Immunological and autonomic profile of ischemic stroke in lowlands
ORIGINAL RESEARCH ARTICLE
Immunological and autonomic profile of ischemic stroke in lowlands
Article Summary
- DOI: 10.24969/hvt.2024.539
- CARDIOVASCULAR DISEASES
- Published: 10/01/2025
- Received: 16/10/2024
- Revised: 03/12/2024
- Accepted: 04/12/2024
- Views: 3105
- Downloads: 2405
- Keywords: ischemic stroke, severity, immunological, autonomic changes, correlation
Address for Correspondence: Nargiza T. Chekeeva, Neurotraumotology Department #1 of the National Hospital under the Ministry of Health of the Kyrgyz Republic, Bishkek, Kyrgyz Republic
Email: Chekeeva.n@gmail.com Phone: +996 777 99 72 27
Nargiza T. Chekeeva1, Svetalana G. Shleifer2, Bolot B. Kulov2, Begimai B. Kadyrova3, Asel T. Jusupova4, Elena V. Andrianova2
1 National Hospital under the Ministry of Health of the Kyrgyz Republic
2 Department of Neurology, Neurosurgery and Clinical Genetics, Kyrgyz-Russian Slavic University named after B.N. Yeltsin
3 Department of Special Clinical Disciplines, International School of Medicine of International University of Kyrgyzstan, Bishkek, Kyrgyz Republic
4 Department of Neurology and Clinical Genetics, Kyrgyz State Medical Academy named after I. K. Akhunbaev, Bishkek, Kyrgyz Republic
Abstract
Introduction: Ischemic stroke (IS) accounts for 80–92% of all stroke cases and is primarily associated with cardiovascular diseases and chronic cerebral ischemia. Stroke severity is influenced not only by the localization and volume of the ischemic focus but also by the degree of autonomic-vascular and neuroinflammatory changes. Investigating the relationship between immune and autonomic system parameters in IS, particularly concerning disease severity, remains a crucial area of research.
Research objective was to study immunological and autonomic parameters in patients with primary IS of varying severity in low-mountain conditions and clarify the pathogenesis and mechanisms underlying stroke severity.
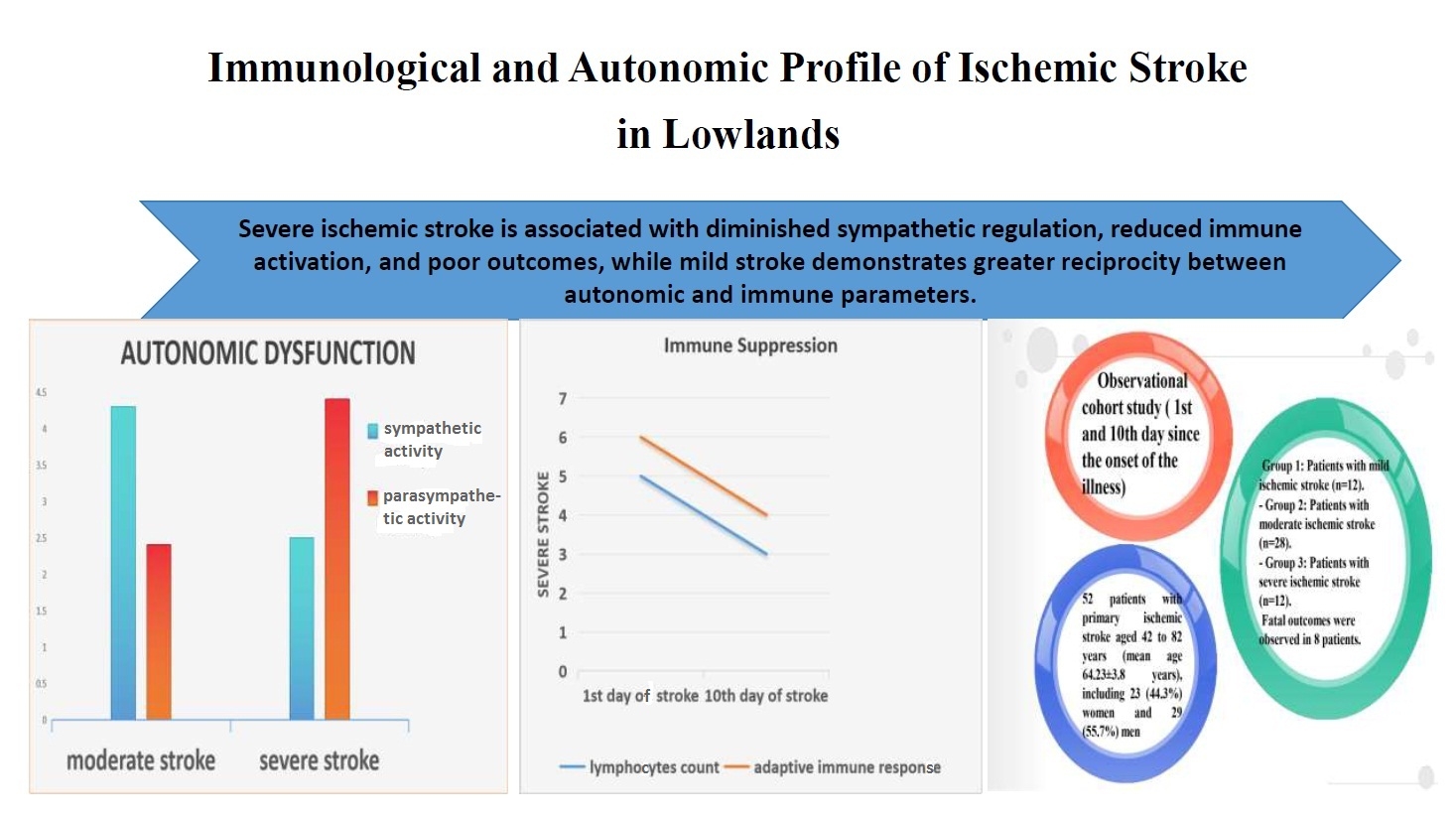
Methods: An observational cohort study was conducted involving 52 patients with primary IS divided into three groups: mild stroke (n = 12), moderate stroke (n = 28), and severe stroke (n = 12). In the acute phase, 44 survivors were observed, of whom 2 (4.5%) had severe neurological deficits, 26 (59%)- moderate deficits, and 16 (36.4%) - mild deficits. Additionally, 8 deceased patients were analyzed separately. Stroke severity was assessed using the Gusev and Skvortsova scale and the NIH Stroke Scale (NIHSS). Heart rate variability (HRV) and immunological parameters, including lymphocyte subpopulations and cytokine profiles, were evaluated on the 1st and 10th days of illness.
Results: In the acutest period, mild stroke was characterized by balanced autonomic activity, with neurohumoral activity at 30.1 (8.0)%, decreasing slightly in the acute period to 27.7 (8.6)%. Moderate stroke showed increased sympathetic activation, with neurohumoral activity reaching 61.6 (11.0)% and 83.7(8.0)% in the acute period (p< 0.05). Severe stroke demonstrated parasympathetic predominance and reduced immune activity, with levels of complement (CD) CD3+, CD16+, CD19+, and interleukin 1β being 1.4–2 times lower than in moderate stroke and 1.5–3 times lower than in mild stroke (p<0.05). Correlation analysis revealed that in mild stroke, 24% of studied parameters showed significant correlations, which was 5 times higher than in moderate and severe stroke. Among the 8 patients with fatal stroke, the number of significant correlations doubled compared to mild stroke, reaching 59.2%.
Conclusion: The interaction between the immune and autonomic systems in IS depends on disease severity. Severe stroke is associated with diminished sympathetic regulation and reduced immune activation, while mild stroke demonstrates greater reciprocity between autonomic and immune parameters. These findings contribute to understanding the pathogenesis of ischemic stroke and offer insights for tailored therapeutic strategies.
The study provides new insights into the interplay between immune and autonomic systems in IS, enriching knowledge of disease pathogenesis and progression. The results of this study can enhance the treatment of IS patients by incorporating HRV and immunological assessments into clinical practice. These methodologies are already implemented at the Angioneurology Department, City Clinical Hospital No. 1, Bishkek.
Key words: ischemic stroke, severity, immunological, autonomic changes, correlation
Graphical abstract
Introduction
Ischemic stroke (IS) accounts for the majority (80-92%) of stroke cases, largely due to the prevalence of cardiovascular conditions and chronic cerebral ischemia (1-5). Atherothrombotic and cardioembolic subtypes are the most common, with a distribution ratio of approximately 4:1 to 5:1 in the carotid versus vertebrobasilar circulation (1, 3, 6-9). Despite advancements in understanding the pathogenesis of IS, critical gaps persist, particularly concerning the role of immune-autonomic interactions in determining stroke severity.
Neuroinflammatory responses, including the release of cytokines such as interleukin-6 (IL-6) and C-reactive protein (CRP), play a pivotal role in the pathophysiology of stroke. Elevated levels of these markers have been linked to increased stroke severity and unfavorable outcomes (10-12). Concurrently, activation of the sympathetic nervous system and changes in heart rate variability (HRV) are noted during stroke, suggesting a reciprocal relationship between the immune and autonomic systems (13-17). However, the extent and dynamics of these interactions, especially in different severities of IS, remain underexplored and, at times, controversial.
Some studies highlight persistent immunosuppression following the initial immune activation phase of stroke as a negative prognostic indicator, potentially leading to fatal outcomes (14-15, 18-19). Others suggest that this immunosuppression may have protective effects, such as reducing autoimmune aggression in response to increased blood-brain barrier permeability (14-17). These divergent findings underscore the need for focused research to clarify the immunological and autonomic mechanisms in stroke pathogenesis.
This study aims to investigate immunological and autonomic parameters in patients with primary ischemic stroke of varying severity in low-mountain conditions. Specifically, the objectives include assessing immunological markers and HRV across mild, moderate, and severe cases during the acutest and acute periods and performing correlation analysis to explore the relationship between immune and autonomic responses in these patients.
This research seeks to bridge knowledge gaps in understanding the interplay of immune and autonomic systems in ischemic stroke, particularly its implications for disease severity and potential therapeutic strategies.
Methods
Study design and population
Study design is observational cohort study (1st and 10th day since the onset of the illness).
Study population was comprised of 52 patients with primary IS and 30 control healthy persons.
All patients were divided into 3 groups according to the severity of stroke:
- Group 1: Patients with mild ischemic stroke (n=12)
- Group 2: Patients with moderate ischemic stroke (n=28)
- Group 3: Patients with severe ischemic stroke (n=12).
Control group included 30 healthy volunteers from whom blood samples were collected.
The study was conducted from 2014 to 2024 in the specialized departments of City Clinical Hospital No. 1, City Clinical Hospital No. 6, and the Hospital of the Government and Presidential Administration of the Kyrgyz Republic in Bishkek. Immunological testing was performed at the laboratory of the Research Institute of Molecular Biology and Medicine at the National Center for Cardiology and Therapy under the Ministry of Health of the Kyrgyz Republic.
Inclusion criteria: primary IS in the acutest phase. Diagnosis confirmed by neurological, clinical, and neuroimaging methods according to the International Classification of Diseases (ICD-10), TOAST classification, and OCSP criteria.
Exclusion criteria: hospitalization more than 24 hours after disease onset, hemorrhagic or recurrent stroke, patient’s refusal to cooperate at any stage, presence of acute and/or chronic infectious diseases, severe heart rhythm disturbances, myocardial infarction, heart failure, oncology, history of traumatic brain injury, alcoholism, or drug addiction. Patients taking β-blockers or neuroleptics were excluded.
Ethics: All patients provided written informed consent for voluntary participation, collection of biological material (blood), and publication of general study results. For incapacitated patients, consent was obtained from relatives.
Study protocol was approved by local Ethics Committee.
Baseline variables
All patients underwent clinical examinations and demographic (age, sex), laboratory and clinical data were collected.
Neurological examination
The neurological examination was performed according to the generally accepted method (5, 20-21). The degree of impaired consciousness was assessed using the Glasgow Coma Scale (5). This involved testing eye-opening response, speech, and motor reactions. A total score of 15 corresponded to clear consciousness, 14-10 points to stupor, 9-8 points to lethargy, and 7 or less to coma.
The severity of IS was assessed using the clinical scales of E.I. Gusev and V.I. Skvortsova, National Institutes of Health Stroke Scale (NIHSS), and Medical Research Council Weakness Scale (MRC) on the 1st and 10th days of the disease. The original scale of E.I. Gusev and V.I. Skvortsova (1991) considers the level of consciousness, higher brain functions, cranial nerves, motor, coordination, and sensory functions, as well as the presence of meningeal symptoms and vital disorders. The more severe the stroke, the fewer points the patient scores on the scale. Less than 30 points was considered extremely severe, 30-35 points moderately severe, and over 35 points mild stroke.
The NIHSS complements the assessment of stroke severity. A total score of 1-4 points corresponded to mild stroke severity, 5-15 points to moderate severity, 16-20 points to moderate-to-severe stroke, and 21-42 points to severe stroke progression.
Motor impairments were evaluated using the MRC as follows:
-0 points – no movement;
- 1 point – muscle contraction without movement in the corresponding joint;
- 2 points – muscle contraction with joint movement but no limb elevation;
- 3 points – muscle contraction with limb elevation but unable to overcome resistance;
- 4 points – the examiner cannot resist the limb movement during extension;
- 5 points – normal limb strength with resistance.
Neuroimaging: magnetic resonance imaging (MRI) and computed tomography (CT)
All patients with stroke underwent neuroimaging within 48 hours of disease onset. MRI of the brain was performed on 48 patients, while CT was performed on 4 patients. MRI was conducted using a "Hitachi 1.5 Tesla" scanner (Japan) in T1- and T2-weighted imaging (WI) modes, T2-FLAIR, and diffusion weighted imaging (DWI), in sagittal, horizontal, and frontal planes. Slice thickness was 5 mm with an interval of 2 mm. The volume of the ischemic focus was considered large if it was more than 50 cm3, average from 10 to 50 cm3, small - up to 10 cm3, while the size of the ischemic focus in the studied groups corresponded to the severity of the disease (22).
Heart rate variability and assessment of autonomic dysfunction
HRV was recorded using an automated system consisting of a personal computer, a registration device, a rhythmograph based on a single-channel electrocardiograph, an analog-to-digital converter, and software. HRV was studied according to the method of R.M. Baevsky (1984) in a resting lying position for 5 minutes, on the first and tenth days from the onset of stroke, in the morning. Analyzed statistical and spectral parameters of HVT are presented in Table 1.
|
Table 1. Heart rate variability parameters |
||
|
Variables |
Unit |
Description |
|
Mode amplitude (AMo) |
% |
Conditional indicator of sympathetic regulation activity |
|
Tension index of regulatory systems (TI) |
c.u. |
Activity of the sympathetic and central regulatory circuits |
|
Total spectral power (TP) |
мs2 \Hz |
Overall heart rate variability spectral power |
|
High-frequency power (HF norm) |
% |
Reflects parasympathetic activity |
|
Low-frequency power(LF norm) |
% |
Defines sympathetic effects on heart rhythm |
|
Sympathetic/parasympathetic ratio (LF/HF) |
c.u |
Ratio index of sympathetic and parasympathetic influences |
|
Very-low-frequency power (VLF) |
ms2 \Hz |
Indicates humoral effects on heart rhythm |
Immunological analysis
The assessment of cellular and humoral immunity involved analyzing T- and B-lymphocyte subpopulations (CD3+, CD4+, CD8+, CD16+, CD19+), the immunoregulatory index (IRI), and the cytokine profile, including interleukins (IL-1β, IL-4, IL-8), tumor necrosis factor-alpha (TNF-α), cytotoxic effect of lymphocytes (CTEL), and CRP in venous blood. The results were compared to established reference values and a control group of healthy volunteers (9, 15).
Subpopulations of T and B lymphocytes in venous blood were determined using immunofluorescence techniques with monoclonal and polyclonal antibody kits ("Status," SORBENT LLC, Moscow, Russia). The immunogram included absolute and relative levels of key lymphocyte subpopulations:
CD3+ T cells: representing the total population of mature T lymphocytes, serving as an integral marker for immune responses.
CD4+ T cells: helper T cells that regulate and enhance both cellular and humoral immune responses.
CD8+ cytotoxic T lymphocytes: responsible for cell-mediated cytotoxicity against target structures or functioning as suppressors under normal immune activity.
CD16+ natural killer (NK) cells: Mediating antibody-independent and complement-independent cytotoxic responses.
CD19+ B lymphocytes: Playing a critical role in humoral immunity through antibody production.
The immunoregulatory index (IRI) was calculated as the ratio of CD3+CD4+ to CD3+CD8+ lymphocytes in venous blood, with reference values ranging from 1.6 to 2.2 units.
Results of the immunological study were benchmarked against standard reference values provided by Kitaev et al. (8). Table 2 presents a comparative analysis of the observed immunological parameters against these reference ranges and control group data.
|
Table 2. Reference values for lymphocyte subpopulation levels in venous blood and control group values |
||||
|
Subpopulation |
Absolute Count |
Relative Content (%) |
Control Group (n=30) Mean (SD), cells/μl |
|
|
cells/μl |
×10⁹/l |
|||
|
CD3+ |
800-2200 |
0.80-2.20 |
55-60 |
1244.0 (110.0) |
|
CD4+ |
600-1600 |
0.60-1.60 |
31-51 |
653.6 (88.6) |
|
CD8+ |
300-800 |
0.30-0.80 |
19-37 |
493.5 (66.0) |
|
CD16+ |
150-600 |
0.15-0.60 |
6-20 |
238.3 (35.0) |
|
CD19+ |
100-500 |
0.10-0.50 |
5-19 |
480.0 (86.0) |
Cytokine content in venous blood plasma was analyzed using solid-phase immunoassay with a Multiscan analyzer (Germany) and Vector-Best reagent kits (Novosibirsk, Russia). The method was based on a sandwich variant of solid-phase immunoassay using mono- and polyclonal antibodies against interleukins 1β, 4, 8, and TNF-α. The cytotoxic effect of lymphocytes in venous blood serum was studied because of their involvement in the pathogenesis of many pathological processes and diseases, through effects like stimulation of prothrombotic mechanisms and the coagulation system, inflammation induction, and alteration of target cells such as erythrocytes and platelets (Table 3).
|
Table 3. Cytokine levels in venous blood serum |
|
|
Cytokine |
Control Group (n=30) Mean (SD), pg/ml |
|
Interleukin-1β (IL-1β) |
7.9 (2.0) |
|
Interleukin-4 (IL-4) |
3.8 (0.9) |
|
Interleukin-8 (IL-8) |
7.0 (1.7) |
|
Tumor Necrosis Factor-α (TNF-α) |
4.3 (0.8) |
|
Cytotoxic effect of lymphocytes (CTEL) |
1.7 (0.1) |
Statistical analysis
Statistical processing was performed using "SPSS for Windows ver. 9.0" (IBM, New York, USA), Microsoft Excel-2010 (Microsoft, USA) softwares according to the standard method (23). The mean value (M) and standard deviation (SD) number and percentages were calculated. Statistical significance was assessed by Student's t-criteria, with the probability of an error of the first kind of no more than 5% (p <0.05). A pair-correlation analysis of the studied indicators was carried out with Pearson's coefficient for metric scales (p <0.01, p <0.05).
Results
Baseline characteristics
Patients` population with primary IS was aged between 42 to 82 years (mean age 64.23 (3.8) years), including 23 (44.3%) women and 29 (55.7%) men. Among them 12 had mild IS (n=12), 28 moderate IS (n=28) and 12 - severe IS (n=12). Additionally, in the acute phase: 2 patients (4.5%) had severe neurological deficits, 26 (59 (2.5)%) had moderate deficits, and 16 (36.4 (2.3)%) had mild deficits. Fatal outcomes were observed in 8 patients.
Neuroimaging data
In 18 patients, the ischemic focus was in the middle cerebral artery basin on the left, in 11 patients- in the middle cerebral artery basin on the right, and in 15 patients- in the vertebrobasilar basin. Also, 8 patients with fatal stroke were observed. The ischemic focus was located in the basin of the middle cerebral artery and internal carotid artery in 5 cases, in the basin of the posterior inferior cerebellar artery in 2 cases, and in one case -in the basin of the posterior cerebral artery.
Heart rate variability data
Table 4 provides a detailed comparison of these findings, illustrating how stroke severity correlates with varying autonomic responses. HRV analysis in stroke patients with mild neurological deficits in the acutest period revealed a predominance of parasympathetic influences on heart rhythm. By the 10th day, a shift towards reduced parasympathetic activity and increased sympathetic influences was observed.
In patients with moderate stroke, the acute period was characterized by heightened ergotropic regulatory activity, with a greater sympathetic influence on heart rhythm compared to mild stroke patients. These parameters showed a shift towards increased parasympathetic activity in the later stages.
Patients with severe stroke or fatal outcomes exhibited distinct HRV patterns, including diminished ergotropic regulation and reduced sympathetic influences, differing significantly from those with milder strokes.
The analysis of HRV in patients with IS revealed distinct patterns depending on the severity of the disease and the time of observation.
In patients with mild IS during the acutest period, parasympathetic activity predominated, as indicated by a low LF/HF ratio and a balanced distribution between low-frequency (LF) and high-frequency (HF) components. By the 10th day, there was an increase in sympathetic activity, with an elevated LF/HF ratio and tension index (TI), reflecting a shift towards ergotropic regulatory mechanisms (Table 4).
Patients with moderate IS displayed higher sympathetic activation during the acutest period, with a significantly higher LF/HF ratio compared to mild stroke patients. Over time, a shift towards parasympathetic dominance was observed by the 10th day, indicated by increased HF and reduced LF power values. The reduction in VLF components suggests decreased neurohumoral activity during this period.
In severe IS cases, HRV parameters showed reduced autonomic balance during the acutest period, with low VLF proportions and moderate LF/HF ratios, indicating impaired autonomic regulation. By the 10th day, in the few surviving patients, parasympathetic regulation mechanisms remained dominant, as evidenced by the proportional decrease in LF/HF ratios and low VLF activity.
|
Table 4. HRV indicators in patients with ischemic stroke depending on the severity of the disease on the 1st and 10th day |
||||||
|
HRV indicators |
Mild ischemic strike |
Moderate ischemic stroke |
Severe ischemic stroke |
|||
|
1st day (n=12) |
10th day (n=16) |
1st day (n=28) |
10th day (n=26) |
1st day (n=12) |
10th day (n=2) |
|
|
TР, мs2 \Hz |
2424.7 (664.0) |
2872.0 (1008.0) |
1457.83 (966.0) |
647.1 (69.4)▪ |
3214.0 (114.6) |
1447.9 |
|
VLF, ms2 \Hz |
729.0 (27.5) |
796.0 (144.0)
|
897.50 (189.0) |
539.4(73.0)* |
1256.4 (128.0)• |
424.9 |
|
LF norm ,% |
50.7 (15.0) |
67.0 (7.6)
|
68.2 (2.2) |
29.9 (1.9)* |
47.0 (2.3)• |
52.0 |
|
HF norm, % |
49.0 (15.0) |
44.0 (14.7)
|
31.8 (2.2) |
70.1 (6.5)* |
52.5 (2.4)• |
48.0 |
|
LF\HF, c.u. |
1.4 (0.6) |
2.9 (0.4)
|
2.14(0.12)▪ |
0.43 (0.03)*▪ |
1.05 (0.1)• |
1.3
|
|
АМо, % |
50.3 (2.3)
|
56.9 (5.3)
|
49.2 (3.7) |
70.2 (13.4)* |
50.1 (11.0) |
56.3 |
|
TI, c.u. |
154.0 (62.0) |
194.0 (58.0)
|
128.2 (30.0)▪ |
71.2 (51.0)*▪ |
147.3 (28.4) |
188.2 |
|
Data are presented as mean (SD) * - difference in heart rate variability parameters in patient groups on the 1st and 10th day of observation (p <0.05); ▪ - difference in heart rate variability parameters in patients with mild and moderate stroke on the 1st and 10th day of observation, respectively (p <0.05); • - difference in heart rate variability parameters in patients with moderate and severe stroke (p <0.05) AMo - mode amplitude, HF - high-frequency power, LF - low-frequency power, LF/HF- sympathetic/parasympathetic ratio, TI - tension index of regulatory systems, TP - total spectral power, VLF - very-low-frequency power |
||||||
These findings highlight that stroke severity correlates with the reserve capacity and activity of the autonomic nervous system. In particular, an unfavorable prognostic indicator may be the predominance of parasympathetic regulatory mechanisms during the acute and acutest periods, which are associated with poorer outcomes in severe stroke cases.
Immunological data
The immunological study (Table 5) revealed variations in lymphocyte subpopulations and cytokine profiles in patients with ischemic stroke, depending on the severity of their neurological deficits.
In the acutest period, patients with mild stroke demonstrated higher levels of lymphocyte subpopulations compared to those with moderate and severe stroke. Specifically, levels of CD3+, CD4+, CD8+, CD16+, and CD19+ were noticeably higher in mild stroke patients than in moderate stroke patients and significantly decreased in severe stroke cases. For example, CD3+ levels in severe stroke were approximately 2 to 6 times lower than reference values and those observed in mild stroke (p<0.05). This trend persisted in the acute period, with lymphocyte subpopulation levels slightly increasing in mild stroke but remaining lower than expected regional reference values in all groups.
The cytokine profile also showed marked differences across IS severity. In the acute period, IL1β levels were significantly lower in severe stroke patients compared to those with mild or moderate stroke (p<0.05). Similarly, CRP levels were substantially higher in moderate stroke patients than in those with mild stroke. However, IL4 levels were elevated in mild stroke patients compared to moderate stroke, suggesting a stronger adaptive immune response in less severe cases (p<0.05). Across all groups, cytokine levels (except IL4) showed a consistent decrease from the acutest to the acute period, dropping by approximately 1.5 to 2 times. The increase in IL4 was most pronounced in the mild stroke group (p<0.05). Due to the limited sample size of severe stroke patients in the acute period, comprehensive cytokine analysis in this group was not feasible.
These findings suggest that stroke severity is inversely correlated with the levels of lymphocyte subpopulations and adaptive immune responses, with more severe cases exhibiting profound immunosuppression and higher systemic inflammation markers.
|
Table 5. Immunological parameters of patients with ischemic stroke depending on the severity of the disease in the acute and pre-acute periods |
|||
|
Immunogram variables |
Group 1 (n=12) |
Group 2 (n=28) |
Group 3 (n=12) |
|
On the 1st day of observation |
|||
|
CD 3+, cells/μl |
656.8 (99.0) |
499.0 (71.0)‣ |
208.4 (98.0)‣• |
|
CD 4+, cells/μl |
417.0 (58.0) |
330.9 (42.0‣ |
220.8 (110.0)‣ |
|
CD 8+, cells/μl |
388.0 (45.4) |
334.6 (34.8) |
209.0 (109.2)‣ |
|
CD 16+, cells/μl |
354.0 (59.0) |
297.0 (40.7) |
130.0 (68.0)‣• |
|
CD 19+, cells/μl |
420.0 (58.4) |
335.0 (36.0)‣ |
180.8 (84.0)‣• |
|
IRI |
1.08 (0.07) |
0.99 (0.05) |
1.21 (0.24) |
|
IL 1β, pg/ml |
20.3 (2.2) |
20.1 (1.3) |
14.2 (1.8)‣• |
|
IL 8, pg/ml |
54.7 (9.5) |
63.6 (7.0) |
51.0 (7.0) |
|
IL 4, pg/ml |
12.0 (1.2) |
11.0 (1.1) |
9.9 (3.5) |
|
CRP, mg/dL |
201.6 (31.8) |
204.6 (24.3) |
211.0 (34.6) |
|
TNF-α, pg/ml |
59.5 (7.8) |
64.6 (7.5) |
46.5 (13.5) |
|
CTEL, IU/ml |
4.0 (0.4) |
3.8 (0.3) |
3.1 (0.3) |
|
On the 10th day of observation |
|||
|
|
Group 1 (n=16) |
Group 2 (n=26) |
Group 3 (n=2) |
|
CD 3+, cells/μl |
707.0(91.0) |
560.9 (69.0)‣ |
240.0 |
|
CD 4+, cells/μl |
431.4 (54.9) |
346.6 (34.6)‣ |
245.6 |
|
CD 8+, cells/μl |
391.0 (51.8) |
324.8 (35.4) |
231.2 |
|
CD 16+, cells/μl |
402.8 (58.6) |
317.9 (50.0) |
282.2 |
|
CD 19+, cells/μl |
440.6 (59.0) |
338.0 (31.8)‣ |
401.6 |
|
IRI |
1.11 (0.05) |
1.08 (0.05) |
0.97 |
|
IL 1β, pg/ml |
11.9 (0.7)Ѳ |
10.1 (0.5) Ѳ |
10.0 |
|
IL 8, pg/ml |
31.0 (3.6)Ѳ |
35.7 (9.2) Ѳ |
40.5 |
|
IL 4, pg/ml |
28.0 (2.0)Ѳ |
19.3 (1.8)‣ Ѳ |
20.0 |
|
CRP |
27.4 (4.5) Ѳ |
46.0 (16.8) ‣Ѳ |
60.0 |
|
TNF-α, pg/ml l |
32.0 (3.8) Ѳ |
30.0 (5.9) Ѳ |
25.0 |
|
CTEL, IU/m |
1.93 (0.14) Ѳ |
1.81 (0.25) Ѳ |
2.05 |
|
Data are presented as mean (SD) ‣ - comparison with indicators of patients with mild stroke (p <0.05) • - comparison with indicators of patients with moderate stroke p <0.05); Ѳ - comparison of indicators of patients with stroke on the 1st and 10th days of observation in the related groups (p <0.05) CRP – C-reactive protein, CTEL- cytotoxic effect of lymphocytes, IL – interleukin, IRI - the ratio of CD3+CD4+ to CD3+CD8+ lymphocytes, TNF-α - tumor necrosis factor-α |
|||
The results indicate that as stroke severity increases, the cellular immune response becomes progressively weaker during both the acutest and acute periods, with particularly low IL4 levels observed in the acute period (Table 5).
A similar pattern was noted in deceased patients, where a marked suppression of T- and B-lymphocyte differentiation was evident, reflecting inadequate regulation of humoral and adaptive immunity. These findings suggest that diminished cellular immune responses are an unfavorable prognostic indicator in IS.
Conversely, patients with mild stroke exhibited
relatively higher nonspecific immune activity and metabolic adaptation, as inferred from HRV parameters. These changes may represent a favorable adaptive mechanism in the dynamics of acute ischemia.
Correlation of stroke severity with immune and autonomic indices (Table 6)
The correlation analysis (Table 6) revealed the following key patterns:
In mild stroke- the most significant correlations were observed in the acute period, where 24% of the parameters showed meaningful associations.
|
Table 6. Correlation of stroke severity with immune and autonomic indices |
|||
|
Variables |
Mild stroke |
Moderate stroke |
Severe stroke |
|
Parasympathetic Activity (HF norm) |
Direct with CD19+ (r=0.725–0.931, p<0.01), CD16+, CD8+ (r=0.725–0.931, p<0.05) |
Direct with IL1β, IL8, CTEL (r=0.9–0.94, p<0.05) |
Direct with CD19+ (r=0.59, p<0.01), inverse with LF norm (r=-0.58, p<0.01) |
|
Sympathetic Activity (LF norm) |
Inverse with CD19+, CD16+, CD8+ (r=-0.725 to -0.931, p<0.05) |
Strong inverse with CD3+ (r=-0.79 to -0.98, p<0.01) |
Inverse with CD19+ (r=-0.58, p<0.01) |
|
Sympathovagal Index (LF/HF) |
Inverse with CD19+, CD16+, CD8+ (r=-0.719 to -0.954, p<0.05) |
Inverse with CD4+ (r=-0.88 to -0.98, p<0.01) |
Inverse with CD19+ (r=-0.54, p<0.05) |
|
VLF |
Direct with CD8+ (r=-0.99, p<0.01) |
Direct with CD4+, IRI, CRP (r=0.72–0.82, p<0.05) |
Direct with CRP, TNF-α, IL8, IL4 (r=0.68–0.95, p<0.01), inverse with CD19+ (r=-0.7, p<0.01) |
|
Total spectrum power (TP) |
Direct with IL4, CTEL (r=0.77–0.89, p<0.05) |
Direct with CRP (r=0.99, p<0.01), IL1β (r=0.79, p<0.01), L8 (r=0.91, p<0.01), CTEL (r=0.8, p<0.05) |
Direct with CD16+ (r=0.5, p<0.05), IL4 (r=0.4, p<0.05), inverse with IL1β (r=-0.44, p<0.05), CTEL (-0.56) |
|
Central regulation circuit (Amo) |
Direct with CD4+, CRP, IL1β, IL8 (r=0.89–0.98, p<0.01), inverse with CD3+ (r=-0.86, p<0.01) |
Inverse with CD4+, IRI (r=-0.88 to -0.98, p<0.01) |
Inverse with TNF-α (r=-0.6, p<0.01), IL4 (r=-0.67, p<0.01) |
|
CRP – C-reactive protein, CTEL- cytotoxic effect of lymphocytes, IL – interleukin, IRI - the ratio of CD3+CD4+ to CD3+CD8+ lymphocytes, TNF-α - tumor necrosis factor-α |
|||
Direct correlations were found between parasympathetic activity (HF norm) and immune markers (CD19+, CD16+, CD8+), while inverse correlations were observed between these markers and sympathetic activity (LF norm) and the sympathovagal index (LF/HF).
The central regulatory circuit (Amo) was associated with inflammatory markers (CRP, IL1β, IL8), indicating the activation of humoral immunity in response to the pathological process.
In moderate stroke: the number of significant correlations decreased, reflecting weakened interaction between the autonomic nervous system and immune regulation. Sympathetic activity (LF norm) and the sympathovagal index (LF/HF) demonstrated strong inverse correlations with T- and B-lymphocytes (CD3+, CD4+, CD19+), suggesting a cellular immune response in adaptation.
In severe stroke: the number of significant correlations was minimal, indicating the dominance of vegetative-metabolic regulation mechanisms typical of critical conditions. Direct correlations between HF norm and CD19+, as well as inverse correlations with LF norm and LF/HF, highlighting the role of parasympathetic adaptation despite suppressed immune activity.
In patients with fatal outcomes, the number of significant correlations doubled compared to mild stroke (59.2%), indicating excessive activation of neurohumoral mechanisms. Immune markers (CRP, TNF-α, IL8, IL4) demonstrated direct correlations with HF norm and VLF but inverse correlations with LF norm and LF/HF, emphasizing the dominance of humoral immune responses.
Discussion
Our study demonstrates that the severity of IS significantly influences both autonomic and immunological functions. Patients with mild stroke exhibited predominant parasympathetic activity during the acutest period, shifting towards sympathetic dominance by the 10th day. Conversely, those with moderate stroke showed heightened sympathetic activity initially, with a subsequent increase in parasympathetic influence. Severe stroke cases were characterized by diminished autonomic balance and reduced ergotropic regulation. Immunologically, mild stroke patients maintained higher levels of lymphocyte subpopulations and adaptive immune responses, whereas severe stroke patients experienced significant immunosuppression, marked by decreased lymphocyte counts and altered cytokine profiles.
These findings align with previous research indicating that stroke severity correlates with autonomic and immune system alterations. For instance, Guan et al. (23) reported that autonomic dysfunction, as measured by heart rate variability (HRV), could predict secondary ischemic events after transient ischemic attack or minor stroke, highlighting the role of autonomic imbalance in stroke prognosis. Similarly, Haeusler et al. (24) observed cellular immunodepression preceding infectious complications in acute ischemic stroke patients, underscoring the impact of stroke on immune function.
Our study contributes novel insights by providing a detailed temporal analysis of both autonomic and immunological changes across different stroke severities.
Study limitations
However, limitations include a small sample size, particularly in the severe stroke group, which may affect the generalizability of the results. Additionally, the observational nature of the study precludes establishing causality between observed changes and clinical outcomes. Further research with larger cohorts is necessary to validate these findings and explore underlying mechanisms.
Clinical Implications
Understanding the dynamics of autonomic and immune responses in stroke can inform targeted therapeutic strategies aimed at improving autonomic balance and immune function. Future studies should explore interventions that modulate these systems to improve outcomes in severe stroke cases.
Further research with larger cohorts and mechanistic approaches is needed to validate these findings and develop novel therapeutic strategies.
Conclusion
1. Impact of stroke severity on autonomic function:
Mild ischemic stroke is characterized by initial parasympathetic dominance, shifting towards sympathetic predominance over time. Moderate stroke shows heightened sympathetic activity during the acutest phase, transitioning to increased parasympathetic activity in the later stages. Severe stroke demonstrates a marked reduction in autonomic balance and diminished ergotropic regulation, indicating impaired autonomic adaptability.
2. Immunological changes and stroke severity:
Patients with mild stroke maintain higher levels of lymphocyte subpopulations (CD3+, CD4+, CD8+, CD19+) and adaptive immune responses. Severe stroke is associated with profound immunosuppression, with significant reductions in lymphocyte counts and altered cytokine profiles (e.g., low IL4, high CRP).
3. Correlation of autonomic and immune functions:
A significant interplay between autonomic and immune responses exists, with direct correlations between parasympathetic activity (HF norm) and adaptive immune markers in mild stroke. Severe stroke shows minimal correlation, reflecting the dominance of metabolic-vegetative regulatory mechanisms.
4. Prognostic indicators
Predominance of parasympathetic regulatory mechanisms in the acute phases of severe stroke correlates with poorer outcomes. Suppression of T- and B-lymphocyte differentiation and reduced cellular immune responses are unfavorable prognostic indicators. Further studies on larger populations are needed.
Ethics: All patients provided written informed consent for voluntary participation, collection of biological material (blood), and publication of general study results. For incapacitated patients, consent was obtained from relatives. Local Ethic Committee approved the study protocol.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: N.T.Ch., S.G.S, B.B.K., B.B.K., A.T. J ., and E.V.A. equally contributed to manuscript preparation and fulfilled the authorship criteria.
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use: Authors declared they did not use A.I.- assisted technologies in preparation of manuscript
Availability of data and material: Not applied
References
| 1. Klochikhin OA, Stakhovskaya LV, Polunina EA, Strakhov OA, Klochikhin MM. Epidemiology and prognosis of incidence and mortality from stroke in different age groups according to the territorial-population registry. Korsakov J Neurol Psychiatry Spec Issues 2019; 119: 5-12. DOI: 10.17116/jnevro20191190825 https://doi.org/10.17116/jnevro20191190825 PMid:31825357 |
||||
| 2. Suslina ZA, Varakin YY. Epidemiological aspects of stroke study: time to sum up. Ann Clin Exp Neurol. 2007; 1: 22-8. | ||||
| 3. Musabekova TO. Diagnostics and treatment of acute cerebrovascular accidents (hospital stage, acute and acute periods) (teaching aid). Bishkek; 2015. 75p. | ||||
| 4. Makarov AY. Clinical neurology: selected. Moscow: Foliant; 2017. 667p. | ||||
| 5. Levin OS, Shtulman DR. Neurology: a practitioner's handbook. 10th ed. Moscow: MED press-inform; 2016. 1024p. | ||||
| 6. Yrysova MB, Kasymov OT. Epidemiological analysis of the prevalence and incidence of cerebrovascular diseases in the Kyrgyz Republic. Avicenna Bull 2019; 21: 472-9. doi:10.25005/2074-0581-2019-21-3-472-479 https://doi.org/10.25005/2074-0581-2019-21-3-472-479 |
||||
| 7. Fadeev PA. Stroke. Moscow: Mir i Obrazovanie; 2020. 727p. | ||||
| 8. Kitaev MI, Soburov KA. Regional norms of immunity indicators and immunological markers in the mountain population of Kyrgyzstan. Bishkek; 2009. 148 p. | ||||
| 9. Lapach SN, Chubenko AV, Babich PN. Statistical methods in biomedical research using Excel. Kyiv: MORION; 2000. 320 p. | ||||
| 10. Barnaby D, Ferrick K, Kaplan DT, Shah S, Bijur P, Ghallagher EJ. Heart rate variability in emergency department patients with sepsis. Acad Emerg Med 2002;14: 661-70. https://doi.org/10.1197/aemj.9.7.661 |
||||
| 11. Ahmad S, Ramsay T, Huebsch L, Flanagan S, McDiarmid S, Batkin I, et al. Continuous multi-parameter heart rate variability analysis heralds onset of sepsis in adults. PLoS One 2009;14: 6642. doi: 10.1371/journal.pone.0006642 https://doi.org/10.1371/journal.pone.0006642 PMid:19680545 PMCid:PMC2721415 |
||||
| 12. Walter U, Kolbaske S, Patejdl R, Steinhagen V, Abu-Mugheisib M, Grossmann A, et.al. Insular stroke is associated with acute sympathetic hyperactivation and immunodepression. Eur J Neurol 2013; 20: 153-9. Doi: 10.1111/ene.12050 https://doi.org/10.1111/ene.12050 PMid:22612423 |
||||
| 13. Murzaliev AM, Musabekova TO, Kim TV. Functional state of the autonomic nervous system in the acute period of ischemic stroke in the carotid and vertebrobasilar arteries in middle-aged patients. Bull Kyrgyz-Russian Slavic Univ 2014; 14: 114-7. | ||||
| 14. Yarets YI. Interpretation of immunogram results. Gomel: State Institution "RSPC RMiECh"; 2020. 38p. | ||||
| 15. Shleifer SG, Kozlov VM, Pervushin VA, Zubarev AS. Cytotoxic effect of lymphocytes with cardiolipin in patients with acute and chronic cerebral ischemia. Cent Asian Med J 2004; 10: 135-7. | ||||
| 16. Bebinov EM, Shcherbak LV, Veiberov VA, Kazantseva, S. N., Popov, V. V., Polikarpov, A. N.. Dynamics of respiratory arrest and its resumption in acute hypobaric hypoxia at different times of mountain adaptation and readaptation. Agadzhanian Readings: Proc II All-Russian Sci Pract Conf. Moscow: Peoples' Friendship Univ Russia 2018. Pp. 51-52. | ||||
| 17. Andrianova EV, Shleifer SG, Lupinskaya, ZA, Sukhareva LA, Ivanov AA. Correlations between systemic hemodynamic parameters and cardiointervalography in patients with atherosclerotic dyscirculatory encephalopathy. Sci New Technol 2011; 6: 82-4. | ||||
| 18. Emsley HCA, Smith CJ, Hopkins SJ. Infection and brain-induced immunodepression after acute ischemic stroke. Stroke 2008; 39: e7. doi: 10.1161/STROKEAHA.107.500447 https://doi.org/10.1161/STROKEAHA.107.500447 PMid:18048842 |
||||
| 19. Candelario-Jalil E. Injury and repair mechanisms in ischemic stroke: considerations for novel neurotherapeutics. Curr Opin Investig Drugs 2009; 10: 644-54. DOI: 10.1016/j.coind.2009.05.003 | ||||
| 20. Piradov MA, Maksimova MYU, Tanashyan MM. Stroke: step-by-step instructions: a guide for doctors. Moscow: GEOTAR-Media. 2019. 267p. | ||||
| 21. Makarov A.Yu. Clinical neurology. Selected. Moscow : Foliant. 2017. 667p. | ||||
| 22. Yabluchansky NI, Kantor BY, Martynenko AV. Heart rhythm variability. Donetsk: Buden; 1997. | ||||
| 23. Guan L, Colet JP, Mazowita G, Claydon VE. Autonomic nervous system and stress to predict secondary ischemic events after transient ischemic attack or minor stroke: possible implications of heart rate variability. Front Neurol 2018; 9: 90. doi: 10.3389/fneur.2018.00090. https://doi.org/10.3389/fneur.2018.00090 PMid:29556209 PMCid:PMC5844932 |
||||
| 24. Haeusler KG. Cellular immunodepression preceding infectious complications after acute ischemic stroke in humans. Cerebrovasc Dis.2008; 25: 50-8. doi: 10.1159/000111499. https://doi.org/10.1159/000111499 PMid:18033958 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

