of the unity of ventilation and circulation of the lungs, the fact noted by us was attributed to pathology. However, physiological emphysema and atelectasis are characteristic of the lungs and are normal. Consequently, alternating areas of emphysema and distelectasis in the lungs, in our material, can be interpreted as a compensatory and adaptive manifestation.
On the part of the microcirculatory bed, intravascular (full-blooded, erythrocyte aggregation, blood separation, formation of fibrin clots, microthrombosis and fatty embolism), vascular (violation of vascular wall permeability, capillary ruptures) and paravasal (diapedesis of shaped blood elements, hemorrhage, edema) changes were noted.
It is important to keep in mind that blood deposition is one of the main non-respiratory functions of the lungs (20, 21).
In our material, this function of the structural and functional units of the lungs was performed not by all acinuses at the same time, but only by those acinuses that were in a state of distelectasis.
At one time, the classic physiologist Claude Bernard wrote: "one should not think that all the blood that passed through the organ served for its function, only one part performed it, the other passed unchanged. It served only to maintain the circulatory mechanism" (21). From this point of view, the deposited blood in the venular link of the microcirculatory bed did not participate in gas exchange; it remained venous and entered vena azygos and vena hemiazygos via bronchopulmonary anastomoses. However, it cannot be excluded that during the deposition period, the blood was cleared of small drops of fat, blood clots and participated in the metabolic function of the lungs, physiological active substances were activated or inhibited in it. And the temporary loss of gas exchange was compensated by its increase in acinuses in a state of physiological emphysema.
The disturbance of blood outflow through the pulmonary venules was complicated by microbleeds in the inter-alveolar septa with the subsequent development of hemosiderosis and foci of pneumonia of interstitial tissue. Since the spilled blood contained a huge amount of chylomicrons, the inflammation of the acinuses resembled lipid interstitial pneumonia.
The rate of oxygen transport by arterial blood taken from the common carotid arteries (VaO2) was reduced with a fat diet compared with the control group. The decrease in the rate of oxygen transport can be explained by changes in the heart and lungs that occurred with an unbalanced diet.
It is interesting to note that at the same time, the rate of oxygen consumption by the body (Vo2), according to the results of our study, remained stable for the body.
The lactate content in arterial blood entering the lungs through a small circle of blood circulation in our study in rats on a fat diet, although it did not exceed the reference values (0.5-0.91mmol/l), nevertheless it was increased 2.5 times compared with the control data. Whereas buffer bases were reduced in animals of the main group. This may be due to the changes in blood gas homeostasis that are presented in Table 2.
Study limitations
Experiments have been conducted to study the short-term effects of an isolated fat diet. To obtain a more complete picture of the effect of an isolated fat diet on the body of the studied object, it is necessary to conduct a study of the effect of a long-term high-fat diet.
Conclusions
A 30-day feeding of male rats exclusively with animal fat leads to an increase in the concentration of lactic acid and a deficiency of blood buffer bases, and also causes a decrease in the rate of oxygen transport of arterial blood and oxygen consumption by its tissues.
Remodeling of the histophysiology of the lungs with an extremely fatty diet is characterized by pronounced heterogeneity at the level of large and medium bronchi; changes are noted mainly in the mucous and submucosal layers, in small and terminal bronchi and bronchioles. At the level of the alveolar tree, foci of atelectasis are combined with blood deposition, emphysema and an inflammatory reaction. At the level of microcirculation, intravascular, vascular and paravasal changes are detected. Changes in the acid-base state and oxygen transport function of the blood are more associated with the membrane component of the alveoli than with the blood component.
Ethics: The study was conducted on the basis of the laboratory of experimental modeling of pathological processes of the Kyrgyz-Russian Slavic University (KRSU) in accordance with the rules of research and laboratory practice approved by the order of the Ministry of Health and Social Development of the Russian Federation No. 708 dated August 23, 2010 "On Approval of the Rules of Laboratory Practice". The animals were cared for and fed by the staff of the vivarium in accordance with international standards for conducting experimental work on animals used for scientific purposes (Consensus Guidelines on Animal Ethics and Welfare 2010).
The protocol of the study was approved by the local Ethics Committee of the Kyrgyz State Medical Academy named after I.K. Akhunbayev.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: A.T. I., E.M.M., Y.Kh-M.S., A.Dj.M., D. A.O,
B.A.U., A. T.A., R.R.T.s equally contributed to manuscript preparation and fulfilled the authorship criteria. All authors approved the final version of the article before publication, and agreed to be responsible for all aspects of the work, implying proper study and resolution of issues related to the accuracy or integrity of any part of the work.
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use: Authors declared they did not use A.I.- assisted technologies in preparation of manuscript
Availability of data and material: Not applied
References
| 1.Fedotova AA, Tichlyak AV, Semyanov AV. The influence of diet as an exposome factor on brain function. Russian J Physiol 2021; 107: 533-67. | ||||
| 2.Feriani A, Bizzarri M, Tir M, Aldawood N, Alobaid H, Allagui MS, Et al. High-fat diet-induced aggravation of cardiovascular impairment in permethrin-treated Wistar rats. Ecotoxicol Environ Safety 2021; 222: 11246. Doi: 10.1016/j.ecoenv.2021.112461 https://doi.org/10.1016/j.ecoenv.2021.112461 PMid:34224971 |
||||
| 3.Birulina JG, Ivanov VV, Buyko EE, Bykov VV, Smagliy LV, Nosarev AV, et al.. High-fat, high-carbohydrate diet-induced experimental model of metabolic syndrome in rats. Bull Siberi Med 2020; 19: 14-20. doi.org: 10.20538/1682- 0363-2020-4-14-20. https://doi.org/10.20538/1682-0363-2020-4-14-20 |
||||
| 4. Israilova AT, Mamytova EM, Shidakov YKH-M, Mamytova AD. The effect of an isolated fat diet on the remodeling of the functional system "lungs-heart" under experimental conditions. Vestnik KRSU 2023: 23: 176-82. https://doi.org/10.36979/1694-500X-2023-23-5-176-184 |
||||
| 5.Saleh NEH, Ibrahim MY, Saad AH. The impact of consuming different types of high-caloric fat diet on the metabolic status, liver, and aortic integrity in rats. Sci Rep 2024; 14: 8602..doi.org: 10.1038/s41598-024-68299-6 https://doi.org/10.1038/s41598-024-68299-6 PMid:39127712 PMCid:PMC11316824 |
||||
| 6.Wachsmuth NB, Aberer F, Haupt S, Schierbauer JR, Zimmer RT, Eckstein ML, et al. The impact of a high-carbohydrate/low fat vs. low-carbohydrate diet on performance and body composition in physically active adults: A cross-over controlled trial. Nutrients 2022; 14: 423. doi.org: 10.3390/nu14030423 https://doi.org/10.3390/nu14030423 PMid:35276780 PMCid:PMC8838503 |
||||
| 7.Cai L, Xia X, Gu. Opposite effects of low-carbohydrate high-fat diet on metabolism in humans and mice. Lipids Health Dis 2023; 191: doi.org: 10.1186/s12944-023-01956-3 https://doi.org/10.1186/s12944-023-01956-3 PMid:37950240 PMCid:PMC10636972 |
||||
| 8.Muth AK, Park SQ, The impact of dietary macronutrient intake on cognitive function and the brain, Clinical Nutrition,Volume 2021; 40: 3999-4010. doi.org: 10.1016/j.clnu.2021.04.043 https://doi.org/10.1016/j.clnu.2021.04.043 PMid:34139473 |
||||
| 9.Sun, Y, Ge X, Li X. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis 2020; 11: 914. //doi.org/10.1038/s41419-020-03122-4 https://doi.org/10.1038/s41419-020-03122-4 PMid:33099578 PMCid:PMC7585574 |
||||
| 10.Alymzhan uulu B, Mamytova EM, Shidakov YX-M, Israilova AT, Mamytova AJ, Tukhvatshin RR, et al. Remodeling of the microcirculation of the small intestine with a fatty diet in rats: an experimental randomized study. Heart Vessels Transplant 2024; 8: doi: 10.24969/hvt.2024.483 https://doi.org/10.24969/hvt.2024.483 |
||||
| 11.Mustafina LR, Logvinov SV, Bogdanova LI, Kurbatov BK. Effects of a high-carbohydrate high-fat diet on liver morphology in young and old rats. Siber J Clin Exp Med 2023; 38: 126-32. doi.org: 10.29001/2073-8552-2023-38-1- 126-32. https://doi.org/10.29001/2073-8552-2023-38-1-126-132 |
||||
| 12.Feingold KR. The effect of diet on cardiovascular disease and lipid and lipoprotein levels. In: Feingold KR, Anawalt B, Blackman MR, et al, editors. Endotext (Internet). South Dartmouth (MA): MDText.com, Inc.; 2000. Available from: URL: https://www.ncbi.nlm.nih.gov/books/NBK570127 | ||||
| 13.Gaeini Z, Mirmiran P, Bahadoran Z. The association between dietary fats and the incidence risk of cardiovascular outcomes: Tehran Lipid and Glucose Study. Nutr Metab (Lond) 2021; 18: 96. Doi: 10.1186/s12986-021-00624-6 https://doi.org/10.1186/s12986-021-00624-6 PMid:34717669 PMCid:PMC8557498 |
||||
| 14.Hong YP, Yu J, Su YR, Mei FC, Li M, Zhao KL, et al. High-fat diet aggravates acute pancreatitis via TLR4-mediated necroptosis and inflammation in rats. Oxid Med Cell Longev 2020; 2020: 8172714. doi: 10.1155/2020/8172714. https://doi.org/10.1155/2020/8172714 PMid:31998444 PMCid:PMC6973188 |
||||
| 15.Wentzel P, Eriksson UJ, Herrera E. High-fat diet in pregnant rats and adverse fetal outcome. Ups J Med Sci 2019; 124: 125-34. doi: 10.1080/03009734.2019.1604588. https://doi.org/10.1080/03009734.2019.1604588 PMid:31063006 PMCid:PMC6567025 |
||||
| 16.Pakiet A, Jakubiak A, Mierzejewska P, Zwara A, Liakh I, Sledzinski T, et al. The effect of a high-fat diet on the fatty acid composition in the hearts of mice. Nutrients 2020; 12: 824. doi.org: 10.3390/nu12030824 https://doi.org/10.3390/nu12030824 PMid:32245049 PMCid:PMC7146498 |
||||
| 17.Aliluev A, Tritschler S, Sterr M. Diet-induced alteration of intestinal stem cell function underlies obesity and prediabetes in mice. Nat Metab 2021; 3: 1202-16. doi.org:10.1038/s42255-021-00458-9 https://doi.org/10.1038/s42255-021-00458-9 PMid:34552271 PMCid:PMC8458097 |
||||
| 18.Song R, Hu M, Qin X, Qiu L, Wang P, Zhang X, et al. The roles of lipid metabolism in the pathogenesis of chronic diseases in the elderly. Nutrients 2023; 15: 3433. doi: 10.3390/nu15153433. https://doi.org/10.3390/nu15153433 PMid:37571370 PMCid:PMC10420821 |
||||
| 19.Xu L, Yang Q, Zhou J. Mechanisms of abnormal lipid metabolism in the pathogenesis of disease. Int J Mol Sci 2024; 25: 8465. doi.org: 10.3390/ijms25158465 https://doi.org/10.3390/ijms25158465 PMid:39126035 PMCid:PMC11312913 |
||||
| 20.Neely ML, Hellkamp AS, Bender S, et al. Lung function trajectories in patients with idiopathic pulmonary fibrosis. Respir Res 2023; 24: 209. doi.org: 10.1186/s12931-023-02503-5 https://doi.org/10.1186/s12931-023-02503-5 PMid:37612608 PMCid:PMC10463468 |
||||
| 21.El-Zayat SR, Sibaii H, El-Shamy KA. Physiological process of fat loss. Bull Natl Res Cent 2019; 43: 208. doi.org: 10.1186/s42269-019-0238-z https://doi.org/10.1186/s42269-019-0238-z |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

