Risk assessment of adverse drug reactions in elderly patients with cardiovascular diseases: Study on diagnostic accuracy
ORIGINAL RESEARCH ARTICLE
Risk assessment of adverse drug reactions in elderly patients with cardiovascular diseases: Study on diagnostic accuracy
Article Summary
- DOI: 10.24969/hvt.2025.547
- CARDIOVASCULAR DISEASES
- Published: 25/02/2025
- Received: 23/12/2024
- Revised: 23/01/2025
- Accepted: 24/02/2025
- Views: 4889
- Downloads: 2321
- Keywords: Adverse drug reactions, polypharmacy, cardiovascular drugs, older adults, GerontoNet scale, diagnostic accuracy, ROC analysis
Address for Correspondence: Ainura T. Sharaeva, Department of Basic and Clinical Pharmacology, Kyrgyz-Russian Slavic University named after the First President of the Russian Federation B.N. Yeltsin, Bishkek, Kyrgyzstan
Email: sharaevaainur@yandex.com
ORCID: Ainura T. Sharaeva - 0000-0003-0955-095X; Aida A. Zurdinova - 0000-0002-7093-981X,
Aida T. Satybaldieva - 0009-0000-5487-5919
Facebook: Ainura T. Sharaeva - ruslan.alimov.5621
Ainura T. Sharaeva, Aida A. Zurdinova, Aida T. Satybaldieva
Department of Basic and Clinical Pharmacology, Kyrgyz-Russian Slavic University named after the First President of the Russian Federation B.N. Yeltsin, Bishkek, Kyrgyzstan
Abstract.
Objective: The aim of the study was to assess prognostic value and diagnostic accuracy of the universal and adapted GerontoNet ADR risk score in prediction of risk of adverse drugs reactions (ADRs) among hospitalized older adult (≥65 years old) patients with cardiovascular (CV) diseases.
Methods: The study design - retrospective observational study on diagnostic accuracy. Medical records of 113 patients over 65 years old with CV diseases were analyzed. Demographic, history, comorbidities, diaries, clinical, laboratory, and instrumental data, prescription sheets were retrieved. To evaluate the risk of ADRs, prescription sheets from the medical histories were analyzed using the "GerontoNet ADR Risk" scale, specialized and validated risk stratification scale "GerontoNet ADR Risk." We used ROC analysis for assessment of diagnostic accuracy of GERONTONET ADR score and calculated sensitivity, specificity, positive and negative predictive values.
Results: History of adverse drug reactions ADRs was noted in 2 (1.76%) patients. During hospitalization, ADRs were identified in 26 patients (23%). An increase in transaminase levels above 2 upper limits during treatment was observed in 7 patients (6.19%), confusion, speech slowness, coordination problems, and drowsiness were noted in 9 patients (7.96%), bradycardia occurred in 5 (4.42%), and electrolyte disturbances in 5 (4.42%), and renal dysfunction was noted in 3 (2.6%). GerontoNet ADR score had a significant prognostic value in identification of adverse reactions development (OR- 10.41, 95% CI 3.53-30.75, p < 0.001) and strong diagnostic accuracy (AUC 0 0.88, 95% CI, 0.83-0.89) based on the ROC curve. Sensitivity and specificity analyses of the GerontoNet scale for predicting the risk of ADRs in our study showed that the sensitivity of the scale was 80.77% and the specificity was 71.26%, positive predictive value (PV) of this - 45.65% and negative PV - 92.5% of cases. The accuracy of the scale was 73.45%.
Conclusion: The study concluded that the GerontoNet scale demonstrates good sensitivity and specificity and can be used as a tool for identifying patients at high risk of ADRs, allowing timely therapy adjustments to optimize pharmacotherapy and reduce the severity of these reactions. Therefore, broader application of the GerontoNet ADR Risk scale is advisable for older adult patients with CV diseases.
Key words: Adverse drug reactions, polypharmacy, cardiovascular drugs, older adults, GerontoNet scale, diagnostic accuracy, ROC analysis
Graphical abstract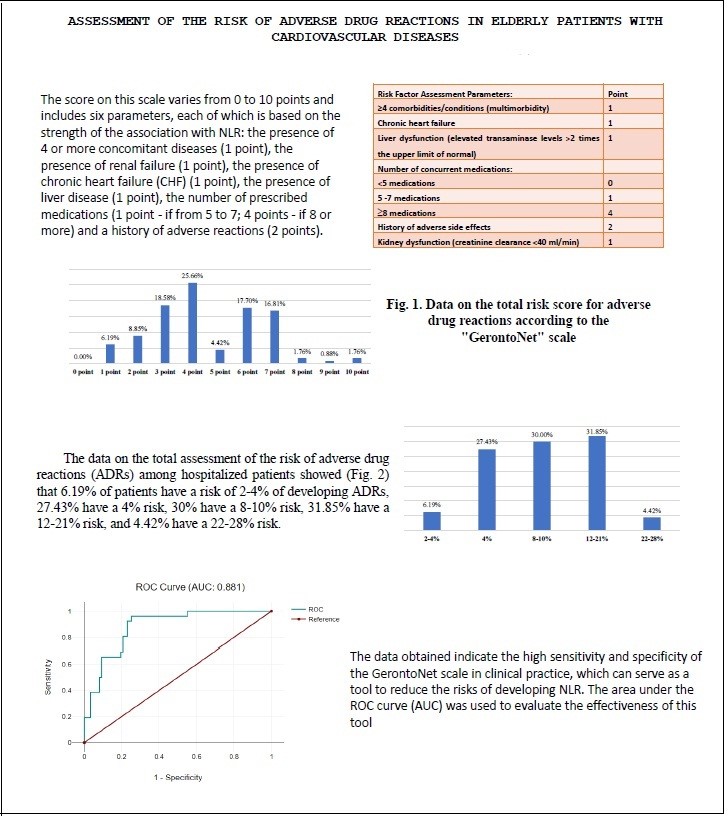
![]()
Introduction
Aging leads to an increase in the prevalence of chronic diseases, including cardiovascular (CV) diseases and the number of medications taken (1). Polypharmacy is commonly observed in elderly hospitalized patients and may lead to the use of potentially inappropriate drugs for older adults, resulting in an increased number of potential drug interactions and adverse drug reactions (ADRs) (2). These factors can reduce the safety of drug use, jeopardize the functionality of elderly individuals, and affect the effectiveness of pharmacotherapy (3). The issue of drug safety is becoming increasingly acute both in our country and globally. This is due to the introduction of new drugs with high biological activity into clinical practice, irrational drug prescribing, polypharmacy, medical errors, and the use of poor-quality and counterfeit drugs. According to the World Health Organization (WHO), ADRs are among the top ten causes of death in patients (4).
The use of any medication can be accompanied by the development of ADRs, and the risk of their occurrence increases when multiple drugs are used. This aspect is related to the concept of polypharmacy, which refers to the simultaneous, usually unjustified, prescription of a large number of medications (5).
Older adults often experience multimorbidity and associated polypharmacy, as well as various physiological changes affecting the pharmacokinetics and pharmacodynamics of drugs, which increase risk of hospitalizations related to ADRs (6, 7). Two systematic reviews suggested that the median frequency of ADR-related hospitalizations is 10% and 11%, respectively, among individuals aged ≥65 years (8, 9).
A meta-analysis of observational studies measuring hospitalizations due to ADRs showed that one in ten hospitalizations of older adult patients was caused by ADRs (10). However, systematic reviews and meta-analyses have shown a wide range of ADR prevalence from 5% to 50%, with heterogeneity in the definition and identification of ADRs considered the main reason for much of this variability (9).
ADRs are difficult to identify in elderly individuals, and hospital reporting systems significantly underreport their occurrence, leading to unreliable estimates of hospitalizations in the elderly due to ADRs (11, 12). In a prospective review, 15% of hospitalizations in elderly individuals were classified as ADR-related (13). Accurate identification of ADRs requires several methods, including in-depth analysis of medical records and assessment of the causal relationship between the medication and the adverse clinical event (14).
Another approach to preventing hospitalizations related to ADRs in elderly individuals is identifying those at greatest risk of ADR-related hospitalizations. Previous risk prediction tools have primarily focused on ADRs occurring in hospital settings, with only a few designed for use in outpatient practice (15). A systematic review found that high risk for ADRs in elderly patients is associated with age, female gender, increased comorbidities, and the number of medications (9).
Given the widespread nature of polypharmacy among elderly and geriatric patients, regular audits of prescribed medications should be conducted to assess their rationality and necessity for each specific patient in a given clinical situation (16). Pharmacotherapy in the modern healthcare system involves not only a comprehensive evaluation of the effectiveness of medications but also their safety. Pharmacotherapy for older adults differs from therapy for middle-aged and younger adults due to the following factors: (1) age-related changes in the body (reduced liver blood flow, liver mass, glomerular filtration rate, etc.), which affect the pharmacokinetics of drugs; (2) multimorbidity, requiring the simultaneous use of multiple medications (polypharmacy); (3) the presence of geriatric syndromes and issues (e.g., geriatric asthenia, sarcopenia, falls, dysphagia, cognitive decline); (4) other end goals of pharmacotherapy. All of these factors can increase the risk of ADRs, including severe and fatal outcomes (17, 18). It should be noted that elderly patients might also experience changes in sensitivity to certain medications, such as cardiac glycosides, neuroleptics, barbiturates, narcotic analgesics, and anticoagulants. Polypharmacotherapy can also lead to enhanced pharmacodynamic drug-drug interactions, which can increase the frequency of drug-related complications, leading to a cascade of further prescriptions. However, due to multimorbidity, it is often impossible to limit treatment to a single medication. Therefore, when prescribing multiple drugs simultaneously, it is essential to consider their drug-drug interactions (16).
One of the main principles of geriatrics is that when a new symptom appears in an elderly patient, differential diagnosis should consider the possibility of an adverse drug reaction (ADR). Advanced age, frailty syndrome, multimorbidity, disorders in the intellectual and amnestic sphere, and the large number of medications are factors that contribute to an increased risk associated with medication use (16, 17). Continuous monitoring and analysis of the prognostic risks for ADR development are also necessary (18, 19). Therefore, a tool for predicting the risk of ADRs in elderly people is highly desirable for real clinical practice (20, 21).
The aim of the study was to assess prognostic value and diagnostic accuracy of the universal and adapted GerontoNet ADR risk score in prediction of risk of adverse drugs reactions among hospitalized older adult (≥65 years old) patients with CV diseases.
Methods
Study design and population
A retrospective observational study on prognostic value and diagnostic accuracy of the universal and adapted ` GerontoNet ADR Risk Score scale (hereafter referred to as the "GerontoNet" scale) in prediction of ADR development.
Study population was comprised of 113 hospitalized elderly patients aged 65 and older (their medical records) with CV diseases. The inclusion criteria were patients aged 65 and older, receiving inpatient treatment for coronary artery disease (CAD) with multimorbidity at a multidisciplinary clinic in Bishkek during the period from January to March 2024.
No patient consent was required for the study, as it was retrospective, and only the medical documentation of the healthcare organization was used.
Baseline data
We collected demographic (age, sex) data, number of patients distributed by age (65-74 years old, from 75 to 84 years old and 85 years and older) and clinical data: history of myocardial infarction and ADRs, comorbidities as hypertension, dyscirculatory encephalopathy heart failure, diabetes mellitus, atrial fibrillation, chronic obstructive pulmonary disease, liver diseases, chronic kidney disease, cardiomyopathy, laboratory and instrumental data, number and type of prescribed drugs and ADRS developed during hospitalization.
GerontoNet ADR score
The GerontoNet scale was developed as a screening tool to assess the risk of adverse events, aimed at preventing ADRs and reducing the risks of polypharmacy before the onset of undesirable events (15). It is also designed to identify patients at high risk for ADRs, who may become the focus of interventions aimed at reducing the risk of ADR development and revising prescriptions.
Each patient's prescription sheet was evaluated using the "GerontoNet" scale, with appropriate scores assigned based on the presence of risk factors for the development of adverse drug reactions (ADR) (Table 1). The risk of ADR according to the GerontoNet scale was assessed in 113 patients who met the inclusion criteria.
|
Table 1. GerontoNet scale for assessing the risk of ADR in hospitalized individuals aged 65 and older |
|
|---|---|
|
Risk factor assessment variables |
Point |
|
≥4 comorbidities/conditions (multimorbidity) |
1 |
|
Chronic heart failure |
1 |
|
Liver dysfunction (elevated transaminase levels >2 times the upper limit of normal) |
1 |
|
Number of concurrent medications: |
|
|
˂5 medications |
0 |
|
5 -7 medications |
1 |
|
³8 medications |
4 |
|
History of adverse side effects |
2 |
|
Kidney dysfunction (creatinine clearance <40 ml/min) |
1 |
The score on this scale varies from 0 to 10 points and includes six parameters, each of which is based on the strength of the association with ADRs: the presence of 4 or more concomitant diseases (1 point), the presence of renal failure (1 point), the presence of chronic heart failure (CHF) (1 point), the presence of liver disease (1 point), the number of prescribed medications (1 point - if from 5 to 7; 4 points - if 8 or more) and a history of adverse reactions (2 points).
The risk of developing ADR was assessed by the sum of the scores of each parameter and interpreted as follows: 0-1 points, the risk of ADR -2-4%, 2-3 points-4%, 4-5 points -8-10%, 6-7 points - 12%, 8-10 points -22-28%, respectively. If the scale shows high score figures, then it is necessary to be wary of the high risk of developing ADR in such patients. In this case, there is a need for careful monitoring of the safety of medicines, to weigh the risk-benefit ratio of prescribed drugs, to carry out.
Statistical analysis
The data was collected in a standardized data collection format (Microsoft Excel® 2016) and used Data Mining software for statistical analysis. We calculated odds ratios with 95% CI to assess probability of risk of ADR development, negative and positive likelihood ratios, sensitivity and specificity, positive predictive and negative predictive value of scale in diagnosis of adverse events. We conducted a ROC analysis to determine diagnostic accuracy of scale with calculation of area under the curve, 95% CI and p value.
Results
Clinical characteristics
As can be seen from Table 2, of 113 patients 59.3% were females, 40.7% - males, majority (52.2%) of patients were age category of 65-74 years old, 36.3% - 75 to 84 years old and 11.5% - 85 years and older. Analysis of multimorbidity in patients with coronary artery disease demonstrated that all patients had hypertension and atherosclerosis of the coronary vessels (100%), dyscirculatory encephalopathy was present in 90.2%.
Heart failure was reported in 44 patients (38.9%), 38 patients had diabetes mellitus (33.6%), 37 patients had atrial fibrillation (32.7%), 24 patients had chronic obstructive pulmonary disease (21.2%), 13 patients had postinfarction cardiosclerosis (11.5%), liver diseases were present in 10 patients (8.8%), chronic kidney disease in 3 patients (2.6%) and cardiomyopathy in 4 patients (3.5%).
Next, we calculated the number of prescribed drugs, where it was revealed that 46 patients out of 113 (40.7%) received 5 drugs, 41 patients (36.2%) took 5-7 drugs, and ≥8 drugs were prescribed to 26 patients (23%). When analyzing the medical records, it was revealed that 2 patients had a history of ADRs (1.7%) and 26 patients (23%) had ADRs during hospital stay.
|
Table 2. Characteristics of the study population |
||
|
Patient Demographics and Medications |
Number of Patients (n = 113) |
% |
|
Age Groups 65-74 75-84 ≥85 |
59 41 13 |
52.2% 36.3% 11.5% |
|
Female Male |
67 46 |
59.3% 40.7% |
|
Multimorbidity (>4 diseases) |
113 |
100% |
|
Comorbidities structure |
||
|
Coronary disease (primary condition) |
113 |
100% |
|
Heart failure |
44 |
38.9% |
|
Hypertension |
113 |
100% |
|
Atrial fibrillation |
37 |
32.7% |
|
Extrasystole |
26 |
23% |
|
Post-infarction cardiosclerosis |
13 |
11.5% |
|
Coronary artery atherosclerosis |
113 |
100% |
|
Diabetes mellitus |
38 |
33.6% |
|
Chronic obstructive pulmonary disease (COPD) |
24 |
21.2% |
|
Discirculatory encephalopathy |
102 |
90.2% |
|
Liver diseases |
10 |
8.8% |
|
Chronic kidney disease (CKD) |
3 |
2.6% |
|
Cardiomyopathy |
4 |
3.5% |
|
Polypharmacy during hospital stay |
||
|
Patients taking <5 medications |
46 |
40.7% |
|
Patients taking 5-7 medications |
41 |
36.2% |
|
Patients taking ≥8 medications |
26 |
23% |
|
Number of patients who previously reported adverse reactions |
2 |
1.7% |
|
Number of patients with adverse drug reactions (ADRs) during hospital stay |
26 |
23% |
GerontoNet ADR scale analysis
The results of the overall analysis using the "GerontoNet" scale are presented in Figure 1.
One of the risk factors for developing ADRs on the GerontoNet scale of eight parameters is "The presence of 4 or more diseases" with a weight of 1 point was found in 113 (100%) patients, among whom 67 (59.3%) women and 46 (40.7%) men.
The presence of a risk factor as “The presence of CHF in a patient” (1 point) was indicated in 44 (38.9%) of hospitalized patients, with 63.6% of women (n=28) and 36.4% of men (n=16). The pharmacotherapy of chronic heart failure CHF has certain features in elderly and senile people. Such patients receive on average three times more drugs than more patients without CHF and middle-aged people, primarily due to the presence of multimorbidity. It should be noted that in the pharmacotherapy of CHF, an elderly patient is prescribed more than 2 diuretics, an angiotensin-converting enzyme inhibitor or angiotensin receptor blockers, beta-blockers, anticoagulants, antiplatelet agents, a proton pump inhibitor, cardiac glycosides (digoxin), which can amount to 6-7 drugs. In addition, for each concomitant disease or condition, the patient takes 1-2 more drugs from different groups (hypoglycemic drugs, painkillers from the nonsteroidal anti-inflammatory drugs group, benzodiazepines, sleeping pills, etc.), and more medications may be prescribed.
The data for the factor "Number of simultaneously taken medications" show that fewer than 5 prescribed medications were noted in the prescription sheets of 46 (40.7%) patients, mostly aged between 65 and 75 years, without comorbidities like CHF. Simultaneously, 5-7 medications were prescribed to 41 (36.2%) patients, and more than 8 medications were prescribed to 26 (23%) patients. Overall, 67 (59.2%) patients were prescribed more than 5 medications simultaneously.
It is also important to note that the analysis of the relationship between the number of existing diseases and the prescription of medications revealed a strong positive correlation (r=0.78; p<0.05), meaning that the greater the number of diseases, the more medications are prescribed.
The presence of the parameter "History of adverse drug reactions (ADRs)" was noted in 2 (1.76%) patients. It should be pointed out that during hospitalization, ADRs were identified in 26 patients (23%), but unfortunately, these were not recognized by the physicians as ADRs, their severity was not determined, and "yellow" cards were not filled out.
For example, an increase in transaminase levels above 2 upper limits during treatment was observed in 7 patients (6.19%), confusion, speech slowness, coordination problems, and drowsiness were noted in 9 patients (7.96%), bradycardia occurred in 5 (4.42%), and electrolyte disturbances in 5 (4.42%). The retrospective analysis showed that these were predictable, dose-dependent ADRs (type A reactions), of moderate severity, and according to the Naranjo scale, they can be attributed to a probable cause-and-effect relationship.
Thus, these ADRs led to the additional prescribing of medications, which increased the risk of polypharmacy and adverse drug-drug interactions.
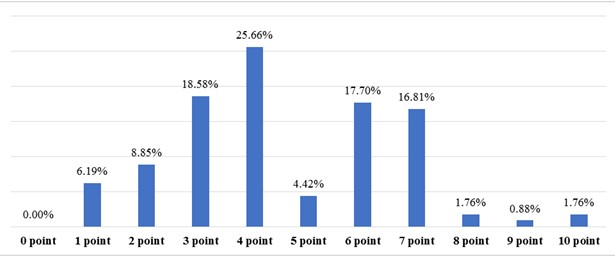
Figure 1. Data on the total risk score for adverse drug reactions according to the GerontoNet scale
point -points for more than 2 points
The factor "Renal dysfunction" was noted in 3 (2.6%) patients with a creatinine clearance of <40 ml/min. Unfortunately, the prescribed doses and administration intervals were not adjusted based on kidney function.
In 10 (8.8%) patients, "Liver disease" was noted (1 point), and an increase in transaminase levels above 2 upper limits of normal was observed in 7 of them (70%). As a result, 1 point was assigned to only 6.19% of patients.
The results of the total risk score calculation based on the "GerontoNet" scale showed (Fig. 1) that 1 point was scored by 6.19% of patients (n=7), 2 points by 8.85% (n=10), 3 points by 18.58% (n=21), 4 points by 25.66% (n=29), 5 points by 4.42% (n=5), 6 points by 17.70% (n=20), 7 points by 16.81% (n=16), 8 points by 1.76% (n=2), 9 points by 0.88% (n=1), and 10 points by 1.76% (n=2). The median total score on the GerontoNet scale was 7 points, with an interquartile range from 1.5 to 18.
Overall, 8 points and higher were scored by only 4.42% of patients (n=5). A total score from 4 to 7 was found in 61.94% of patients (n=70), and a total score from 1 to 3 was found in 33.62% (n=38). That is, the higher the score a is associated with the higher risk of developing ADRs.
The data on the total assessment of the risk of adverse drug reactions (ADRs) among hospitalized patients showed (Fig. 2) that 6.19% of patients have a risk of 2-4% of developing ADRs, 27.43% have a 4% risk, 30% have a 8-10% risk, 31.85% have a 12-21% risk, and 4.42% have a 22-28% risk.
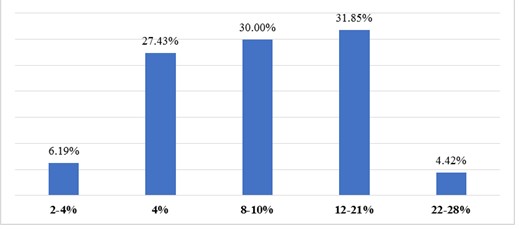
Figure 2. Results of the total assessment of the risk of adverse reactions using the "GerontoNet" scale
In our study, 26 patients (23%) had identified ADRs, of which 18.58% of patients (n=21) were assigned to the group with a total score ≥5 (higher), and 4.42% of patients (n=5) to the group <5 (lower). The odds ratio (OR) was 10.41 (95% CI 3.53-30.75; p<0.0001), meaning the chances of identifying risk factors for ADRs are higher in the group with ADRs. Thus, the presence of the factor has a direct correlation with the likelihood of the outcome. The Fisher criterion = 0.000005; χ²=22.45, indicating a statistically significant difference.
Sensitivity and specificity analyses of the GerontoNet scale for predicting the risk of ADRs in our study showed that the sensitivity of the scale was 80.77% (i.e., the scale can identify the proportion of patients who truly have ADRs among those with total scores above 5), and the specificity was 71.26% (i.e., the scale can identify the proportion of patients who do not have ADRs among those with total scores below 5).
The positive predictive value (PPV) of this scale showed that in 45.65% of cases, there is a likelihood of a positive result in patients with ADRs. The negative predictive value (NPV) indicated that in 92.5% of cases, there is a likelihood of obtaining a false negative result. The accuracy of the scale was 73.45%.
The use of the GerontoNet scale helped us determine the positive likelihood ratio (PLR), which is the ratio of the probability of developing ADRs in patients with ADRs to the probability of ADRs among patients without ADRs. The result was 2.81. This indicates that the PLR >1, meaning patients with ADRs are more likely to have a total score >5 (positive test result) compared to patients without ADRs. The higher the total score (above 5 points), the higher the risk of developing ADRs.
The negative likelihood ratio (NLR), which is the ratio of the probability of a negative result in patients with ADRs to the probability of a negative result in patients without ADRs (NLR), was 0.26. This indicates that the NLR <1, and accordingly, observed ADRs were much less likely in patients with a total score below 5 compared to those with a score above 5.
The data obtained suggest a high sensitivity and specificity of the GerontoNet scale in clinical practice, indicating that it can serve as a tool to reduce the risks of ADR development. To assess the effectiveness of this tool, the area under the ROC curve (AUC) was used (Fig. 3).
Figure 3. ROC curve illustrates how the number of false positives is plotted on the X-axis, and the number of true positives is plotted on the Y-axis
The area under the curve (AUC) derived from the ROC curve was 0.88 (95% CI, 0.83-0.89). This indicates the overall ability of the model to distinguish between positive and negative results and is interpreted as showing that the risk assessment using the GerontoNet scale is better than random guessing (0.5 < AUC < 1).
Discussion
ADRs are a common clinical issue among the elderly and contribute significantly to morbidity and mortality. They are often unrecognized in elderly patients due to competing comorbidities and a lack of awareness among treating physicians about the variability of drug actions in this population. In our study, 26 patients (23%) were retrospectively identified with ADRs, and these were analyzed. The analysis of real clinical practice revealed issues with the monitoring of treatments, particularly in assessing safety. There is a lack of practice in identifying ADRs and completing yellow cards when they occur. Risk factors for ADR development are not being taken into account, possible drug-drug interactions are not considered, and there is no multidisciplinary approach to patient management. This leads to a "cascade" of prescriptions due to miscoordination between specialists in pharmacotherapy, which, unfortunately, increases the risk of ADRs. These ADRs are often not recognized, deprescribing is not performed, and the next cascade of prescriptions follows.
Additionally, monitoring the condition of eliminating organs is not being conducted.
According to the results of our study, 3 patients (2.6%) had an estimated glomerular filtration rate (eGFR) of less than 40 mL/min, and 7 patients (6.9%) had elevated transaminase levels exceeding two upper limits of normal. However, treatment was not reviewed. According to the recommendations of the developers of the GerontoNet scale (15), when eGFR falls below 60%, it is necessary to review the dosages and dosing frequency of medications, as disturbances in biotransformation, elimination, and excretion of drugs lead to accumulation, which in turn increases the concentration of drugs in the blood and the risk of ADRs. Due to age-related declines in this function, the frequency of this risk factor emphasizes the clinical importance of monitoring the function of excretory organs in elderly patients.
Treating physicians should consider potential ADRs as part of every differential diagnosis. New medications should be prescribed with a clear therapeutic goal. Drugs that are ineffective or no longer indicated should be discontinued. Standardized tools, such as "appropriateness criteria" or "risk prediction tools," are useful additions but do not replace sound clinical judgment, supported by training in pharmacotherapy for elderly patients.
According to Onder and co-authors (15), as developers of the GerontoNet scale, it is considered that 8 or more points in 21.7% of cases may be associated with adverse side effects, while scores in the range of 3 to 4 points are considered the best indicators of sensitivity and specificity in predicting the increased risk of potential complications. In our study, 8 points and higher were scored by only 4.42% of patients (n=5). A total score from 4 to 7 was found in 61.94% of patients (n=70), and a total score from 1 to 3 was found in 33.62% (n=38). That is, the higher the score a patient receives on this scale, the higher the risk of developing adverse reactions.
Polypharmacy has always been widespread among elderly individuals due to the necessity of treating various medical conditions that develop as patients aged. Unfortunately, with the increased use of multiple medications comes an elevated risk of negative health outcomes, such as higher healthcare costs, adverse events, drug interactions, non-compliance with treatment regimens, reduced functional status, and geriatric syndromes. Regular review of prescribed medications can significantly reduce the likelihood of medical errors, including those related to organizational processes, and improve the safety of pharmacotherapy in the elderly.
When evaluating the odds ratio (OR) of identifying risk factors for ADRs in patients who already had ADRs (26 patients), it was found to be 10.41 (95% CI 3.53-30.75; p < 0.0001), indicating a significant difference and a direct relationship between the presence of risk factors and the likelihood of ADR occurrence. The identification and prediction of ADRs in elderly patients are based on monitoring and regular review of prescribed medications. ADRs are a common cause of cognitive or functional decline, falls, gastrointestinal bleeding, heart failure, and orthostatic hypotension.
Due to the universal applicability of the GerontoNet scale in healthcare institutions, it has been evaluated by various researchers for its effectiveness in different medical settings, with good sensitivity and specificity (15, 18). According to our study, the GerontoNet scale demonstrated good sensitivity (80.77%) and specificity (71.26%), which means that the risk assessment for ADRs using this scale is better than random guessing (0.5 < AUC < 1), with an AUC of 0.88 (95% CI, 0.83-0.89) based on the ROC curve.
The efforts made by our country's government and the Ministry of Health to develop and implement modern digital technologies in medicine can contribute to improved continuity when transitioning patients from one stage of healthcare to another (e.g., from hospital discharge to outpatient follow-up). Further research on the implementation of these methods is needed to demonstrate that practical solutions to polypharmacy problems can be applied across various healthcare institutions where elderly patients receive care. The results obtained highlight the importance of careful monitoring of drug therapy safety in patients aged 65 and older, optimizing treatment in cases of high risk for drug-related complications, and improving the quality of care, which can have a positive impact on the healthcare organization’s budget.
Study limitations
The small sample size and ts restrospective nature are the maim limitations. Further prospective studies on a larger sample are needed.
Conclusion
After analyzing the data from the study, we concluded that the GerontoNet scale should be implemented in geriatric practice for routine use in everyday clinical practice when admitting multimorbid elderly patients to the hospital.
Ethics: All patients provided written informed consent for the procedures during hospitalization. No informed patients` consent approval of the study by Ethics committee were for the study, as it was retrospective and data were collected from patients records.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: A.T.S, A.A.Z, and A.T.S. equally contributed to manuscript preparation and fulfilled the authorship criteria.
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use: Authors declared they did not use A.I.- assisted technologies in preparation of manuscript
Availability of data and material: We are committed to transparency and collaboration. Relevant data supporting our work is available upon request. If you are interested in collaborating or require specific data for further research, please feel free to reach out. We welcome discussions and partnerships to enhance the impact of our findings.
If data will be shared – rules of fair use (proper citation, acknowledgement of source) and ethics (for collaboration in studies co- authorship should be sought) apply
References
| 1.Baldoni ADO, Chequer FMD, Ferraz ERA, Oliveira DPD, Pereira LRL, Dorta DJ. Elderly and drugs: risks and necessity of rational use. Br J Pharm Sci 2010; 46: 617-32. https://doi.org/10.1590/S1984-82502010000400003 |
||||
| 2.Cruciol-Souza JM, Thomson JC. Prevalence of potential drug-drug interactions and its associated factors in a Brazilian teaching hospital. J Pharm Pharm Sci 2006; 9: 427-33. | ||||
| 3.Petrovic M, Somers A, Onder G. Optimization of geriatric pharmacotherapy: role of multifaceted cooperation in | ||||
| the hospital setting. Drugs Aging 2016; 33: 179-88. https://doi.org/10.1007/s40266-016-0352-7 PMid:26884392 |
||||
| 4.Factors associated with drug interactions in elderly hospitalized in high complexity hospital - https://www.scielo.br/j/csc/a/SMYQ4RzJKDXgbjckzBsvYgw/?lang=en# | ||||
| 5.General principles of pharmacotherapy in elderly and senile age: Methodological recommendations. Editor Tkacheva ON. Moscow: Prometey, 2019. 66 p. | ||||
| 6.Franceschi M, Scarcelli C, Niro V, Seripa D, Pazienza AM, Pepe G, et al. Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit: A prospective study of 1756 patients. Drug Saf 2008: 31; 545-56. doi: 10.2165/00002018-200831060-00009. https://doi.org/10.2165/00002018-200831060-00009 PMid:18484788 |
||||
| 7.Lehnert T, Heider D, Leicht H, Heinrich S, Corrieri S, Luppa M, et al. Review: Health care utilization and costs of elderly persons with multiple chronic conditions. Med Care Res Rev 2011; 68: 387-420. doi:10.1177/1077558711399580. 8.Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: A systematic review of prospective observational studies. Ann Pharmacother 2008; 42: 1017-25. doi:10.1345/aph.1L037. https://doi.org/10.1345/aph.1L037 PMid:18594048 |
||||
| 9.Alhawassi TM, Krass I, Bajorek BV, Pont LG. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interventions Aging 2014; 9: 2079-86. doi:10.2147/CIA.S71178. https://doi.org/10.2147/CIA.S71178 PMid:25489239 PMCid:PMC4257024 |
||||
| 10.Oscanoa, TJ, Lizaraso F, Carvajal A. Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur J Clin Pharmacol 2017; 73: 759-70. doi:10.1007/s00228-017-2225-3 https://doi.org/10.1007/s00228-017-2225-3 PMid:28251277 |
||||
| 11.Waller P, Shaw M, Davidson H, Shakir S, Ebrahim S. Hospital admissions for 'drug-induced' disorders in England: A study using the hospital episodes statistics (HES) database. Br J Clin Pharmacol 2004; 59: 213-9. doi:10.1111/j.1365-2125.2004.02236.x. https://doi.org/10.1111/j.1365-2125.2004.02236.x PMid:15676044 PMCid:PMC1884760 |
||||
| 12.Sari B-A, Sheldon TA, Cracknell A, Turnbull A. Sensitivity of routine system for reporting patient safety incidents in an NHS hospital: Retrospective patient case note review. BMJ 2007; 334: 79. doi:10.1136/bmj.39031.507153 https://doi.org/10.1136/bmj.39031.507153.AE PMid:17175566 PMCid:PMC1767248 |
||||
| 13.Parameswaran N, Chalmers L, Bereznicki BJ, Curtain CM, Bereznicki LR. Repeat adverse drug reaction-related hospital admissions in elderly Australians: A retrospective study at the Royal Hobart Hospital. Drugs Aging 2017; 34: 777-83. doi:10.1007/s40266-017-0490-6 https://doi.org/10.1007/s40266-017-0490-6 PMid:28952130 |
||||
| 14.Williams DJ, Olsen S, Crichton W, Witte K, Flin R, Ingram J, et al. Detection of adverse events in a Scottish hospital using a consensus-based methodology. Scott Med J 2008; 53: 26-30. doi:10.1258/RSMSMJ.53.4.26 https://doi.org/10.1258/RSMSMJ.53.4.26 PMid:19051661 |
||||
| 15.Onder G, Petrovic M, Tangiisuran B, Meinardi MC, Markito-Notenboom WP, Somers A., et al. (2010). Development and validation of a score to assess risk of adverse drug reactions among in-hospital patients 65 years or older: The GerontoNet ADR risk score. Archives Intern Med 2010; 170: 1142-8. doi:10.1001/archinternmed.2010.153 https://doi.org/10.1001/archinternmed.2010.153 PMid:20625022 |
||||
| 16.Mangoni A, Jackson S. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol 2004; 57: 6-14. https://doi.org/10.1046/j.1365-2125.2003.02007.x PMid:14678335 PMCid:PMC1884408 |
||||
| 17.Yakovlev AA. Risk factors of polypharmacy in elderly people with chronic heart failure. Modern Probl Health Care Med Stat 2021; 4: Available at: URL: http://healthproblem.ru/magazines?text=726 | ||||
| 18.Hefner G, Hahn M, Roll SC, Klimke A, Hiemke C. Application of the GerontoNet ADR risk score in a psychiatric setting. International J Clin Med Res 2018; 5: 7-14. | ||||
| 19.Krasnov GS, Davydov IV, Bulgakova SV, Treneva EV, Romanchuk NP, Kurmaev DP, Solyanova NA. Geriatric syndromes that cause difficulties in medical practice: results of an e-survey, proposed solutions, and deprescribing. Modern Probl Health Care Med Stat 2021; 4: Available at: URL: http://healthproblem.ru/magazines?text=718 | ||||
| 20.Lavan AH, Gallagher P. Predicting risk of adverse drug reactions in older adults. Ther Adv Drug Saf 2016; 7: 11-22. doi: 10.1177/2042098615615472 https://doi.org/10.1177/2042098615615472 PMid:26834959 PMCid:PMC4716390 |
||||
| 21.Laroche ML, Van Ngo TH, Sirois C, et al. Mapping of drug-related problems among older adults conciliating medical and pharmaceutical approaches. Eur Geriatr Med 2021; 12: 485-97. doi: 10.1007/s41999-021-00482-8 https://doi.org/10.1007/s41999-021-00482-8 PMid:33745106 |
||||
| 22.Al-Ragavi A, Zyrjanov S, Ushkalova E, Butranova O, Pereverzev A. Prediction of adverse drug reactions and assessment of polypharmacy in elderly patients: A retrospective study at the Russian Gerontological Center. OBM Geriatrics 2019; 3: 038; doi:10.21926/obm.geriatr.1901038 https://doi.org/10.21926/obm.geriatr.1901038 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

