A potential innovative surrogate marker for hypoxic injury: Shehata marker of angiogenesis
ORIGINAL RESEARCH ARTICLE
A potential innovative surrogate marker for hypoxic injury: Shehata marker of angiogenesis
Article Summary
- DOI: 10.24969/hvt.2024.540
- CARDIOVASCULAR DISEASES
- Published: 12/01/2025
- Received: 31/10/2024
- Revised: 08/12/2024
- Accepted: 12/12/2024
- Views: 3322
- Downloads: 2263
- Keywords: Angiogenesis, ischemia, hypoxia, nitric oxide synthase, vascular endothelial growth factor, endothelin-1
Address for Correspondence: Shehata A. Mohamed, Medical School Brandenburg & MSAM Clinic, Center Of Translational Medical Research, D-14770 Hochstraße 29, Brandenburg, Germany.
Email: mohammed.shehatta1@gmail.com Phone: +49 15201043716
Mohamed S.A. Mohamed
Medical School Brandenburg & MSAM Clinic, Center ofTranslational Medical Research, Brandenburg, Germany
Abstract
Objective: Many factors regulate the process of angiogenesis under physiological and pathological conditions. However, no reliable marker is currently available that can predict the patient's pro-angiogenic status, which might reliably reflect or predict certain clinical outcomes. This paper targetly reviews the response of certain important vascular effectors to hypoxia and their roles in angiogenesis, trying to introduce a reliable surrogate marker that can reflect the pro-angiogenic status of a patient, based on experimental and clinical published studies.
Methods: The adopted approach is a mix of preclinical testing and narration of published examples of individual correlations of the marker components. As tumor-associated angiogenesis is the most prominently and clinically studied, this model constitutes the significant base for the notions of this work.
Results: Under hypoxic conditions, a decrease of tissue nostrin, along with increased production of nitric oxide synthase (eNOS), vascular endothelial growth factor (VEGF)-A, sVEGFR1 and endothelin-1 (ET-1) are to be expected.
Conclusion: Based on the experimentally proven changes in the selected angiogenesis-related effectors, as well as their experimentally and clinically proven roles and correlations in angiogenesis, the following conclusion can be stated; Low tissue nostrin, high circulating eNOS, high circulating VEGF-A, high circulating ET-1 can indicate a pro-angiogenic status, i.e. hypoxic injury.

Key words: Angiogenesis, ischemia, hypoxia, nitric oxide synthase, vascular endothelial growth factor, endothelin-1
Introduction
Angiogenesis is the formation of new blood vessels that involves organized migration, growth, and differentiation of endothelial cells. Various signals control this process, where some, such as vascular endothelial growth factor (VEGF) signaling, are promoters, while others are inhibitors (1). Under physiological conditions, the angiogenesis stimulating and inhibiting signals are balanced, so that new blood vessels form only when and where needed, for example during growth and healing. Sometimes, however, these signals may become unbalanced, which contributes to pathological conditions or diseases, such as angiogenesis in cancer and metastasis or angiogenesis in age-related wet macular degeneration (2).
The aim of this work is to provide an innovative hypothesis regarding a potential angiogenesis marker, along with an initial proof of it. The adopted approach is a mix of preclinical testing and narration of published examples of individual correlations of the marker components.
Methods
Study design: Experimental study
Experiment
The preclinical testing was achieved through experiments on cell lines, where hypoxia was chemically induced and the changes of the marker components (proteins of interest) were assessed using immunoblotting.
Cells used in this work are HEK293T and HuH7 cells. Cells were obtained from neighbor research groups. Cells were harvested, washed with PBS and three independent experimental repeats were conducted, where 200K cells were seeded in culture wells and incubated in 2 ml supplemented medium overnight (DMEM medium supplemented with: 10% BSA (Gibco), 1X sodium pyruvate, 1X penicillin – streptomycin (Gibco), 1X Glutamax-Supplement (Gibco) and 25mM HEPES). Cells were treated with CoCl2 (Sigma Aldrich) according to the following design:
• Control non treated cells
• Cells treated with 200uM CoCl2
• Cells treated with 300uM CoCl2
• Cells treated with 400uM CoCl2
Cells were incubated at 37Cо for additional 72 hours before harvesting and further processing. Collected samples were divided into cell lysates and media. The cell lysate samples were used to investigate the intracellular changes to various treatments, while the same proteins in media were considered secreted, as media were collected before cell lysis in RIPA buffer supplemented with proteases inhibitors. To ensure equal protein loading, prior estimation of samples' protein concentrations was conducted using the Bradford method. To test and document equal loading, certain internal controls were tested, to which the target protein bands were normalized. The effects of the treatments on hypoxia inducible factor-1 alpha (HIF1-α) and target proteins were then assessed using immunoblotting. Used primary antibodies were purchased from Proteintech Germany. Bands were analyzed with ImageJ software for detection of band density.
Statistical analysis
Unpaired T-test was used to compare the values of various experimental arms to the control. P values less than 0.05 were considered significant.
Evidence collection
The selected studies for the narration were reached through searching the Pubmed and Scopus Databases using the corresponding key words, namely; hypoxia, angiogenesis and the protein of interest.
Results
The basic observations
One of the most important and well-studied angiogenesis stimulators is VEGF (3), which has been a target for many angiogenesis inhibitors that have been approved for clinical application. Many proangiogenic factors (PFs) have been studied, which could be divided in two subgroups (4):
•Classical, for example: VEGF, fibroblast growth factor-2 (FGF-2), platelet derived growth factor (PDGF), platelet derived endothelial cell growth factor/thymidine phosphorylase (PD-ECGF/TP), angiopoietins (Ang), hepatocyte growth factor (HGF), insulin like growth factors (IGFs), tumor necrosis factor (TNF) and interleukin-6 (IL-6).
•Non-classical, such as stem cell factor (SCF), tryptase and chymase.
Hypoxia leads to induction of HIF1-α and significant reduction in tissue nostrin levels (5). Under hypoxic conditions, HIF-1α forms a dimer complex with HIF-1β through nuclear translocation. This complex binds the hypoxia response element, through interacting with coactivator p300, increasing the expression of VEGF, MMPs, angiopoietin, and PDGF (6). Low nostrin leads to enhanced activity of endothelial nitric-oxide synthase (eNOS) (7). Hypoxia leads to increased VEGF-A (8), which induces sVEGFR-1(sflt-1) (9) along with an accompanying enhanced endothelin-1 (ET-1) activity (10). Thus, under hypoxic conditions, a decrease of tissue nostrin, along with increased production of eNOS, VEGF-A, sVEGFR1 & ET-1 are to be expected.
Role of eNOS/VEGF axis in angiogenesis
During the conversion of l-arginine to l-citrulline eNOS works as a catalyst and nitric oxide (NO) is produced. NO is important for mediating the angiogenic activity of several factors, including VEGF, where eNOS activation is regulated in part by the upstream Akt/protein kinase B signaling. Meanwhile, the VEGF family includes seven known members, namely; VEGF-A, VEGF-B, VEGF-C, VEGF-D, placental growth factor (PlGF), non-human genome encoded VEGF-E and svVEGF (snake venom VEGF). VEGF-A supports the vascular endothelium and is an essential regulator of angiogenesis. It participates in tumor growth, proliferation, invasion, metastasis, angiogenesis, and drug resistance. VEGF-B promotes neuronal survival and cardiovascular growth through angiogenesis in specific organs, while the importance of VEGF-C and VEGF-D is seen in tumor growth and metastasis, being involved in VEGFR-3- mediated lymphangiogenesis and lymphatic metastasis (11).
Role of endothelin -1 axis in angiogenesis
The endothelin system includes 3 endothelin isoforms; ET-1, ET-2 and ET-3, which interact with 2 types of specific receptors; endothelin receptor-A (ETRA) and endothelin receptor-B (ETRB). ET-1 and ET-2 interact more potently with ETRA than ET-3 does, while the 3 isoforms can interact equipotently with ETRB. Mature ET-1 is the isoform expressed mainly in vascular endothelial cells and smooth muscles (12). ET-1 has a direct angiogenic effect on the endothelial and peri-vascular cells. It is important for cell growth and proliferation and exerts its actions through the mitogen-activated protein kinase (MAPK) pathway activation. ET-1 can increase VEGF expression and stimulate angiogenesis, through its endothelin A receptor (ETAR), integrin-linked kinase (ILK), Akt and HIF-1α signaling cascades (13).
The innovative marker (hypothesis):
Based on the published studies (5-10), concomitant changes in the levels of the above- mentioned angiogenesis – related effectors, in response to hypoxia, can be noticed, which enclose enhanced activities of both eNOS/VEGF and ET-1 axes (Fig. 1).
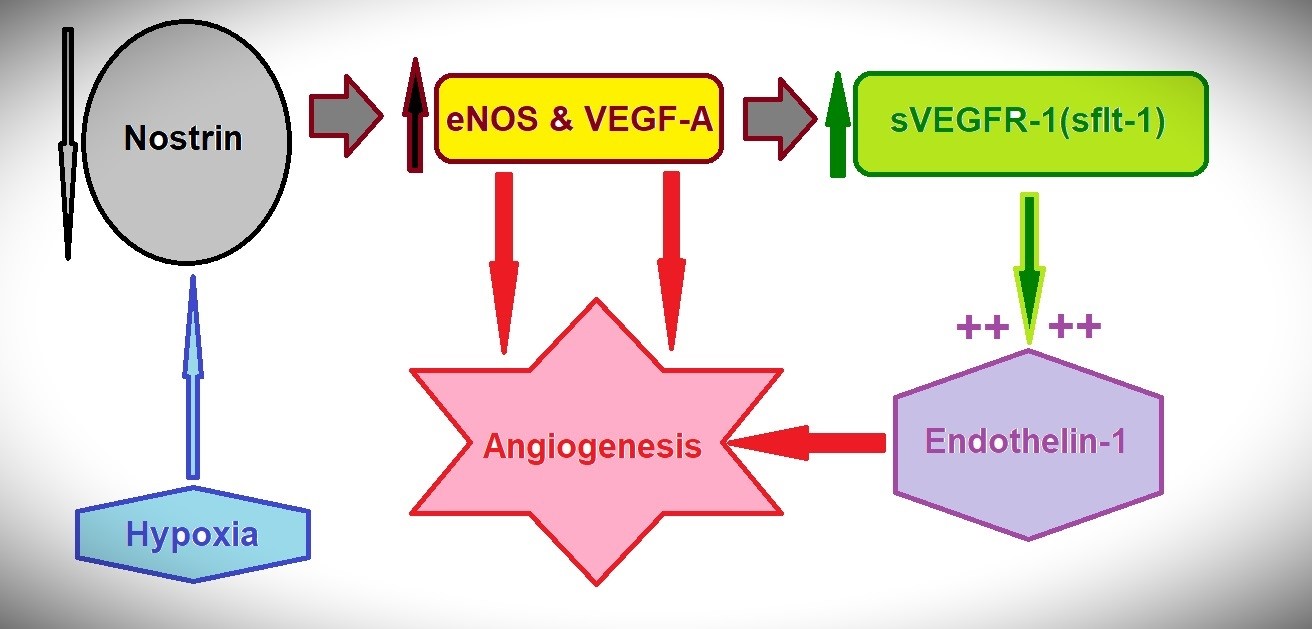
Figure 1. Hypoxia is associated with decreased nostrin, increased secretion of eNOS & VEGF-A, increased sVEGFR-1, which augments the actions of the concomitantly increased ET-1. All favor the angiogenesis
eNOS - endothelial nitric-oxide synthase, ET – endothelin, VEGF -vascular endothelial growth factor
Based on the experimentally proven changes in the angiogenesis-related effectors displayed in Figure 1, as well as the experimentally and clinically proven roles and correlations in angiogenesis, the following conclusion can be stated;
(a)Low tissue nostrin, high circulating eNOS, high circulating VEGF-A, high circulating ET-1 can indicate a pro-angiogenic status, i.e. hypoxic injury
(b)Vice versa, high tissue nostrin, low circulating eNOS, low circulating VEGF-A, low circulating ET-1 may indicate a poor pro-angiogenic status.
Evidence through relevant published studies
As relative ischemia and hypoxia usually accompany the rapid growth rates of neoplasms, thus always present in the tumor microenvironment, tumor-associated angiogenesis has gained great interest in the last decades, where the individual components of the proposed marker have been extensively studied and anti-angiogeneic agents are often integrated as adjuvant therapeutic regimes (14).
Table 1 shows some examples of the published evidence of the individual correlations of the marker components with tumor-associated angiogenesis.
|
Table 1. Examples of the published evidence of the individual correlations of the marker components with tumor-associated angiogenesis |
|||
|
Study |
Marker/Findings |
Year |
Origin |
|
Nostrin |
|||
|
Wang et al. (15) |
A study included 50 samples from pancreatic cancer patients, concluded that nostrin is a negative regulator of disease aggressiveness in pancreatic cancer. Additional cell line experimental work revealed that the over-expression of nostrin suppressed migration and invasion of pancreatic cancer cells and increased sensitivity to the chemotherapy drug Gemcitabine. |
2017 |
USA |
|
Paul et al. (16) |
Nostrin is a novel negative regulator of colon cancer progression in cell line experiments, which were confirmed clinically. Patient samples (n=144), where nostrin expression was assessed by PCR. The onset of colorectal cancer (CRC) is associated with significantly (p<0.05) reduced nostrin transcripts compared to control samples. Nostrin transcripts decreased as disease stages progressed, reaching their minimum level in stage IIB (p<0.001), after which they plateaued in stages IIIB, IIIC and IV. |
2022 |
India |
|
|
Endothelin-1 (ET-1) |
|
|
|
Asham et al. (17) |
Increased ET -1 production in CRC: ET-1 plasma levels in patients with CRC were measured by radioimmunoassay: Group 1 = controls (n = 22), Group 2 = primary CRC only (n = 39), Group 3 = liver metastases only (n = 26). ET-1 plasma levels were significantly higher in groups 2 and 3, compared to group 1 (p<0.01, 3.9 (1.4), 4.5 (1.5), vs. 2.75 (1.37) pg/ml, respectively). Increased ET-1 expression in primary CRC samples (n = 10). In a rat model, after treatment with the ET(A) antagonist (BQ123), there was a significant reduction in liver tumor weight compared to controls, 30 minutes after intraportal inoculation of tumor cells (p<0.05). |
2001 |
UK |
|
Hoosein et al. (18) |
Altered ET receptor subtypes in CRC: Frozen sections from colon cancer (n=9) and normal colon (n=9) samples. ETRA is upregulated in all cell types except nerves in cancer compared to normal colon (1:1.59 normal vs cancer). ETRB are the predominant receptors in the normal colon (1:0.59 normal vs CRC) and were significantly downregulated in cancer-associated blood vessels, fibroblasts and, to a lesser extent, epithelial cells. |
2007 |
UK |
|
Cianfrocca et al. (19) |
Blocking ET-1 receptors by macitentan (a dual ETAR and ETBR antagonist) sensitizes to chemotherapy in CRC (Preclinical mouse study). |
2017 |
Italy |
|
Kappes et al. (20) |
Ambrisentan, an ET receptor type A-selective antagonist, inhibits both spontaneous and induced cancer cell migration, invasion, and metastasis capacity of different tumor cells (COLO-357 metastatic pancreatic adenocarcinoma, OvCar3 ovarian carcinoma, MDA-MB-231 breast adenocarcinoma, and HL-60 promyelocytic leukemia) |
2020 |
Germany |
|
|
VEGF and eNOS |
|
|
|
Miyazaki et al. (21) |
Plasma levels of VEGF-C in patients with CRC (n = 127) and normal healthy volunteers (n = 23) were determined by ELISA. Plasma VEGF-C levels were higher in patients who experienced recurrence (n = 35) than in patients who did not experience recurrence (n = 74) (p=0.04). Disease-free (p=0.02) and overall survival times (p =0.02) were shorter in patients with high plasma VEGF-C levels (>1840 pg/ml) than in patients with low plasma VEGF-C levels. Multivariate analysis using Cox proportional hazard model showed that plasma VEGF-C level, along with Dukes stage, was an independent factor affecting overall survival (p=0.03). |
2008 |
Japan |
|
Continued from page… |
||||
|
Study |
Marker/Findings |
Year |
Origin |
|
|
VEGF and eNOS |
||||
|
Alabi et al. (22) |
Serum VEGF-A levels were determined by ELISA in 93 CRC patients preoperatively: Nodal negative (n = 41) and nodal positive (n = 52). Patients with local recurrence had significantly higher serum VEGF-A levels (p=0.01). Patients with preoperative serum VEGF-A levels > 575 pg/mL are more likely to have recurrence. |
2009 |
UK |
|
|
Chin et al. (23) |
Preoperative serum VEGF level can select patients for adjuvant treatment after curative resection for CRC. Serum VEGF was examined by quantitative ELISA in 81 patients before curative resection for nodal negative (n = 53) and nodal positive (n = 28) disease. Preoperative serum VEGF was significantly higher in patients, who later developed metastases than in patients, who did not develop metastases. Using multivariate Cox regression analysis, preoperative serum VEGF was the most important prognostic factor, independent of nodal status and adjuvant chemotherapy, and was superior to nodal status in predicting the clinical outcome (p<0.0001). At 575 pg ml-1 preoperative serum VEGF was 64% sensitive and 89% specific in predicting the development of metastases after curative resections, with a positive predictive value of 73% and a negative predictive value of 85%. |
2000 |
UK |
|
|
Marisi et al. (24) |
Circulating VEGF and eNOS variations as predictors of the clinical outcome in metastatic CRC patients receiving bevacizumab. Total participants: 129 patients Arm 1: Receiving FOLFOX4/FOLFIRI (chemotherapy) with bevacizumab (64 patients). Arm 2: Receiving FOLFOX4/FOLFIRI (chemotherapy) without bevacizumab (65 patients). Bevacizumab-treated patients with greater than 30% reduction in eNOS and VEGF levels from baseline to first clinical assessment demonstrated better overall survival. |
2017 |
Italy |
|
|
George et al. (25) |
Clinical study involving 70 CRC samples and 20 adenomatous polyp samples. VEGF-D mRNA expression was significantly lower in both polyps and CRC samples compared to normal mucosa (p=0.0002 and 0.002, respectively). VEGF-A and VEGF-C are significantly increased in CRC samples (p=0.006 and 0.004, respectively), but not in polyp samples (p=0.22 and p=0.5, respectively). Receptor expression was similar in tumor tissue and normal mucous membranes. Tumors with lymph node metastasis had significantly higher VEGF-A levels compared to non-metastatic tumors (p=0.043). No connection was observed between VEGF-C or VEGF-D and lymphatic spread. |
2001 |
UK |
|
|
Hanrahan et al. (26) |
Samples: normal colorectal tissue (n = 20), adenomas (n = 10) and CRC (n = 71), representing different Duke stages. VEGF-A is the most abundant in colorectal tissue, followed by VEGF-B, VEGF-C and VEGF-D. VEGF-A and VEGF-B are significantly more common in adenomas (p=0.0003 and p=0.04, respectively) than in normal tissue. VEGF-A and VEGF-C were significantly increased in carcinomas compared to normal tissue (p=0.0006 and p=0.0009, respectively). |
2003 |
New Zealand |
|
|
CRC – colorectal cancer, eNOS - endothelial nitric-oxide synthase, ET – endothelin, ETRA - endothelin receptor-A, ETRB - endothelin receptor-B, VEGF -vascular endothelial growth factor |
||||
Evidence through own experiments
Nevertheless, when the chemical induction of HIF-1α under cobalt chloride (CoCl2) treatment was used to evaluate the corresponding effects on nostrin, eNOS, VEGF-A and ET-1 in HEK293T (kidney) and HuH7 (liver) cells (author's original work), the results confirmed the innovative potential, where the chemical induction of hypoxia resulted in statistically significant reduction of nostrin, along with statistically significant increase in secreted eNOS, VEGF-A and ET-1 (Fig. 2).
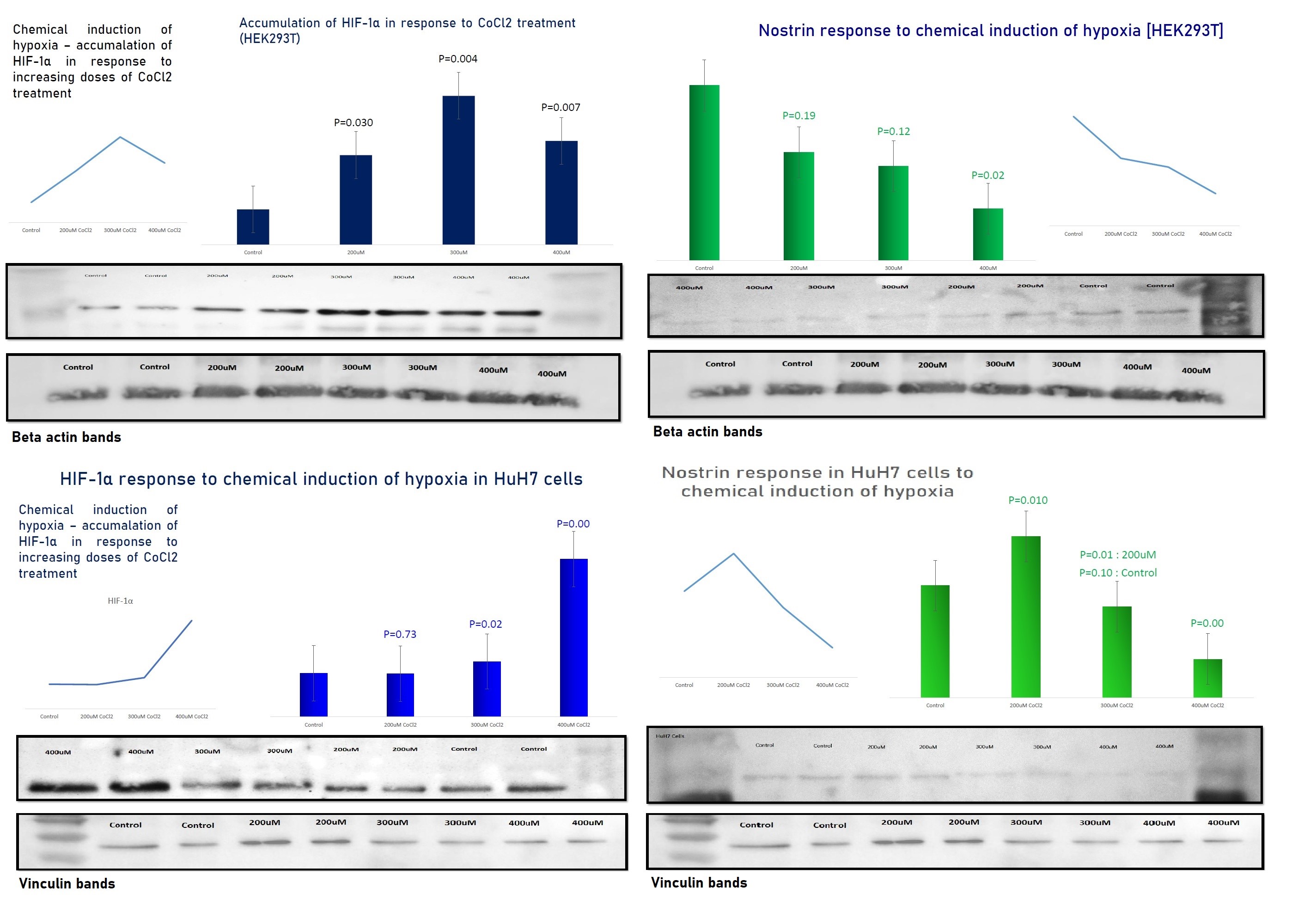
A)
B)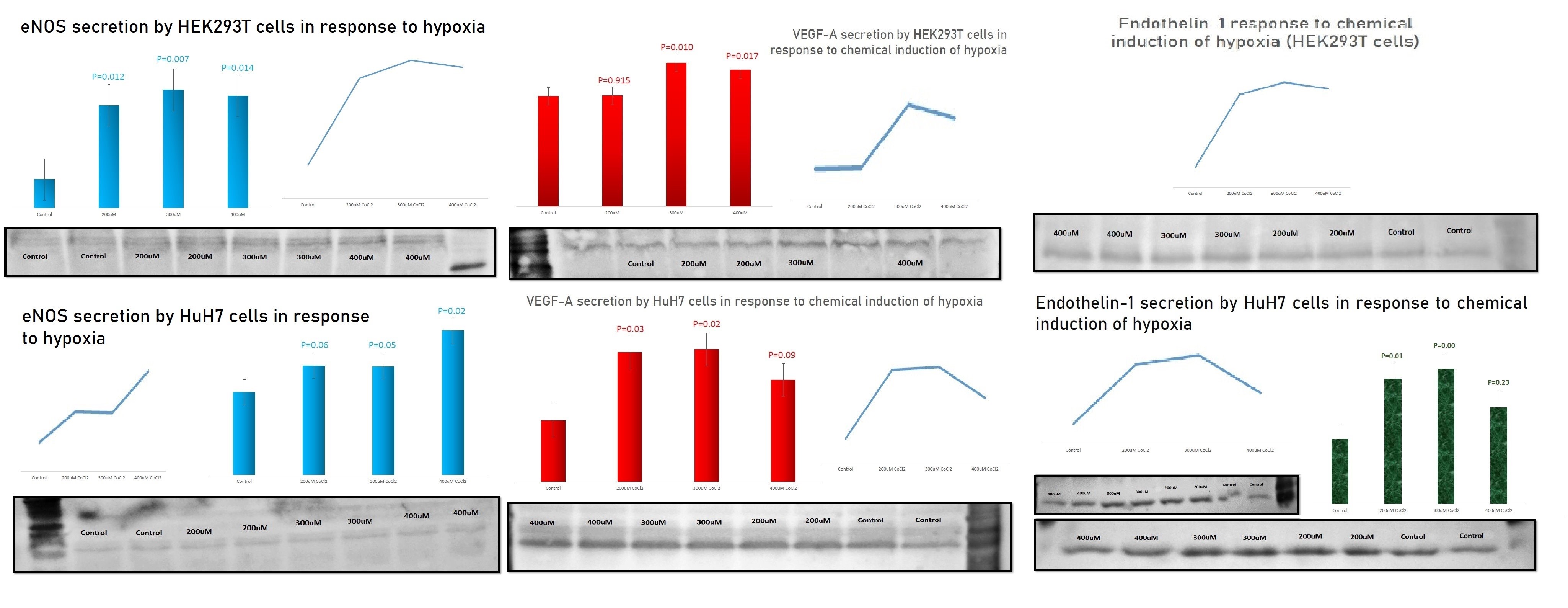
Figure 2 (A and B); Effect of hypoxia on the target proteins in HEK293T and HuH7 cells. With almost equal loading, β-actin or vinculin as loading controls showed no significant difference among bands.
-HIF-1α was induced in response to CoCl2 treatments compared to control non-treated cells.
-Nostrin decreased in response to hypoxia induction in both cell lines
-The production of VEGF-A, eNOS and ET-1 increased compared to the corresponding control non-treated cells
The results are the outcome of 3 independent biological repeats.
Although other techniques, such as ELISA, may have been more sensitive to detect the corresponding significant changes, being usually delicate and measured in picograms, immunoblotting was still able to detect significant changes, denoting strong reliability of the results. In addition, other in-vivo preclinical and clinical studies reported similar findings, where intermittent hypoxia led to enhanced ET-1 expression in animals (27) and chronic intermittent hypoxia led to increased circulating ET-1 levels in patients of sleep apnea (28, 29). Moreover, elevated circulating ET-1 has been associated with vascular dysfunction in transplant patients (30).
Discussion
Angiogenesis is not just a vital task for existence and survival of any tissue, but also a procedure that participates in many vital processes within the human body, starting from wound healing up to the determination of the clinical outcomes of the ischemic and neoplastic conditions. Hence, it would be very important to introduce a reliable marker that can reflect the pro-angiogenic status of a tissue or even an individual. The clinical interpretation of such a marker can be very useful. For example; it may contribute to early detection of neoplasm, which is a major cause of post-transplant morbidity and mortality with 3 times higher incidence risk due to immunosuppression (31), and or graft dysfunction. Thus, clinical studies are essential to determine the cut-off levels and the degree of changes accompanying various clinical conditions.
In addition, this marker can be very efficient in monitoring the therapeutic response in angiogenesis-related diseases, especially with adjuvant therapy and organ transplantation. The persistence of the high pro-angiogenic marker pattern after surgery would mean persistent hypoxic injury and or pro-angiogenic status, which may indicate remnant primary tumor and or metastasis in oncological conditions, or an in-vivo perfusion dysfunction in solid organ transplantation. On the other side, the subside of a pre-interventional high marker pattern to normal would indicate a good response to the therapeutic interventions.
Recently, hypoxia and angiogenesis have been accused for playing important roles in the initiation of inflammation (32), hence graft rejection reactions (33). Indeed, the selected marker components are heavily involved in many steps of inflammation. For example, eNOS and VEGF-A are involved in increased vascular permeability, facilitating leukocytic migration and infiltration, though VEGF itself has immune modulatory activities (32, 33). Likewise, nostrin can suppress NFκB signaling, thus its reduction affects the production of many inflammatory cytokines, including interleukin-6 (IL-6) (34). In addition, ET-1 is also known to have proinflammatory activities (35). Hence, the proposed marker can be extraordinary important for the clinical practice of organ transplantation.
Relation to transplantation
In the field of solid organ transplantation, neovascularization is desired for graft survival. As a part of pretransplant ischemia, some degree of graft hypoxia would result in the above presented pro-angiogenic pattern of changes. Increased VEGF affects the innate and adaptive immune mechanisms through its interaction with the immune and endothelial cells, modulating the vascular permeability and endothelial protein expression (36). Among the immune modulatory activities of VEGF are:
-The polarization of macrophages into an M2 phenotype, which is involved in immunosuppressive activities, in contrast to the M1 phenotype, which is mainly proinflammatory (36, 37);
-Decrease the maturation of the dentritic cells (DC) (36);
-Upregulation of checkpoint inhibitors on DC and CD8+ T cells (36);
-Attenuation of the cytotoxicity of the natural killer (NK) cells through VEGF-C/VEGFR-3 signaling (36);
-Inhibition of the proliferation, cytotoxicity, and recruitment of CD3+ T cells (36).
Contradicting the above immunemodulatory effects is not wanted in solid organ transplantation, where immunosuppression is a clinical target to minimize rejection reactions. Thus, a pretransplant limited degree of hypoxia may be beneficial. On the other side, there is the ET-1 axis, which expresses proinflammatory activities, including functioning as a chemokine that promotes chemotaxis and concomitantly increases vascular permeability. In addition, ET-1 increases the monocytes' release of TNF-α, IL-1 and IL-6, whose roles in inflammation and transplant clinical outcomes are well studied (36, 38, 39). Thus, the clinical application of Shehata marker of angiogenesis may aid in the prediction of, not only the pro-angiogenic potential essential for graft survival, but also the possible direction of the immune response. In other words, the personalized and individual reaction to hypoxia may differ from person to person, i. e. from graft to graft. The use of the proposed marker may allow the prior detection of any imbalance between the involved axes; VEGF axis and ET-1 axis. Thus, the proper interventions can be applied.
Study limitations
This article represents the initial introduction of a potential innovative marker that can reflect the hypoxic injury and the proangiogenic status. As a perspective article, the introduced notion was supported with published evidence and experimental results. The reported evidence is based on individual correlations and or involvements of each marker component. There are no published studies that involve the combined marker components in relation to hypoxia or angiogenesis, hence the presented notion is innovative. However, systemic reviews to discuss each component by itself may provide stronger evidence, but cannot be enclosed in one article. Likewise, the presented experimental results were limited to commercial cell lines. In-vivo results on the preclinical and clinical levels are essential for more reliable confirmations.
Conclusion
To the limit of this perspective article, the notion of combining the above-mentioned effectors into a single marker that can reflect the hypoxic response and the pro-angiogenic profile of a patient or a tissue (organ) has been introduced for the first time. The individual roles played by each component of the marker can be reviewed and discussed separately, both on the vascular functional, hypoxic and angiogenic levels, as well as on the inflammatory and immunological levels. Gaining more of this knowledge confirms the enormous relativity of those proteins to the clinical practice in the field of organ transplantation. Future studies are intended to confirm the above-discussed principles, reporting the preclinical reliability and clinical utilities of the proposed marker.
Ethics: There were no animal or patients experiments conducted. Thus, ethical agreements do not apply.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: M.A.S.
Author is the sole author of this work. He created and conducted the work from scratch, including making all substantial contributions to the conception, searching the literature, development of the target questions, development of the hypothesis, designing the experimental work, conduction of the experiments, interpretation of the results, performing the statistical analyses, drafting the manuscript, visualization of the results, final revision and submission.
Intellectual rights:
All the innovative notions and statements included in this work or being based on them belong intellectually solely to the author and are his own property.
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use: No AI tools were used in the preparation of this work.
Availability of data and material: There are no new patients' data associated with this work. All provided information are based on experimental results, either published or author's own work on commercial cell lines
References
| 1.Larionova I, Kazakova E, Gerashchenko T, Kzhyshkowska J. New angiogenic regulators produced by TAMs: perspective for targeting tumor angiogenesis. Cancers (Basel) 2021; 13: 3253. https://doi.org/10.3390/cancers13133253 PMid:34209679 PMCid:PMC8268686 |
||||
| 2.Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci 2020; 77: 1745-70. https://doi.org/10.1007/s00018-019-03351-7 PMid:31690961 PMCid:PMC7190605 |
||||
| 3.Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2011; 2: 1097-105. https://doi.org/10.1177/1947601911423031 PMid:22866201 PMCid:PMC3411125 |
||||
| 4.Marech I, Leporini C, Ammendola M, Porcelli M, Gadaleta CD, Russo E, et al. Classical and non-classical proangiogenic factors as a target of antiangiogenic therapy in tumor microenvironment. Cancer Lett 2016; 380: 216-26. https://doi.org/10.1016/j.canlet.2015.07.028 PMid:26238184 |
||||
| 5.Wade BE, Zhao J, Ma J, Hart CM, Sutliff RL. Hypoxia-induced alterations in the lung ubiquitin proteasome system during pulmonary hypertension pathogenesis. Pulm Circ 2018; 8: 2045894018788267. https://doi.org/10.1177/2045894018788267 PMid:29927354 PMCid:PMC6146334 |
||||
| 6.Ikeda H, Kakeya H. Targeting hypoxia-inducible factor 1 (HIF-1) signaling with natural products toward cancer chemotherapy. J Antibiot 2021; 74: 687-95. https://doi.org/10.1038/s41429-021-00451-0 PMid:34331027 |
||||
| 7.Kovacevic I, Müller M, Kojonazarov B, Ehrke A, Randriamboavonjy V, Kohlstedt K, et al The F-BAR protein NOSTRIN dictates the localization of the muscarinic m3 receptor and regulates cardiovascular function. Circ Res 2015; 117: 460-9. https://doi.org/10.1161/CIRCRESAHA.115.306187 PMid:26169369 |
||||
| 8.Manukjan N, Majcher D, Leenders P, Caiment F, van Herwijnen M, Smeets HJ, et al. Hypoxic oligodendrocyte precursor cell-derived VEGFA is associated with blood-brain barrier impairment. Acta Neuropathol Commun 2023; 11: 128. https://doi.org/10.1186/s40478-023-01627-5 PMid:37550790 PMCid:PMC10405482 |
||||
| 9.Saito T, Takeda N, Amiya E, Nakao T, Abe H, Semba H, et al. VEGF-A induces its negative regulator, soluble form of VEGFR-1, by modulating its alternative splicing. FEBS Lett 2013; 587: 2179-85. https://doi.org/10.1016/j.febslet.2013.05.038 PMid:23711375 |
||||
| 10.Amraoui F, Spijkers L, Hassani Lahsinoui H, Vogt L, van der Post J, Peters S, et al. SFlt-1 elevates blood pressure by augmenting endothelin-1-mediated vasoconstriction in mice. PLoS One 2014; 9: e91897. https://doi.org/10.1371/journal.pone.0091897 PMid:24632840 PMCid:PMC3954828 |
||||
| 11.Liu ZL, Chen HH, Zheng LL, Sun LP, Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther 2023; 8: 198. https://doi.org/10.1038/s41392-023-01460-1 PMid:37169756 PMCid:PMC10175505 |
||||
| 12.Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, et al. Endothelin. Pharmacol Rev 2016; 68: 357-418. https://doi.org/10.1124/pr.115.011833 PMid:26956245 PMCid:PMC4815360 |
||||
| 13.Wu MH, Huang CY, Lin JA, Peng C-Y, Cheng H-C, Tang C-H. Endothelin-1 promotes vascular endothelial growth factor-dependent angiogenesis in human chondrosarcoma cells. Oncogene 2014; 33, 1725-35. https://doi.org/10.1038/onc.2013.109 PMid:23584483 |
||||
| 14.Xu M, Fan R, Fan X, Shao Y, Li X. Progress and challenges of anti-VEGF agents and their sustained-release strategies for retinal angiogenesis. Drug Des Devel Ther 2022; 16: 3241-62. https://doi.org/10.2147/DDDT.S383101 PMid:36172053 PMCid:PMC9512290 |
||||
| 15. Wang J, Yang S, He P, Schetter A, Gaedcke J, Ghadimim M, et al. Endothelial nitric oxide synthase traffic inducer (NOSTRIN) is a negative regulator of disease aggressiveness in pancreatic cancer. Clin Cancer Res 2016; 22: 5992-6001. https://doi.org/10.1158/1078-0432.CCR-16-0511 PMid:27401251 PMCid:PMC5161709 |
||||
| 16. Paul M, Kanti Gope T, Das P, Ain R. Nitric oxide ynthase traffic inducer (NOSTRIN) is an emerging negative regulator of colon cancer progression. BMC Cancer 2022; 22: 594. https://doi.org/10.1186/s12885-022-09670-6 PMid:35642021 PMCid:PMC9158178 |
||||
| 17. Asham E, Shankar A, Loizidou M, Frederiks S, Miller K, Boulos B, et al. Increased endothelin -1 in colorectal cancer and reduction of tumor growth by ET(A) receptor antagonism. Br J Cancer 2001; 85:1759-63. https://doi.org/10.1054/bjoc.2001.2193 PMid:11742499 PMCid:PMC2363991 |
||||
| 18. Hoosein MM, Dashwood MR, Dawas K, Ali HMMDA, Grant K, Savage F, et al. Altered endothelin receptor subtypes in colorectal cancer. Eur J Gastroenterol Hepatol 2007; 19: 775-82. https://doi.org/10.1097/MEG.0b013e3282c563de PMid:17700263 |
||||
| 19. Cianfrocca R, Rosana L, Tocci P, Sestito R, Carpara V, et al. Blocking endothelin -1-receptor/b-catenin circuit sensitizes to chemotherapy in colorectal cancer. Cell Death Differ 2017; 24:1811-20. https://doi.org/10.1038/cdd.2017.121 PMid:28708138 PMCid:PMC5596423 |
||||
| 20. Kappes L, Amer RL, Sommeriatte S, Bashir G, Plattfaut C, Gieseler F, et al. Ambrisentan, and endothelin receptor type A-selective antagonist, inhibits cancer cell migration, invasion and metastasis. Sci Rep 2020; 10: 15931. https://doi.org/10.1038/s41598-020-72960-1 PMid:32985601 PMCid:PMC7522204 |
||||
| 21. Miyazaki T, Okada N, Ishibashi K, Ogata K, Ohsawa T, Ishiguro T, et al. Clinical significance of plasma level of vascular endothelial growth factor - C in patienst with colorectal cancer. Jpn J Clin Oncol 2008; 38: 839-43. https://doi.org/10.1093/jjco/hyn106 PMid:18923001 |
||||
| 22. Alabi AA, Suppiah A, Madden LA, Monson JR, Greenman J. Preoperative serum vascular endothelial growth factor -a is a marker for subsequent recurrence in colorectal cancer patients. Dis Colon Rectum 2009; 52: 993-9. https://doi.org/10.1007/DCR.0b013e31819ed3bc PMid:19502868 |
||||
| 23. Chin KF, Greenman J, Gardiner E, Kumar H, Topping K, Monson J, et al. Br J Cancer 2000; 83: 1425-31. https://doi.org/10.1054/bjoc.2000.1508 PMid:11076648 PMCid:PMC2363412 |
||||
| 24. Marisi G, Scarpi E, Passardi A, Nanni O, Ragazzini A, Valgiusti M, et al. Circulating VEGF and eNOS variatiosn as predictors of outcome in metastatic colorectal cancerpatients receiving bevacizumab. Sci Rep 2017; 7: 1293. https://doi.org/10.1038/s41598-017-01420-0 PMid:28465540 PMCid:PMC5431064 |
||||
| 25. George ML, Tutton MG, Janssen F, Arnaout A, Abulafi AM, Eccles SA, et al. VEGF-A, VEGF-C and VEGF-D in colorectal cancer progression. Neoplasia 2001; 3 : 420-7. https://doi.org/10.1038/sj.neo.7900186 PMid:11687953 PMCid:PMC1506210 |
||||
| 26. Hanrahan V, Currie M, Gunnigham SP, Morrin HR, Scott PAE, Robinson BA, et al. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF -B, VEGF-C and VEGF - D in the adenocarcinoma sequence during colorectal cancer progression. J Pathol 2003; 200: 183-94. https://doi.org/10.1002/path.1339 PMid:12754739 |
||||
| 27.Belaidi E, Morand J, Gras E, Pépin J-L, Godin-Ribuot D. Targeting the ROS-HIF-1-endothelin axis as a therapeutic approach for the treatment of obstructive sleep apnea-related cardiovascular complications. Pharmacol Ther 2016; 168: 1-11. https://doi.org/10.1016/j.pharmthera.2016.07.010 PMid:27492897 PMCid:PMC5643507 |
||||
| 28. Kosacka M, Brzecka A. Endothelin-1 and LOX-1 as markers of endothelial dysfunction in obstructive sleep apnea patients. Int J Environ Res Public Health 2021; 18: 1319. https://doi.org/10.3390/ijerph18031319 PMid:33535693 PMCid:PMC7908073 |
||||
| 29. Schoen T, Aeschbacher S, Leuppi JD, Miedinger D, Werthmüller U, Estis J, et al. Subclinical sleep apnoea and plasma levels of endothelin-1 among young and healthy adults. Open Heart 2017; 4: e000523. https://doi.org/10.1136/openhrt-2016-000523 PMid:28409007 PMCid:PMC5384465 |
||||
| 30.Radeau T, Lebel M, Houde I, Larivière R, Mauriège P, Kingma I, et al. Endothelin-1 levels and cardiovascular risk factors in renal transplant patients. Clin Biochem 2004; 37: 1072-8. https://doi.org/10.1016/j.clinbiochem.2004.08.001 PMid:15589812 |
||||
| 31. Ietto G, Gritti M, Pettinato G, Carcano G, Gasperina DD. Tumors after kidney transplantation: a population study. World J Surg Oncol 2023; 21: 18. https://doi.org/10.1186/s12957-023-02892-3 PMid:36691019 PMCid:PMC9869548 |
||||
| 32. Aguilar-Cazares D, Chavez-Dominguez R, Carlos-Reyes A, Lopez-Camarillo C, Hernadez de la Cruz ON, Lopez-Gonzalez JS. Contribution of angiogenesis to inflammation and cancer. Front Oncol 2019; 9: 1399. https://doi.org/10.3389/fonc.2019.01399 PMid:31921656 PMCid:PMC6920210 |
||||
| 33. Riesner K, Shi Y, Jacobi A, Kräter M, Kalupa M, McGearey A, et al. Initiation of acute graft-versus-host disease by angiogenesis. Blood 2017; 129: 2021-32. https://doi.org/10.1182/blood-2016-08-736314 PMid:28096092 |
||||
| 34. Chakraborty S, Ain R. Nitric-oxide synthase trafficking inducer is a pleiotropic regulator of endothelial cell function and signaling. J Biol Chem 2017; 292: 6600-20. https://doi.org/10.1074/jbc.M116.742627 PMid:28235804 PMCid:PMC5399110 |
||||
| 35.Banecki KMRM, Dora KA. Endothelin-1 in health and disease. Int J Mol Sci 2023; 24: 11295. https://doi.org/10.3390/ijms241411295 PMid:37511055 PMCid:PMC10379484 |
||||
| 36.Geindreau M, Ghiringhelli F, Bruchard M. Vascular endothelial growth factor, a key modulator of the anti-tumor immune response. Int J Mol Sci 2021; 22: 4871. https://doi.org/10.3390/ijms22094871 PMid:34064508 PMCid:PMC8124522 |
||||
| 37.Strizova Z, Benesova I, Bartolini R, Novysedlak R, Cecrdlova E, Foley LK, et al. M1/M2 macrophages and their overlaps - myth or reality? Clin Sci (Lond) 2023; 137: 1067-93. https://doi.org/10.1042/CS20220531 PMid:37530555 PMCid:PMC10407193 |
||||
| 38.Shen H, Goldstein DR. IL-6 and TNF-alpha synergistically inhibit allograft acceptance. J Am Soc Nephrol 2009; 20: 1032-40. https://doi.org/10.1681/ASN.2008070778 PMid:19357252 PMCid:PMC2678042 |
||||
| 39.Mohamed MS. Translational insights on lung transplantation: learning from immunology. Iran J Immunol 2015; 12: 156-65. | ||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

