Analysis of taurolidine as a preventive solution for CIED infection: Preliminary results from a single centre
ORIGINAL RESEARCH ARTICLE
Analysis of taurolidine as a preventive solution for CIED infection: Preliminary results from a single centre
Article Summary
- DOI: 10.24969/hvt.2024.538
- CARDIOVASCULAR DISEASES
- Published: 05/01/2025
- Received: 10/11/2024
- Revised: 11/11/2024
- Accepted: 12/11/2024
- Views: 4057
- Downloads: 2474
- Keywords: Cardiac implantable electronic device, Taurolidine-containing antimicrobial adjunct, tauropace, infection, Infection prevention strategies
Address for Correspondence: Elkin Gonzalez Villegas, Department of Cardiovascular Surgery, University Hospital La Paz, P.º de la Castellana, 261, 28046 Madrid, Spain
Email: elgovi17@hotmail.com
Elkin Gonzalez Villegas1*, Alvaro Gilsanz2, José Romero Carmona1
1Department of Cardiovascular Surgery, University Hospital La Paz, Paseo de la Castellana, 261 - 28046 Madrid, Spain
2Centro de Biología Molecular Severo Ochoa (CSIC-UAM), Nicolás Cabrera 1, Campus de Cantoblanco, 28049 Madrid, Spain
Objective: Cardiac implantable electronic device (CIED) infection is the most devastating adverse event related to any invasive CIED procedure. Our study investigates the adjunct use of a novel taurolidine containing antimicrobial solution during cardiac implantable electronic device placement to prevent cardiac implantable electronic device infection.
Methods: The authors prospectively evaluate all consecutive CIED procedures performed with adjunct use of the taurolidine containing antimicrobial adjunct in our centre. Cardiac implantable electronic device, procedure and patient related risk factors are recorded. Primary endpoint was major CIED infection. Patients were followed-up three months after implantation for acute CIED infections and one year after for subacute CIED infections.
Results: From September 2023 to January 2024, 26 high-risk CIED procedures were conducted with the taurolidine-based antimicrobial adjunct. Throughout the 3-month follow-up period, no infections were observed. Additionally, only one adverse event related to the CIED was recorded, with no adverse events of any severity related to the procedures or the antimicrobial adjunct, nor was any all-cause mortality reported during the follow-up period.
Conclusions: The use of the taurolidine containing antimicrobial adjunct for any CIED procedure is safe. No adverse event in relation could be recorded.
Key words: Cardiac implantable electronic device, Taurolidine-containing antimicrobial adjunct, tauropace, infection, Infection prevention strategies
List of abbreviations
AE Adverse Event
BLISTER Bristol Implantable Systems Transvenous Endocarditis Risk
CE Conformité Européenne (European Conformity)
CHX Chlorhexidine Gluconate
CIED Cardiac Implantable Electronic Device
CIEDI Cardiac Implantable Electronic Device Infection
CRT Cardiac Resynchronization Therapy
ESC European Society of Cardiology
GDPR General Data Protection Regulation
ICH GCP International Council for Harmonization Good Clinical Practice
IRB Institutional Review Board
KOLEK Klebsiella, Organisms, Lead Extraction, Klebsiella Risk Model
L-IE Lead-associated Infective Endocarditis
NNT Number Needed to Treat
PADIT Prevention of Arrhythmia Device Infection Trial
PACE DRAP Prevention of Arrhythmia Device Infection Prediction Model
RCT Randomized Controlled Trial
RI-AIAC Risk Index for Antibacterial Infection After Cardiac Device Implantation
SHARIFF Score for Healthcare-Associated Risk of Infection Following Implantation of Cardiac Rhythm Devices
TP TauroPace™
TYRX A brand of antibiotic-eluting envelopes for CIEDs
WRAP-IT Worldwide Randomized Antibiotic Envelope Infection Prevention Trial
Graphical abstract
Introduction
Cardiac implantable electronic devices (CIEDs) play a critical role in treating arrhythmias and preventing sudden cardiac death (1). Despite their therapeutic importance, CIED procedures carry risks of adverse events (AEs), including procedural complications (e.g., hematoma formation, lead dislodgement) and device- or patient-related issues (2-5). One of the most severe AEs is CIED-associated infection (CIEDI), which, although infrequent (1–4% incidence), leads to significant morbidity, mortality, and healthcare burden (6-10). Infections may remain localized to the surgical site or pocket but can extend to transvenous leads, causing infective endocarditis (L-IE). Upon diagnosis, complete removal of the device system is necessary, further compounding health risks and costs (11).
The rising incidence of CIEDI, surpassing the growth rate of implantation procedures, is attributed to the increased fragility of patients (e.g., advanced age, comorbidities) and the complexity of newer devices, which necessitate longer and more intricate surgeries (12-14).
Preventive measures such as preoperative intravenous antibiotic prophylaxis and antibiotic-eluting envelopes have demonstrated efficacy in reducing infection rates and are recommended by clinical guidelines (15-17). However, evidence for other interventions, such as pocket irrigation with antibiotics or antiseptic solutions, remains inconclusive, and guidelines generally discourage their routine use due to variable study outcomes.
TauroPace™ (TP), a taurolidine-based antimicrobial solution, offers a novel approach to infection prevention (18-20). Taurolidine, derived from taurine, exhibits broad-spectrum antimicrobial activity without promoting resistance. Upon in vivo metabolism, it produces active compounds that disrupt bacterial adhesion, inhibit biofilm formation, and neutralize toxins (21-27). Unlike many antiseptics, TP can be safely exposed to the bloodstream, enhancing its application as both a surgical antiseptic and a disinfectant for medical devices (28, 29). It is increasingly being used throughout Europe in different healthcare settings (30-33).
This study evaluates the efficacy of TP in reducing CIEDI rates during implantation or revision procedures under standard clinical conditions. By addressing an unmet need for innovative, evidence-backed infection control measures, TP has the potential to improve outcomes in a high-risk patient population.
Methods
Study design and population
Study design is prospective observational, open-label, single-center study.
Patient population and observation duration: The study enrolled 26 consecutive patients undergoing CIED placement with the use of taurolidine-containing antimicrobial adjunct, following them prospectively until hospital discharge and through specific intervals for a 3-month follow-up.
Inclusion criteria: Inclusion criteria for this study were based on the presence of at least five recognized risk factors for CIED infections, identified through a comprehensive analysis of patient-, procedural-, and device-related risks as well as a Charlson Comorbidity index of 12 points and above. These criteria were chosen to align with clinical observations and literature, acknowledging their imperfect alignment with existing risk stratification models such as PADIT, SHARIFF, RI-AIAC, BLISTER, PACE DRAP, or KOLEK.
Patient-related risks included renal impairment reflecting their strong association with immunosuppression and wound healing complications. Additional risk factors comprised systemic conditions such as congestive heart failure, diabetes, neoplasia and immunosuppression, as well as a history of prior infections involving implants, which heightened susceptibility due to microbial colonization.
Procedural and device-related risk factors incorporated the complexity and duration of the implantation process. Criteria included complex devices (e.g., defibrillators or cardiac resynchronization therapy systems), revision procedures (e.g., lead replacement or system upgrades), and procedure durations exceeding 60 minutes. The presence of abandoned leads in situ was also included due to its recognized impact on infection risk.
Ethical Considerations: This study was conducted in accordance with the highest ethical standards, fully complying with the principles outlined in the Declaration of Helsinki (World Medical Association, 2013). The study utilized fully de-identified data, ensuring that no individual could be re-identified at any stage. In line with Recital 26 of the General Data Protection Regulation (GDPR) of the European Union, such data, anonymized to the extent that individuals are no longer identifiable, falls outside the scope of data protection laws. Consequently, the relevant institutional review board (IRB) granted a waiver for informed consent, as the use of completely de-identified data posed no risk to individual privacy. All research activities adhered to applicable clinical research guidelines, including the International Council for Harmonization (ICH) Good Clinical Practice (GCP) standards.
Baseline characteristics
The following demographic, anthropometric and clinical characteristics were recorded: age, sex, body mass index, history and risk factors as therapy with oral anticoagulation and dual antiplatelet therapy, heart failure, diabetes, cardiomyopathy, chronic obstructive pulmonary disease, previous device infections, and immunocompromized state.
All patients underwent diagnostic tests such as physical examination, echocardiography, complete blood count, and blood cultures were conducted if infection was suspected, with samples sent for microbiological analysis.
Test device
The study utilized a CE-marked taurolidine-containing antimicrobial adjunct (TauroPace™ ; Tauropharm, Bavaria, Germany) in accordance with the manufacturer's guidelines. This device is routinely employed as the standard of care at our institution for high-risk procedures.
Alternative measures, such as antibiotic-eluting or extracellular matrix envelopes and antiseptic irrigation solutions (e.g., aqueous chlorhexidine or povidone-iodine), are not implemented in our center's practice.
Physicians: Surgeons from the Department of Cardiovascular Surgery University Hospital La Paz, P.º de la Castellana, 261, 28046 Madrid, Spain performed the procedures, with those conducting fewer than 50 CIED procedures annually and fewer than 200 within three years considered a procedural risk for CIED infections.
Procedure packs: All-inclusive CIED-placement procedure packs tailored to meet specific needs were introduced a decade ago at the center, ensuring proper sterile conditions during placement procedures.
Sterile barrier conditions: Procedures were conducted in a positive-pressure operating room with maximum sterile barrier precautions, including wearing caps, masks, sterile gowns, gloves, and employing large sterile drapes made of polypropylene (PP).
Skin disinfection: Antimicrobial compound containing 2% chlorhexidine gluconate in ethanol 70% was used for skin disinfection by the attending surgeons.
Placement site selection: For de novo placement, the pocket was prepared latero-caudal to the left collarbone, while intermuscular positioning was adopted for subcutaneous implantable cardioverter defibrillator placement. Vein preparation for transvenous lead placement was performed in the all patients. Procedures were conducted under local anesthesia, involving infiltration of the device implantation area, venous puncture, skin incision, subcutaneous dissection, and creation of the pocket.
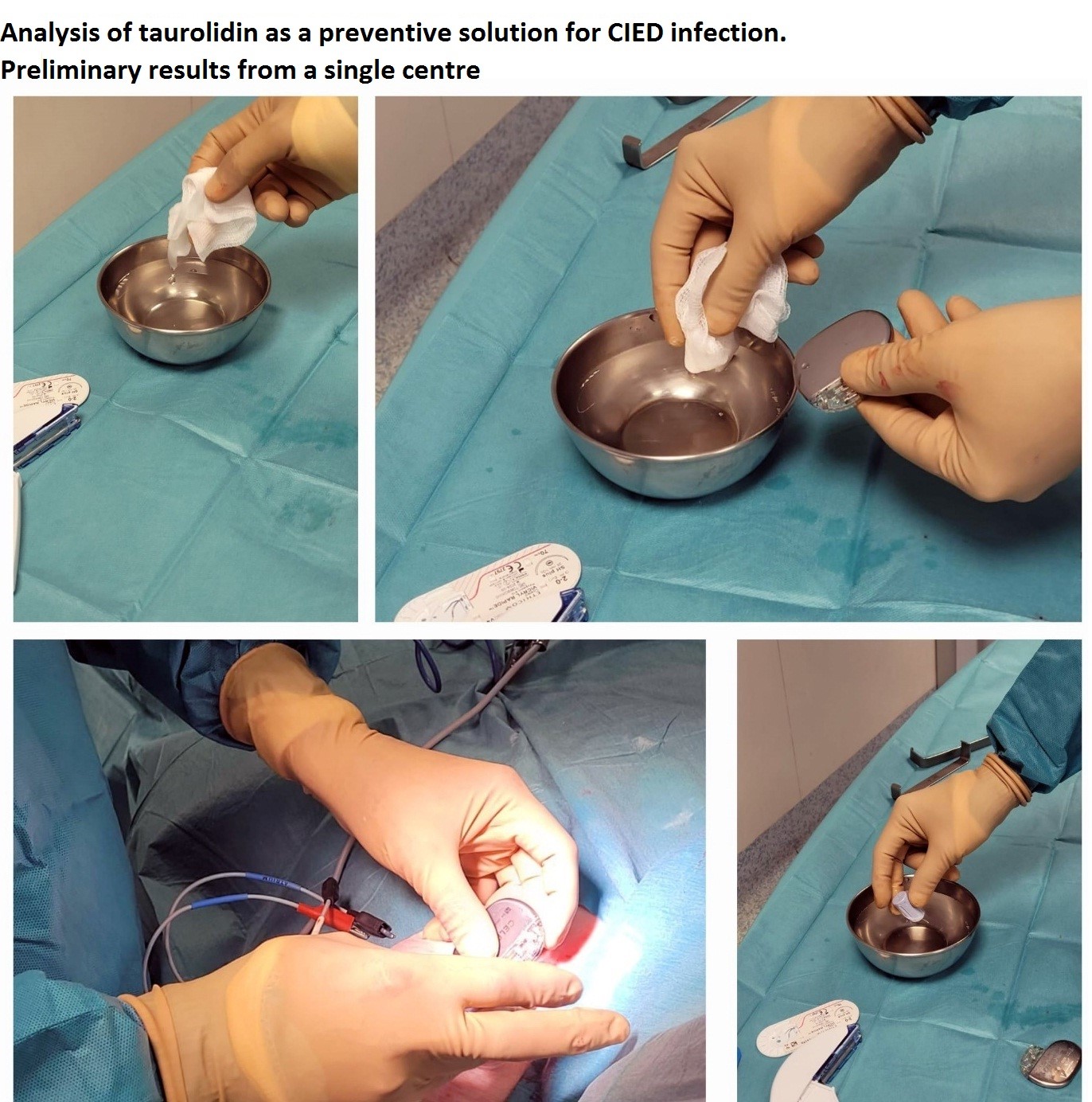
Surgical Technique: All implant procedures were carried out in accordance with the guidelines set forth by the European Society of Cardiology (ESC). Representative images of the procedure, including preparatory steps and the procedure itself, are provided in Figures 1-3.
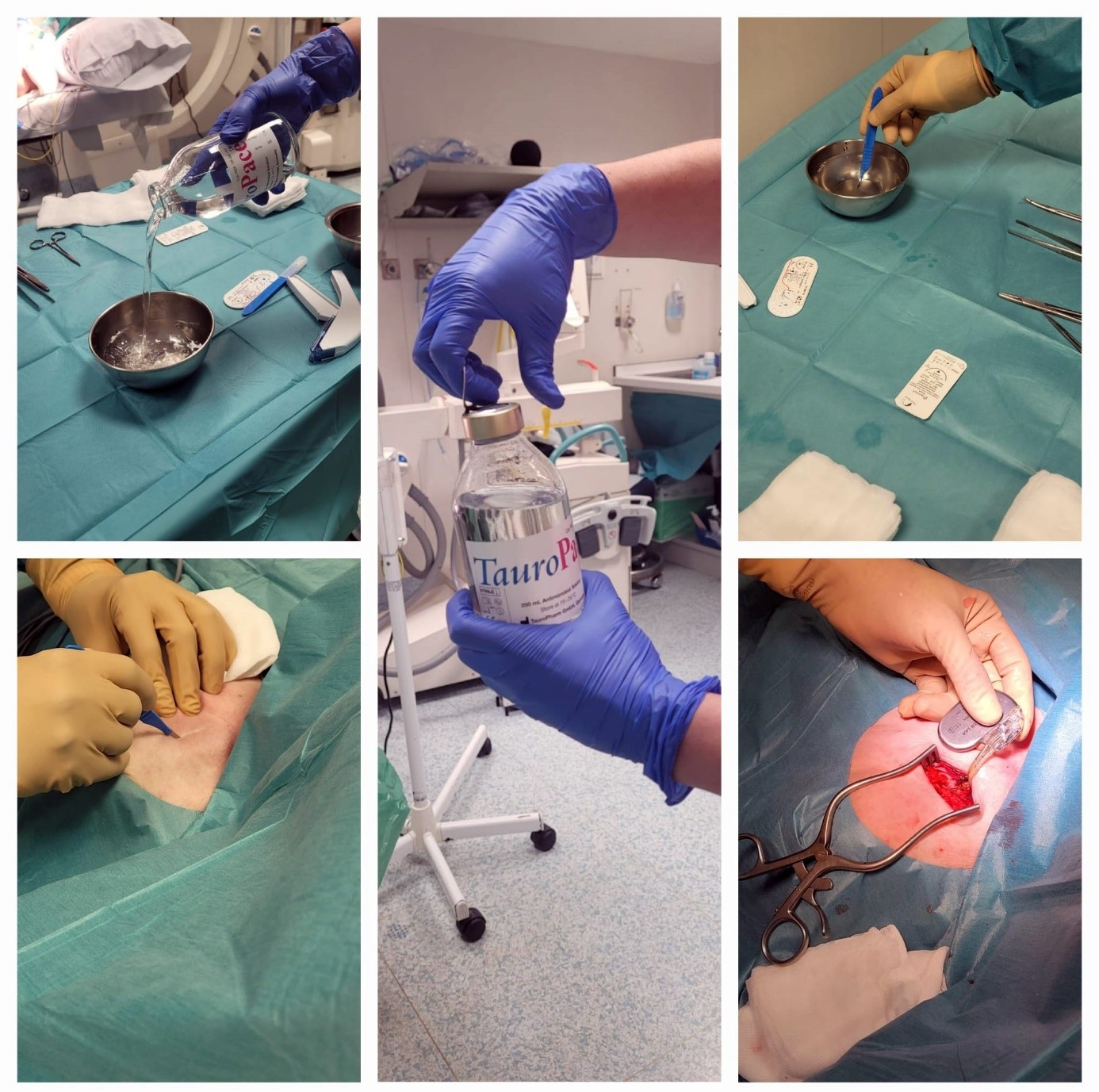
Figure 1. Procedural details, highlighting the use of the antimicrobial adjunct TauroPace™
.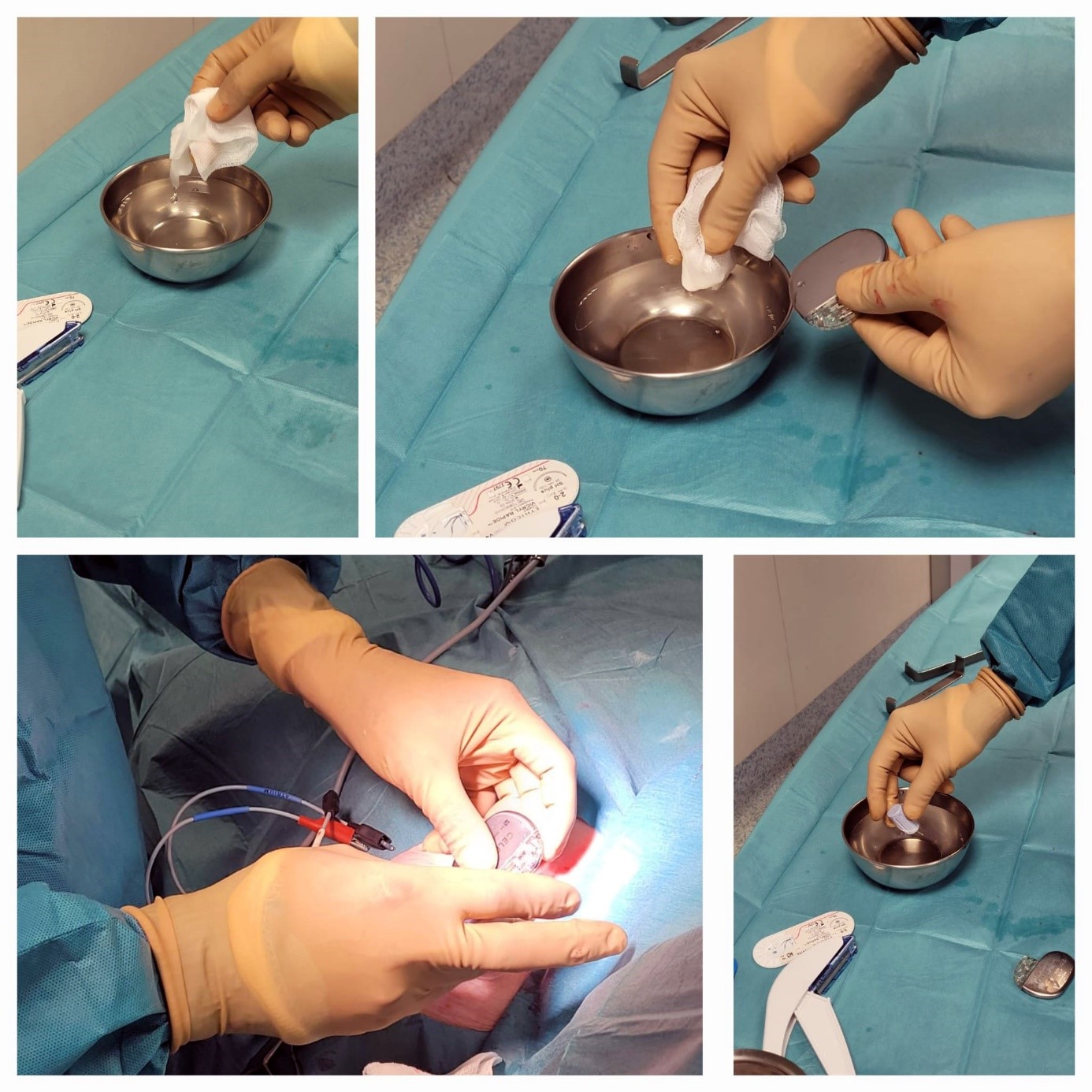
Figure 2. Preparation of the procedure, including immersion of all hardware in TauroPace™ solution and application of TauroPace™ -soaked swabs. The surgical site was also irrigated with TauroPace™ to ensure comprehensive antimicrobial coverage throughout the procedure
We recorded following procedural variables as procedure duration, procedure >60 minutes, revision and de novo device implantation, and type of CIEDs (pacemaker -PM, implantable cardioverter defibrillator – ICD, cardiac resunchronization therapy device - CRT).
Endpoints and follow –up
Endpoints: The primary endpoint was major CIED infection within the 3-month follow-up, while secondary endpoints included all-cause mortality and adverse events.
Endpoint assessment: Diagnostic tests such as physical examination, echocardiography, complete blood count, and blood cultures were conducted if infection was suspected, with samples sent for microbiological analysis.
Statistical analysis
Data are presented as mean (SD) and number (%).
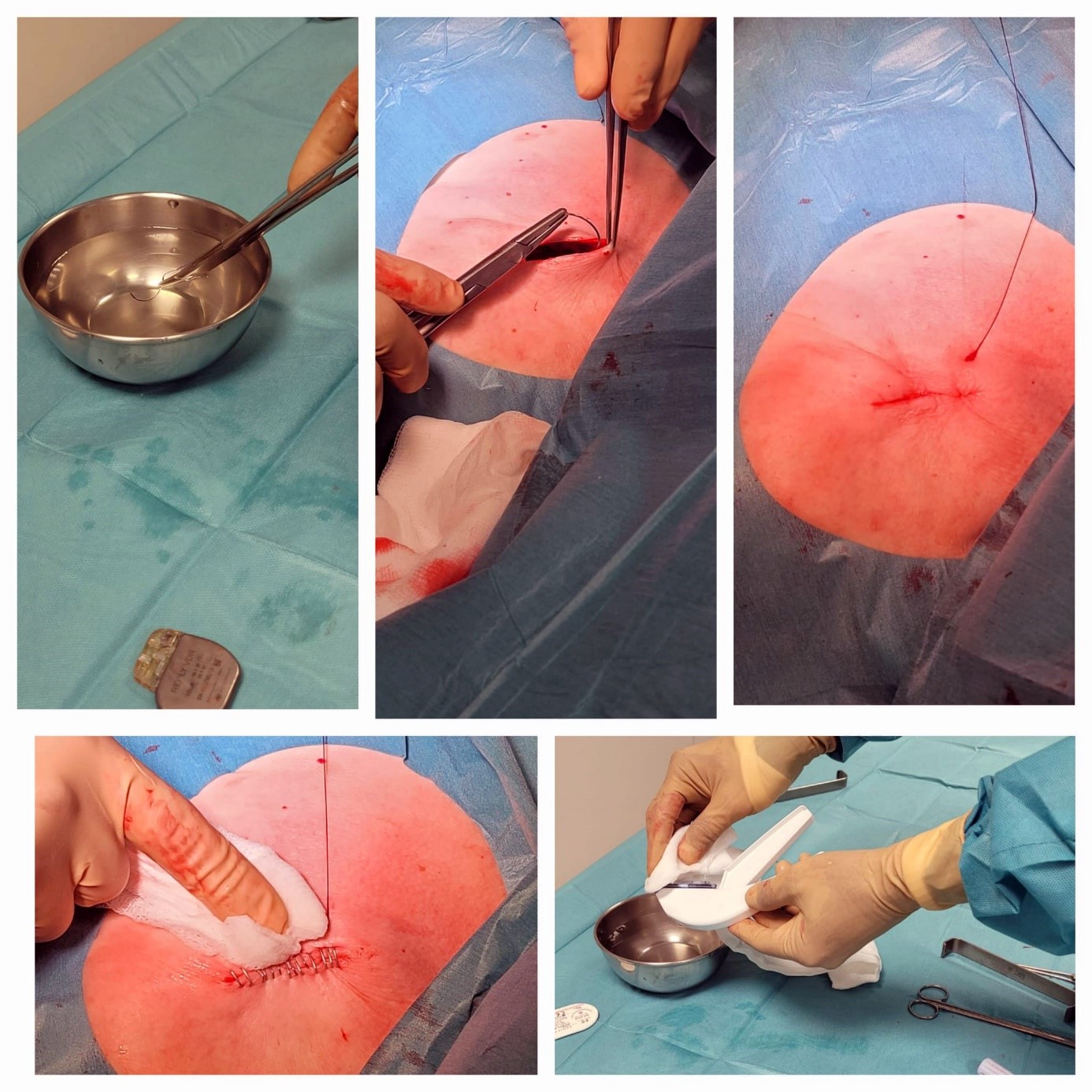
Figure 3. Completion of the procedure, demonstrating wound closure followed by the application of CHX gluconate for skin antisepsis post-closure
Results
Baseline characteristics of the patients, procedures, and CIEDs are provided in Table 1.
As can be seen from Table 1, our study population included 54% males and 46% females with mean age of 73 years. Among them 38% underwent PM, 30% - ICD and 32% CRT device implantations. There were 62% de novo implantations and 30% - revision. More than half of patients had heart failure, 1/3 - diabetes and 1/3 renal impairment.
Procedural and follow –up outcomes
Analysis of 26 patients demonstrated no major CIED infections during the follow-up period. Additionally, no adverse events, regardless of severity, were associated with the procedures, or the antimicrobial adjunct used. One early revision due to CRT dysfunction was necessary. No patient succumbed during the observation.
Discussion
This study adopted a prospective observational approach, characterized by an open-label design, and was executed at a single center. It enlisted 26 consecutive patients undergoing CIED procedures, administered with a taurolidine-containing antimicrobial adjunct, and prospectively followed after hospital discharge, with a minimum 3-month follow-up period. Analysis of the 26-patient cohort unveiled no major CIED infections during the follow-up period
The cohort encompassed patients with diverse comorbidities and an anticipated increased risk for CIED infection, with no serious complications in relation to the antimicrobial adjunct detected.
|
Table 1. Baseline characteristics and the most important risks in relation to devices, procedures and patients
|
|
|
Variables |
Mean (SD) or n(%) |
|
Age, years |
73.2 (15.8) |
|
Male sex, n(%) |
14 (54) |
|
Body mass index, m/kg2 |
25.2 (4.3) |
|
Risk factors, n(%) |
|
|
Heart failure |
14 (53) |
|
Cardiomyopathy |
16 (62) |
|
Diabetes |
8 (30) |
|
Chronic obstructive pulmonary diseases |
5 (20) |
|
Renal impairment |
8 (30) |
|
Immunocompromized state |
16 (62) |
|
Previous device infection |
2 (8) |
|
Oral anticoagulation therapy |
9 (35) |
|
Dual antiplatelet inhibition |
5 (20) |
|
Devices, n(%) |
|
|
Pacemaker |
10 (38) |
|
Implantable cardioverter defibrillator |
8 (30) |
|
Cardiac resynchronization therapy device |
10 (32) |
|
Procedural details |
|
|
Revision, n(%) |
8 (30) |
|
De novo implantation, n(%) |
18 (62) |
|
Procedure >60 minutes, n(%) |
4 (15) |
|
Procedure duration, min |
36.0 (20.5) |
CIED infections pose significant challenges, with recent data indicating increased incidence and underdetection (34-36). Various factors contribute to infection risk, including patient comorbidities and procedural complexity (12). While guidelines offer recommendations for prevention, the effectiveness of certain preventive measures remains uncertain (16, 17, 37).
The absence of major CIED infections in the 26-patient cohort observed in this study is a significant finding that warrants attention in the context of existing literature on CIED infections. The utilization of a taurolidine-containing antimicrobial adjunct during CIED procedures, coupled with meticulous follow-up for a minimum of 3 months post-procedure, suggests a promising avenue for infection prevention.
Prospective observational studies, such as this one, are valuable in providing real-world insights into the effectiveness of preventive measures (19). The open-label design and execution at a single center enhance the study's internal validity, allowing for a focused evaluation of the intervention's impact on infection rates.
The diverse nature of the patient cohort, characterized by the presence of various comorbidities and an anticipated increased risk for CIED infection, reflects the real-world complexity of CIED implantation scenarios. Despite these challenges, the absence of major infections underscores the potential efficacy of the taurolidine-containing antimicrobial adjunct in reducing post-procedural infectious complications.
These findings align with existing literature that recognizes the importance of infection prevention strategies in CIED procedures employing taurolidine (18). Studies have highlighted the substantial morbidity, mortality, and healthcare costs associated with CIED infections, emphasizing the need for effective preventive measures (38).
Taurolidine is a compound with broad-spectrum antimicrobial properties that has been investigated for its potential in preventing various types of infections, including those associated with medical device implantation (20, 39-41).
The extant literature on taurolidine indicates its general tolerability as an antimicrobial agent. Potential side effects may encompass a metallic taste subsequent to its central venous administration; however, the adverse events reported are attributed to adjunctive fibrinolytics or anticoagulants (i.e., citrate) in commercially available antimicrobial central line locks, as no such occurrences have been documented thus far for taurolidine itself (29, 42, 43).
Within the framework of the described study, the lack of adverse events associated with taurolidine employment emerges as a significant discovery bolstering its safety profile. The absence of serious complications within the cohort, notwithstanding the inclusion of patients exhibiting varied comorbidities and undergoing prolonged procedural durations, provides reassurance.
However, it is important to acknowledge that the study may not have captured all potential adverse events, particularly rare or long-term complications. Additionally, the duration of follow-up in this study was limited to a minimum of three months, which may not be sufficient to detect delayed or uncommon adverse reactions.
A three-month follow-up period is sufficient for assessing CIED infections, as the majority occur within this timeframe, aligning with findings from large randomized controlled trials (RCTs) such as the WRAP-IT and PADIT trials (36, 44). These studies demonstrated that early infections, often linked to procedural factors, typically manifest within the first 90 days post-implantation. Late-onset infections beyond this period are comparatively rare and often associated with patient-specific factors or unrelated events (35). Thus, a three-month period captures the clinically relevant window for device-related infections, providing a robust basis for evaluating infection rates in both research and clinical practice.
As with any medical intervention, the safety of taurolidine should be evaluated in the context of its benefits. While the findings from different studies suggest that taurolidine may effectively prevent major CIED infections, further research is needed to fully assess its efficacy and safety profile over longer periods and in larger patient populations.
Although the lack of significant infection complications is promising, it is imperative to recognize the constraints of this study, such as its relatively modest sample size and confinement to a single center. Further investigation, encompassing larger multicenter studies and ideally randomized controlled trials that directly compare with the gold standard, is necessary to substantiate these results and ascertain the broader effectiveness of taurolidine-containing antimicrobial adjuncts in CIED procedures.
Compared to antibiotic-eluting envelopes, such as TYRX, which demonstrated a 40% relative risk reduction in infections in the WRAP-IT trial (36) taurolidine offers a simpler, less costly approach with fewer logistical requirements, though it currently lacks validation through RCTs. While antibiotic envelopes provide sustained, localized antibiotic delivery for up to seven days, their high number-needed-to-treat (NNT = 200) limits cost-effectiveness in low-risk populations (45). In contrast, taurolidine may offer a broader applicability if substantiated by further studies.
Study limitations
This study, while providing valuable insights into the use of the taurolidine-based antimicrobial adjunct, faces several limitations. First, the cohort size of 26 patients is relatively small, which may limit the generalizability of the findings. A larger sample size would be required to confirm these results and better assess the effectiveness of the antimicrobial adjunct across diverse patient populations and procedural contexts. Second, the observational, open-label design of the study lacks a control group, which may introduce potential biases in the interpretation of outcomes, such as the absence of infections or adverse events. Moreover, the study's short follow-up period of 3 months restricts the ability to evaluate long-term efficacy and safety. Additionally, while the use of a taurolidine-containing antimicrobial adjunct was standardized, the absence of a direct comparison with other infection prevention strategies limits the ability to draw definitive conclusions on its superiority. Finally, the study relied on clinician-based assessments for infection and adverse event outcomes, which may not fully account for all potential subclinical infections or complications that could have occurred post-procedure. Future research with a randomized controlled design, longer follow-up, and a larger patient cohort is needed to address these limitations and further validate the findings.
Conclusions
In conclusion, the results of this study contribute to the growing body of evidence supporting the potential role of taurolidine-containing antimicrobial adjuncts in preventing or fighting CIED infections (20, 30-33, 46, 47). While the study provides valuable insights into the safety of taurolidine as an antimicrobial adjunct in CIED procedures, continued vigilance and research are necessary to ensure its safe and effective use in clinical practice. Continued research efforts in this area are essential for refining infection prevention strategies and improving outcomes for patients undergoing CIED procedures. Close monitoring of patients and ongoing evaluation of adverse events will be essential in establishing the risk-benefit balance of taurolidine in infection prevention strategies.
Ethics: This study was conducted in accordance with the highest ethical standards, fully complying with the principles outlined in the Declaration of Helsinki (World Medical Association, 2013). The study utilized fully de-identified data, ensuring that no individual could be re-identified at any stage. In line with Recital 26 of the General Data Protection Regulation (GDPR) of the European Union, such data, anonymized to the extent that individuals are no longer identifiable, falls outside the scope of data protection laws. Consequently, the relevant institutional review board (IRB) granted a waiver for informed consent, as the use of completely de-identified data posed no risk to individual privacy. All research activities adhered to applicable clinical research guidelines, including the International Council for Harmonization (ICH) Good Clinical Practice (GCP) standards.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: E.G.V., A.G., and J.R.C. equally contributed to manuscript preparation and fulfilled the authorship criteria.
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use: Authors declared they did not use A.I.- assisted technologies in preparation of manuscript
Availability of data and material: Not applied
References
| 1.Kusumoto FM, Calkins H, Boehmer J, Buxton AE, Chung MK, Gold MR, et al. HRS/ACC/AHA Expert Consensus Statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation 2014; 130: 94-125. https://doi.org/10.1161/CIR.0000000000000056 PMid:24815500 |
||||
| 2.Baldauf BJ, Bode K, Assadian O, Giaccardi M, Cemin R, Chevalier P, et al. Incidence of infections related to cardiac implantable electronic devices in Germany. Europace 2024; 26: euae102.504 https://doi.org/10.1093/europace/euae102.504 PMCid:PMC11120737 |
||||
| 3.Baldauf B, Lau EW, Giaccardi M, Bonnemeier H. An unusual cause of inappropriate shocks delivered by an implantable cardioverter defibrillator. BMC Cardiovasc Dis 2024; 24: 380. https://doi.org/10.1186/s12872-024-04038-z PMid:39039491 PMCid:PMC11264391 |
||||
| 4.Baldauf B, Bonnemeier H. Focal aneurysm formation in a coronary bypass graft following permanent pacemaker implantation. Heart Rhythm 2024; doi: 10.1016/j.hrthm.2024.07.018 https://doi.org/10.1016/j.hrthm.2024.07.018 PMid:39019382 |
||||
| 5.Baldauf B, Vonthein R, Borov S, Lau E, Giaccardi M, Assadian O, et al. Acute cardiac implantable electronic device infections in Germany. Heart 2024; 110: A17-A18. https://doi.org/10.1136/heartjnl-2024-ICS.18 |
||||
| 6.Rennert-May E, Chu A, Kuriachan V, Somayaji B. Epidemiology of cardiac implantable electronic device infections in the United States: A population-based cohort study. Heart Rhythm 2020; 17: 1125-31. https://doi.org/10.1016/j.hrthm.2020.02.012 PMid:32087358 |
||||
| 7.El-Chami M, Liu Y, Griffiths R, Knight B, Weiss R, Mark G, et al. Device‐related infection associated with increased mortality risk in de novo transvenous implantable cardioverter‐defibrillator Medicare patients. J Cardiovasc Electrophysiol 2022; 33: 725-30. https://doi.org/10.1111/jce.15385 PMid:35066954 |
||||
| 8.El-Chami MF, Soejima K, Piccini JP, Reynolds D, Ritter P, Okabe T, et al. Incidence and outcomes of systemic infections in patients with leadless pacemakers: Data from the Micra IDE study. Pacing Clin Electrophysiol 2019; 42: 1105-10. https://doi.org/10.1111/pace.13752 PMid:31232461 |
||||
| 9.Greenspon AJ, Eby EL, Petrilla AA, Sohail MR. Treatment patterns, costs, and mortality among Medicare beneficiaries with CIED infection. Pacing Clin Electrophysiol 2018; 41: 495-503. https://doi.org/10.1111/pace.13300 PMid:29411401 |
||||
| 10.Sohail MR, Eby EL, Ryan MP, Gunnarsson C, Wright LA, Greenspon AJ. Incidence, treatment intensity, and incremental annual expenditures for patients experiencing a cardiac implantable electronic device infection: evidence from a large US Payer database 1-year post implantation. Circ Arrhythm Electrophysiol 2016; 9: e003929. https://doi.org/10.1161/CIRCEP.116.003929 PMid:27506820 |
||||
| 11.Baddour LM, Esquer Garrigos Z, Rizwan Sohail M, Havers-Borgersen E, Krahn AD, et al., American Heart Association Council on Lifelong Congenital Heart D, Heart Health in the Y and Council on Clinical C. Update on cardiovascular implantable electronic device infections and their prevention, diagnosis, and management: A Scientific Statement From the American Heart Association: Endorsed by the International Society for Cardiovascular Infectious Diseases. Circulation 2024; 149: e201-e16. https://doi.org/10.1161/CIR.0000000000001187 PMid:38047353 |
||||
| 12.Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace 2015; 17: 767-77. https://doi.org/10.1093/europace/euv053 PMid:25926473 |
||||
| 13.Olsen T, Jørgensen OD, Nielsen JC, Thøgersen AM, Philbert BT, Frausing MHJP, et al. Risk factors for cardiac implantable electronic device infections: a nationwide Danish study. Eur Heart J 2022; 43: 4946-56. https://doi.org/10.1093/eurheartj/ehac576 PMid:36263789 PMCid:PMC9748591 |
||||
| 14. Olsen T, Nielsen JC, Thøgersen AM, Johansen JB. Incidence of device-related infection in 97 750 patients: clinical data fromthe complete Danish device-cohort (1982-2018). Eur Heart J 2019; 40: 1862-9. https://doi.org/10.1093/eurheartj/ehz316 PMid:31155647 PMCid:PMC6568207 |
||||
| 15.Fowler VG, Durack DT, Selton-Suty C, Athan E, Bayer AS, Chamis AL, et al. The 2023 Duke-International Society for Cardiovascular Infectious Diseases Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin Infect Dis 2023; 77: 518-26. https://doi.org/10.1093/cid/ciad271 PMid:37138445 PMCid:PMC10681650 |
||||
| 16.Blomstrom-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MG, et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID), and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2020; 22: 515-49. https://doi.org/10.1093/eurheartj/ehaa010 https://doi.org/10.1093/europace/euz246 https://doi.org/10.1093/ejcts/ezz296 PMid:31724720 |
||||
| 17.Traykov V, Bongiorni MG, Boriani G, Burri H, Costa R, Dagres N, et al. Clinical practice and implementation of guidelines for the prevention, diagnosis and management of cardiac implantable electronic device infections: results of a worldwide survey under the auspices of the European Heart Rhythm Association. Europace 2019; 21: 1270-9. https://doi.org/10.1093/europace/euz137 PMid:31209483 |
||||
| 18.Vonthein R, Baldauf B, Borov S, Lau EW, Giaccardi M, Assadian O, et al. Taurolidine-containing solution for reducing cardiac implantable electronic device infection-early report from the European TauroPace™ registry. J Cardiothorac Surg 2024; 19: 592. https://doi.org/10.1186/s13019-024-03059-1 PMid:39367427 PMCid:PMC11451193 |
||||
| 19.Vonthein R, Baldauf B, Borov S, Lau EW, Giaccardi M, Cemin R, et al. The European TauroPace Registry. Methods Protoc 2023; 6: 86. https://doi.org/10.3390/mps6050086 PMid:37736969 PMCid:PMC10514882 |
||||
| 20.Borov S, Baldauf B, Henke J, Pavaci H, Perani A, Zrenner B, et al. Use of a taurolidine containing antimicrobial wash to reduce cardiac implantable electronic device infection. Europace 2023; 25: euad306. https://doi.org/10.1093/europace/euad306 PMid:37831737 PMCid:PMC10616572 |
||||
| 21.Perry JD, Riley G, Johnston S, Dark JH, Gould FK. Activity of disinfectants against Gram-negative bacilli isolated from patients undergoing lung transplantation for cystic fibrosis. J Heart Lung Transplant 2002; 21: 1230-1. https://doi.org/10.1016/S1053-2498(02)00434-5 PMid:12431498 |
||||
| 22.Blenkharn JI. Sustained anti-adherence activity of taurolidine (Taurolin) and noxythiolin (Noxyflex S) solutions. The J Pharmacy Pharm 1988; 40: 509-11. https://doi.org/10.1111/j.2042-7158.1988.tb05288.x PMid:2904994 |
||||
| 23.Gorman SP, McCafferty DF, Woolfson AD, Jones DS. Reduced adherence of micro-organisms to human mucosal epithelial cells following treatment with Taurolin, a novel antimicrobial agent. J Appl Bacteriol 1987; 62: 315-20. https://doi.org/10.1111/j.1365-2672.1987.tb04926.x PMid:3298185 |
||||
| 24.Browne MK. Pharmacological and clinical studies with Taurolin. In: W. L. Brückner and R. W. Pfirrmann, eds. Taurolin: in neues Konzept zur antimikrobiellen Chemotherapie chirurgischer Infektionen München Wien Baltimore: Urban und Schwarzenberg; 1985: 51 - 60. | ||||
| 25.Skellern GG. Pharmacokinetics of Taurolidine. In: W. L. Brückner and R. W. Pfirrmann, eds. Taurolin: Ein neues Konzept zur antimikrobiellen Chemotherapie chirurgischer Infektionen München Wien Baltimore: Urban und Schwarzenberg; 1985: 48 - 50. | ||||
| 26.Thomas LLM, Sturk A, Büller HR. The In-vitro-Inactivation of Endotoxins by Taurolin. In: W. L. Brückner and R. W. Pfirrmann, eds. Taurolin: Ein neues Konzept zur antimikrobiellen Chemotherapie chirurgischer Infektionen München Wien Baltimore: Urban und Schwarzenberg; 1985: 78-81. | ||||
| 27.Erb F, Imbenotte M, Huvenne JP, Vankemmel M, Scherpereel P, Pfirrmann RW. Structural investigation of a new organic antiseptic: Taurolidine. Eur J Drug Metabol Pharm 1983; 8: 163-73. https://doi.org/10.1007/BF03188742 |
||||
| 28.Zeriouh M, Sabashnikov A, Patil NP, Schmack B, Zych B, Mohite PN, et al. Use of taurolidine in lung transplantation for cystic fibrosis and impact on bacterial colonization. Eur J Cardiothorac Surg 2017; 53: 603-9. https://doi.org/10.1093/ejcts/ezx359 PMid:29048473 |
||||
| 29.Redmond HP, Neary PM, Jinih M, O'Connell E, Foley N, Pfirrmann RW, et al. RandomiSed clinical trial assessing Use of an anti-inflammatoRy aGent in attenUating peri-operatiVe inflAmmatioN in non-meTastatic colon cancer - the S.U.R.G.U.V.A.N.T. trial. BMC Cancer 2018; 18: 794. https://doi.org/10.1186/s12885-018-4641-x PMid:30081854 PMCid:PMC6091184 |
||||
| 30.Giudice M, Catuzzo B, Berlier N, Lau EW, Bonnemeier H, Assadian O, et al. Use of Taurolidine in a patient with a cardiac implantable electronic device protrusion. JACC: Case Rep 2023; 14: 101835. https://doi.org/10.1016/j.jaccas.2023.101835 PMid:37152697 PMCid:PMC10157148 |
||||
| 31.Weichsel J, Baldauf B, Bonnemeier H, Lau EW, Dittrich S, Cesnjevar R. Eradication of ventricular assist device driveline infection in paediatric patients with Taurolidine. J Cardiovasc Develop Dis 2022; 9: 18, https://doi.org/10.3390/jcdd9010018 PMid:35050228 PMCid:PMC8779719 |
||||
| 32. Bonnemeier H. Salvage of infected cardiac implantable electronic device with taurolidine-a case report. Cardiothorac Surgeon 2022; 30: 7. https://doi.org/10.1186/s43057-022-00068-5 |
||||
| 33.Giaccardi M, Baldauf B, Lau EW, Borov S, Bonnemeier H. Salvage of cardiac implantable electronic device pocket infection with skin erosion in frail 92-year-old. J Cardiovasc Develop Dis 2022; 9: 81. https://doi.org/10.3390/jcdd9030081 PMid:35323629 PMCid:PMC8955956 |
||||
| 34.Sohail MR, Corey GR, Wilkoff BL, Poole JE, Mittal S, Kennergren C, et al. Clinical presentation, timing, and microbiology of CIED Infections: An analysis of the WRAP-IT Trial. JACC Clin Electrophysiol 2021; 7: 50-61. https://doi.org/10.1016/j.jacep.2020.07.021 PMid:33478712 |
||||
| 35.Mittal S, Wilkoff BL, Kennergren C, Poole J, Corey R, Bracke F, et al. The World-wide Randomized Antibiotic EnveloPe Infection prevenTion (WRAP-IT) trial: Long-term follow-up. Heart Rhythm 2020; 180: 12-21. https://doi.org/10.1016/j.hrthm.2020.02.011 PMid:32087357 |
||||
| 36.Tarakji KG, Mittal S, Kennergren C, Corey R, Poole JE, Schloss E, et al. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med 2019; 380: 1895-905. https://doi.org/10.1056/NEJMoa1901111 PMid:30883056 |
||||
| 37.Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al.. [2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA)]. G Ital Cardiol (Rome) 2022; 23: e1-e94. | ||||
| 38.Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo R, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017; 14: e503-e551. https://doi.org/10.1016/j.hrthm.2017.09.001 PMid:28919379 |
||||
| 39.Minford JL. TauroLock for the prevention of recurrent infections in tunnelled central venous catheters in children. The PATCH study. (Prohylactic Administration of TauroLock in Children). 13/55 HTA CET Open Call, Outline Form, closing 2 Sept 2013. From: 01/10/2014 to: 28/02/2019 (53 months). | ||||
| 40.Agarwal AK, Roy-Chaudhury P, Mounts P, Hurlburt E, Pfaffle A, Poggio EC. Taurolidine/heparin lock solution and catheter-related bloodstream infection in hemodialysis: a randomized, double-blind, active-control, phase 3 study. Clin J Am Soc Nephrol 2023; 18: 1446-55. https://doi.org/10.2215/CJN.0000000000000278 PMid:37678222 PMCid:PMC10637459 |
||||
| 41.Vernon-Roberts A, Lopez RN, Frampton CM, Day AS. Meta-analysis of the efficacy of taurolidine in reducing catheter-related bloodstream infections for patients receiving parenteral nutrition. J Parenter Enteral Nutr 2022; 46: 1535-52. https://doi.org/10.1002/jpen.2363 PMid:35233792 PMCid:PMC9541343 |
||||
| 42.Ledson MJ, Gallagher MJ, Robinson M, Cowperthwaite C, Williets T, Hart CA et al. A randomized double-blinded placebo-controlled crossover trial of nebulized taurolidine in adult cystic fibrosis patients infected with Burkholderia cepacia. J Aerosol Med 2002; 15: 51-7. https://doi.org/10.1089/08942680252908575 PMid:12006145 |
||||
| 43.Korzilius JW, Gillis V, Wouters Y, Wanten GJA. Taurolidine-related adverse events in patients on home parenteral nutrition frequently indicate catheter-related problems. Clin Nutr 2022; 41: 2178-84. https://doi.org/10.1016/j.clnu.2022.07.025 PMid:36067590 |
||||
| 44.Krahn AD, Longtin Y, Philippon F, Birnie DH, Manlucu J, Angaran P, et al.. Prevention of arrhythmia device infection trial: The PADIT Trial. J Am Coll Cardiol 2018; 72: 3098-109. https://doi.org/10.1016/j.jacc.2018.09.068 PMid:30545448 |
||||
| 45.Rennert-May E, Raj SR, Leal J, Exner DV, Manns BJ, Chew DS. Economic evaluation of an absorbable antibiotic envelope for prevention of cardiac implantable electronic device infection. Europace 2020; 23: 767-74. https://doi.org/10.1093/europace/euaa291 PMid:33554239 |
||||
| 46.Henke J, Baldauf B, Lau EW, Dietl HPJ, Perani A, Mehilli J, et al. Taurolidine containing antimicrobial wash to prevent cardiac implantable electronic device infection. Eur J Arrhyth & Electrophysiol 2022; 8. | ||||
| 47.Casorelli E, Pescatori I, Ruocco G, Bonnemeier H, Assadian O, Bui F. [Pacemaker infection in fragile patients]. Herzschrittmacherther Elektrophysiol 2023; 34: 161-4. https://doi.org/10.1007/s00399-023-00940-9 PMid:37115248 PMCid:PMC10229671 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

