Successful treatment of heparin-induced thrombocytopenia after tricuspid valve replacement: A case report
CASE REPORT
Successful treatment of heparin-induced thrombocytopenia after tricuspid valve replacement: A case report
Article Summary
- DOI: 10.24969/hvt.2024.548
- CARDIOVASCULAR DISEASES
- Published: 08/03/2025
- Received: 21/01/2024
- Revised: 27/09/2024
- Accepted: 29/09/2024
- Views: 480
- Downloads: 217
- Keywords: Heparin-induced- thrombocytopenia, Ebstein anomaly, tricuspid valve replacement, atrioventricular block, cardiac surgery procedures, complications, treatment, pacemaker
Address for Correspondence: Zhamalbek I. Ashimov, International School of Medicine, International University of Kyrgyzstan, Bishkek, Kyrgyzstan
Email: transplant@mail.ru
Zhamalbek I. Ashimov, Zhanybek Zh. Gaibyldaev, Aiperi K. Kurmanbekova
International School of Medicine, International University of Kyrgyzstan, Kyrgyzstan Bishkek
Abstract
Objective: In this regard, we would like to present a case from our practice with heparin-induced thrombocytopenia developed after tricuspid valve surgery.
Case presentation: A 41-year-old patient was admitted with a diagnosis of congenital heart disease: Ebstein anomaly, atrial septal defect, tricuspid regurgitation grade II – III and congestive heart failure. Her laboratory tests were unremarkable. She underwent tricuspid valve replacement surgery. On the 4th day after operation she developed sign of encephalopathy, discoloration of skin, hematomas all over the body, bradycardia 35 beats/min and atrioventricular (AV) block 2nd degree. She was transferred to intensive care unit and temporary pacemaker was inserted. Blood tests demonstrated marked reduction in hemoglobin levels (Hg – 91 and 78 g/l), red blood count to 3.0 and 2.53×1012/l, drop in hematocrit to 27.3 and 24.7%, thrombocytopenia (59×109 /l), leukocytosis, lymphopenia, and hyperfibrinogenemia. Morphology of erythrocytes showed signs of anisocytosis, polychromatophilia. Her biochemistry tests indicated kidney and liver dysfunction (increase in creatinine, bilirubin, transaminases). She was consulted by hematologist and vascular surgeon and diagnosis of disseminated intravascular coagulation was done. Her vascular ultrasound of peripheral vessels was normal. She received treatment including fresh frozen plasma and blood transfusions, anticoagulant, antibiotics, spironolactone, torasemide, and medicines for improving liver and kidney function. She showed improvement of condition on day 12, the blood parameters returned to normal, echocardiography demonstrated improvement in ejection fraction and normal size heart chambers. However, bradycardia and AV block sustained, therefore dual-chamber permanent pacemaker was implanted. Patients was discharged in satisfactory condition.
Conclusion
Because both unfractionated heparin and low molecular weight heparins are widely used in medicine, all physicians should be aware of the life-threatening complication of heparin treatment, HIT. Platelet counts should be monitored for 3 to 5 days in patients receiving heparin. Also, patients with Ebstein anomaly should be examined before surgery more comprehensively, including coagulation tests, hemostasis system, bleeding time, determination of factor antigens. In the case of HIT type 2, immediately discontinue heparin therapy and replace it with an alternative anticoagulant. If a patient who had experienced HIT type 2 and requires future cardiac surgery, direct thrombin inhibitors should be prescribed.
Key words: Heparin-induced- thrombocytopenia, Ebstein anomaly, tricuspid valve replacement, atrioventricular block, cardiac surgery procedures, complications, treatment, pacemaker
Graphical abstract
Graphical abstract
Introduction
Heparin-induced thrombocytopenia (HIT) is a specific complication of the use of low molecular weight and unfractionated heparins, which is characterized by a decrease in platelets and an increased tendency to thrombosis (1-3).
The probability of its occurrence is no more than 5% of the total number of patients receiving unfractionated heparin (UFH), mainly patients who have undergone cardiac surgery or orthopedic surgery. HIT occurs much less frequently when using low molecular weight heparin.
Causes of heparin-induced thrombocytopenia
According to the development mechanism, this pathology is divided into 2 types:
Heparin-induced thrombocytopenia type 1.
Non-immune in nature, associated with platelet aggregation as a result of the action of heparin molecules on specific receptors. In the first type of HIT, a direct (non-immune) interaction of UFH molecules with the platelet membrane occurs, subsequently causing platelet activation and aggregation with the development of thrombocytopenia. Due to the non-immune nature of the interaction, clinical manifestations are noted on days 1-3 of the disease, characterized by an isolated drop in the platelet count of at least 100 × 109 /l. With timely withdrawal of UFH, the platelet count recovers spontaneously within 2-4 weeks and does not require any additional treatment (1).
Heparin-induced thrombocytopenia type 2.
Immune-mediated, associated with the production of immunoglobulins of isotype G (IgG), which interact with the platelet factor 4 (PF4) heparin complex. The process involves platelets and large white cells, which are necessary to clear the human body from foreign antigens (monocytes). This phenomenon contributes to the formation of a macromolecular structure that provokes platelet aggregation, the release of biogenic amines (serotonin and histamine) and procoagulants (substances that reduce blood clotting).
Heparin-induced thrombocytopenia type 2 is a rare immune-mediated reaction that occurs during the first 4-15 days from the first use of heparin, characterized by the production of specific antibodies and manifested by a sharp drop in platelet levels below 100 × 109 /l and paradoxical thrombosis (2).
The key link in the pathogenesis is the unexplained synthesis of specific IgG antibodies to the heparin/PF 4 complex (this factor is released from heparin-activated platelet α-granules). The appearance of IgG in plasma occurs on average on days 4-10, which coincides with the onset of clinical manifestations. Antibodies to the heparin/PF 4 complex are present in the blood of almost all patients with type 2 HIT, however, it is necessary to note the presence of antibodies (≈70%) in the blood of patients who were connected to a cardiopulmonary bypass machine (4, 5).
Subsequently, the interaction of IgG, PF4, and heparin occurs with the formation of an immune complex (IC), capable of adsorption on the platelet membrane, platelet activation occurs (the Fc region of IgG binds to the Fc receptors of the platelet), followed by destruction and release of vasoactive substances (serotonin, histamine, adenosine diphosphate), procoagulant substances that increase thrombin levels. In addition, ICs can activate/damage endothelial cells, resulting in the formation of tissue thromboplastin, which increases thrombin synthesis and the risk of thrombotic complications (3).
In this regard, we would like to present a case from our practice with heparin-induced thrombocytopenia developed after tricuspid valve surgery.
Case report
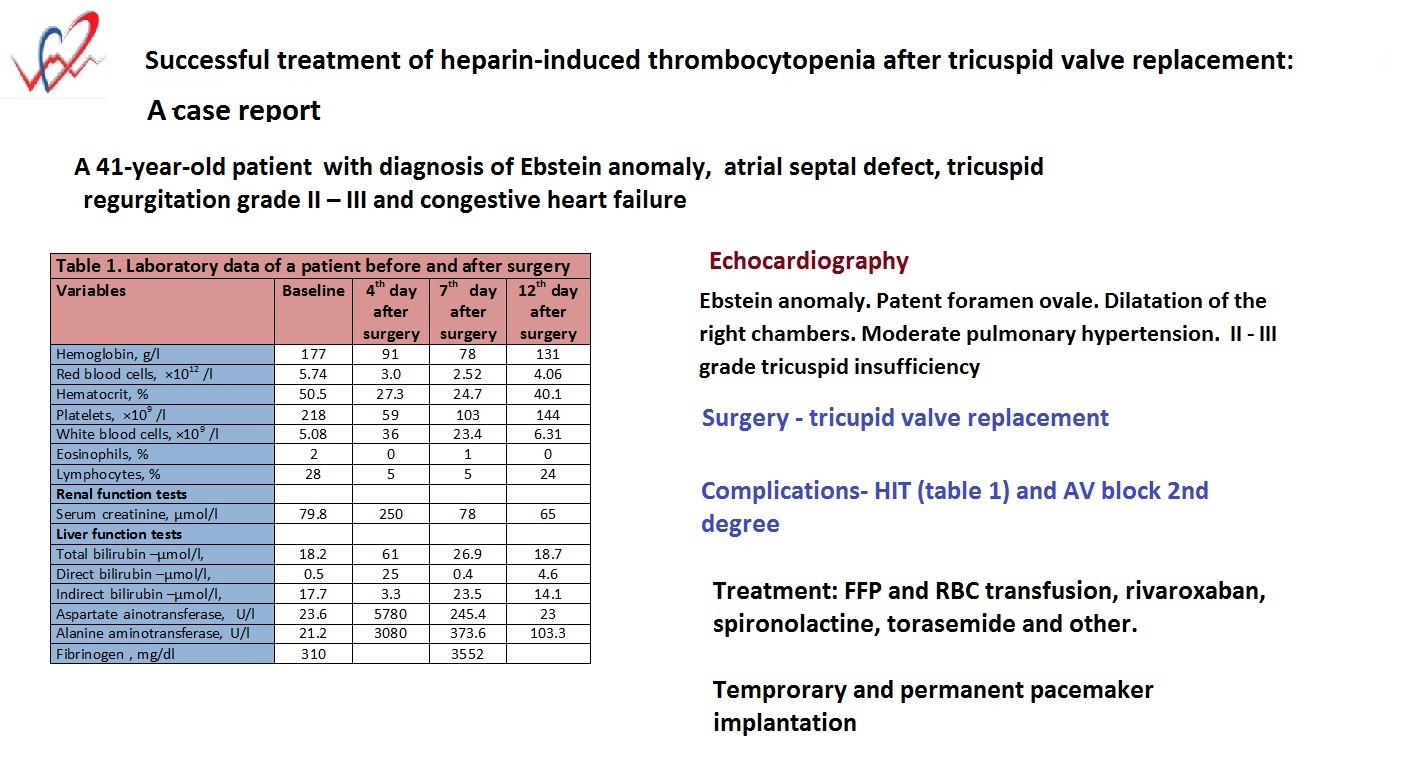
A 41-year-old patient was admitted with a diagnosis of congenital heart disease: Ebstein anomaly, atrial septal defect, tricuspid regurgitation grade II – III and congestive heart failure. She was admitted with complaints of general weakness during insignificant physical activity, palpitations, constant dull pain in the heart area, headaches, dizziness, joint pain, nausea, sweating. Anamnesis: these complaints have been disturbing her for many years.
Laboratory tests on admission (Table 1) demonstrated unremarkable values for complete blood count, renal and liver tests, lipid profile, coagulation tests, and glucose level. Her antistreptolysin O level was 200 IU/ml, C-reactive protein - negative, rheumatoid factor - 48 IU/ml. Markers for hepatitis, HIV, Wassermann reaction were negative.
|
Table 1. Laboratory data of a patient before and after surgery |
||||
|
Variables |
Baseline |
4th day after surgery |
7th day after surgery |
12th day after surgery |
|
Hemoglobin, g/l, |
177 |
91 |
78 |
131 |
|
Red blood cells, ×1012 /l |
5.74 |
3.0 |
2.52 |
4.06 |
|
Hematocrit, % |
50.5 |
27.3 |
24.7 |
40.1 |
|
Platelets, ×109 /l |
218 |
59 |
103 |
144 |
|
White blood cells, ×109 /l |
5.08 |
36 |
23.4 |
6.31 |
|
Eosinophils, % |
2 |
0 |
1 |
0 |
|
Lymphocytes, % |
28 |
5 |
5 |
24 |
|
Monocytes, % |
6 |
4 |
8 |
8 |
|
Erythrocyte segmentation rate , mm/h |
3 |
2 |
4 |
2 |
|
Morphology of erythrocytes |
|
|
|
|
|
Polychromatophilia |
|
++ |
++ |
|
|
Anisocytosis |
|
+ |
+ |
|
|
Renal function tests |
|
|
|
|
|
Serum creatinine, μmol/l |
79.8 |
250 |
78 |
65 |
|
Urea, mmol/l |
5.1 |
|
14.1 |
5 |
|
Blood urea nitrogen, mmol/l |
17.7 |
|
36.6 |
17.5 |
|
Liver function tests |
|
|
|
|
|
Total bilirubin –μmol/l, |
18.2 |
61 |
26.9 |
18.7 |
|
Direct bilirubin –μmol/l, |
0.5 |
25 |
0.4 |
4.6 |
|
Indirect bilirubin –μmol/l, |
17.7 |
3.3 |
23.5 |
14.1 |
|
Aspartate ainotransferase, U/l |
23.6 |
5780 |
245.4 |
23 |
|
Alanine aminotransferase, U/l |
21.2 |
3080 |
373.6 |
103.3 |
|
Lipid profile |
|
|
|
|
|
Total cholesterol, mmol/l |
4.0 |
|
|
|
|
Low-density lipoprotein, mmol/l |
2.5 |
|
|
|
|
High-density lipoprotein, mmol/l |
1.2 |
|
|
|
|
Triglycerides, mmol/l |
0.7 |
|
|
|
|
Atherogenic index of plasma |
-0.234 |
|
|
|
|
Serum glucose, mmol/l |
5.1 |
|
|
|
|
Coagulation tests |
|
|
|
|
|
Partial thromboplastin time, sec |
21 |
|
20 |
|
|
Prothrombin time, sec |
14 |
|
17 |
|
|
Prothrombin index, % |
90 |
|
74 |
|
|
International normalized ratio |
1.1 |
|
1.46 |
|
|
Fibrinogen , mg/dl |
310 |
|
3552 |
|
|
Rheumatic tests |
|
|
|
|
|
Antistreptolysin O, IU/ml |
200 |
|
|
|
|
C-reactive protein |
negative |
|
|
|
|
Rheumatoid factor, IU/ml |
4 |
|
|
|
On echocardiography: the aorta was normal, aortic annulus diameter was 23 mm, the proximal ascending aorta diameter - 24 mm. The aortic valve leaflets were normal. There was no regurgitation. Mitral valve prolapse. Minimal regurgitation. Tricuspid valve: leaflets ere elongated, septal leaflet is displaced apically by 47 mm, posterior leaflet by 21 mm, II - III grade regurgitation (central flow, with two jets, vena contracta - 7 mm). The pulmonary artery diameter was 18 mm. The pulmonic valve is normal. Minimal regurgitation. The left atrial (LA) diameter was 23 mm, the right atrium is dilated. Left ventricular end-diastolic diameter (LVEDD) - 32 mm, left ventricular end-systolic diameter (LVESD) - 20 mm, ejection fraction (EF) - 66%. Interventricular septum 8 mm, posterior wall of the left ventricle 8 mm. There are no zones of hypokinesis. Diastolic function of the left ventricle is normal. Right ventricular diameter (RVD)- 37 mm, thickness of the right ventricular wall - 4 mm, TAPSE - 21 mm. RVD with atrialized part: RVD 1 - 46 mm, RVD 2 - 37 mm, RVD 3 - 74 mm. The right ventricle without the atrialized part is 31×30 mm. The pericardium and pleural cavities are not changed. Average pulmonary arterial pressure (PAP) 38 mm Hg. The inferior vena cava is 19 mm, its collapse - 50%. Interatrial septum: blood shunt from left to right through patent foramen ovale (diameter 7 mm). Interventricular septum: intact. Conclusion: Congenital heart defect. Ebstein anomaly. Patent foramen ovale. Dilatation of the right chambers. Moderate pulmonary hypertension. II - III grade tricuspid insufficiency.
On November 11, 2022, a sternotomy was performed. Revision of the tricuspid valve. Due to the impossibility of performing cone reconstruction of the tricuspid valve, it was decided to perform - tricuspid valve replacement (Bioprostheses - Hancock TM II E 510-31, Medtronic) utilizing normothermic cardiopulmonary bypass. The patient was extubated on the 2nd day and transferred to the hospital ward.
On the 4th day after the operation, the general condition progressively began to deteriorate. Blood tests showed marked thrombocytopenia, increased levels of serum creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST). She showed signs of encephalopathy, discoloration of the skin of the legs on both sides, and development of the 2nd degree AV block. Heart rate reduced to 35 beats per minute.
The patient was transferred to the intensive care unit (ICU) for further treatment. A temporary pacemaker was inserted. Examination: hematomas appeared all over the body, the skin of the lower extremities was cold, dark brown in color, swollen on both sides.
Further laboratory tests (Table 1) on the 4th day after surgery revealed marked decrease in hemoglobin (Hg - 91 g/l) red blood count (RBC) to 3.0×1012 /l), drop in hematocrit (HCT) to 27.3%, reduction in platelets count (59×109 /l, leukocytosis, lymphopenia. Morphology of erythrocytes showed signs of anisocytosis, polychromatophilia. Her biochemistry tests indicated kidney and liver dysfunction (increase in creatinine, bilirubin, ALT, AST ).
She was examined by a hematologist and diagnosed with disseminated intravascular coagulation (DIC). She was also examined by a vascular surgeon and had a Duplex ultrasonography of major blood vessels of the neck, upper and low extremities was done. The vessels were without obstruction, passable, the blood flow was normal.
A treatment was prescribed including fresh frozen plasma and red blood cell transfusions, Gyno-Tardyferon (ferrous sulfate + folic acid), TAD-600 (glutathione), anicoagulant rivaroxaban, pentoxifylline, aldestorone inhibitor -spironolactone, antibiotics - ceftriaxone, amoxicillin, ciprofloxacin, ademetionine, diuretic torasemide, Panangin (potassium and magnesium aspartate), pantoprazole. After stabilization of the patient's condition, she was transferred to the department.
Further tests on the 7th day (Table 1) after surgery showed still lower Hg - 78 g/l, RBC – 2.52×1012/l, and HCT – 24.7%, however platelets increase to 103×109/l, leukocytes decreased to 23.4x109/l. We observed high levels of fibrinogen - 3552 mg/l, however kidney and liver tests started returning to normal values. On electrocardiogram, AV block 2nd degree sustained, that is why the pacemaker remained implanted.
During treatment, there was a significant improvement in general condition. Laboratory tests on the 12th day after surgery and treatment returned to normal (Table 1).
On control echocardiography: Left atrium diameter - 32 mm, Right ventricle diameter - 28 mm, LVEDD 38mm, LVESD - 21 mm, EF - 75%. Mitral valve – minimal regurgitation – grade 1. Tricuspid valve echoed from the bioprosthesis, minor tilt to the left, gradient 10 mm Hg. Average 6 mm Hg. Regurgitation grade I. Systolic PAP was 45 mm Hg. The pericardium had an echo-negative space as a thin strip behind the left ventricle (minimal effusion). The pleural cavities have an echo-negative space of 16 mm on the right (effusion), none on the left.
On electrocardiogram: AV block. Heart rate 35 beats/min. Due to high degree AV block, a dual-chamber pacemaker was implanted as planned. The ventricular electrode was installed in the coronary sinus. Heart rate 60 beats/min.
She was discharged home in satisfactory condition.
Discussion
In general, the diagnosis of HIT is often difficult, due to a wide range of underlying health conditions that are indications for heparin therapy, as well as due to the concurrent use of drugs that potentially lead to the development of thrombocytopenia (abciximab, tirofiban (aggrastat), clopidogrel, procainamide, gold drugs, NSAIDs, penicillin and anticonvulsants).
The diagnosis of HIT should be based on evidence-based clinical criteria (thrombocytopenia, arterial or venous thrombosis) and laboratory data (dynamics of platelet count, detection of IgG2 antibodies). To assess the likelihood of HIT2 in each individual patient, a scoring scale based on thrombocytopenia, time of manifestation, thrombosis or other complications and other causes of thrombocytopenia are currently used (2).
For our patient, 7 points were calculated.
The doctor’s algorithm for suspecting the development of HIT should include (5-11):
-Exclusion of any source of heparin use (“heparin plugs” and other hidden sources of heparin), including LMWH due to possible cross-reaction;
-prescription of alternative anticoagulants;
-testing for the presence of antibodies to heparin-PF4 by any available method;
-limit platelet transfusions;
-conducting duplex scanning of arteries and veins to exclude thrombosis, performing an electrocardiography and troponin test.
Treatment of HIT
The key to suspecting HIT is immediate discontinuation of heparin (5, 7). Regardless of the presence or absence of thrombosis, once the diagnosis of HIT is confirmed or suspected, alternative non-heparin anticoagulant therapy should be prescribed, which can last up to 3 months. When choosing alternative non-heparin anticoagulants, it is necessary to take into account their availability (varies by country), the clinical features of HIT (venous or arterial thrombosis, therapeutic or prophylactic regimen), and the clinical condition of the patient (liver and kidney function).
Thrombin generation plays a major role in the pathogenesis of HIT, which suggests the advisability of using direct thrombin inhibitors and drugs with anti-factor Xa activity (for example, danaparoid), which inhibit thrombin generation in the treatment of HIT (10).
Unlike the treatment of thrombosis not associated with HIT, which often involves the use of warfarin within 24 hours of starting unfractionated heparin, low-molecular-weight heparin, or fondaparinux, in HIT it is important to delay warfarin therapy until the platelet count has recovered to at least 150×109 /l. Initiating warfarin therapy before platelet levels are controlled carries the risk of precipitating venous gangrene of the limb as a result of acute decreases in protein C and protein S levels. If necessary, for example, long-term treatment of deep vein thrombosis, warfarin should be started while the patient is receiving alternative anticoagulant treatment without less than 4-5 days combination (5). Moreover, alternative anticoagulants should not be discontinued until the two INRs (24 hours apart) are within the target interval and the platelet count has reached a stable plateau in the normal range. After discontinuation of direct thrombin inhibitors or danaparoid, warfarin therapy is continued for 3-6 months or longer if clinically warranted.
Direct Xa inhibitors (rivaroxaban, dabigatran, apixaban) are new generation oral anticoagulants for the prevention and treatment of thromboembolic disorders. These drugs do not cross-react with HIT antibodies (10, 11).
Conclusion
Because both unfractionated heparin and low molecular weight heparins are widely used in medicine, all physicians should be aware of the life-threatening complication of heparin treatment, HIT. Platelet counts should be monitored for 3 to 5 days in patients receiving heparin. Also, patients with Ebstein anomaly should be examined before surgery more comprehensively, including coagulation tests, hemostasis system, bleeding time, and determination of factor antigens. In the case of heparin-induced thrombocytopenia type 2, remember to immediately discontinue heparin therapy and replace it with an alternative anticoagulant. If a patient who had experienced HIT type 2 and requires future cardiac surgery, direct thrombin inhibitors should be prescribed.
Ethics: There were no animal or patients experiments conducted. Informed consent was obtained from patient for all procedures, surgery and treatment.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: Zh.I.A., Zh.Zh.G., and A.K.K. equally contribute to case management and manuscript preparation, thus fulfilled authorship criteria
Acknowledgement: With deep gratitude to our teachers and colleagues working with these difficult patients
Funding: None to declare
Statement on A.I.-assisted technologies use: No AI tools were used in the preparation of this work.
Availability of data and material: Contact authors. If data will be shared for collaboration or other purposes, fair use proper citation and authors, source acknowledgement)and ethical rules apply.
References
| 1. Franchini M. Heparin-induced thrombocytopenia: an update Thromb J 2005; 3: 14. https://doi.org/10.1186/1477-9560-3-14 PMid:16202170 PMCid:PMC1262784 |
||||
| 2. Greinacher A, Warkentin TE. Heparin-induced thrombocytopenia. New York: Marcel Dekker. 2004. https://doi.org/10.1201/9780203027363.ch11 |
||||
| 3. Messerli AW, Askari AT. Management strategies in antithrombotic therapy. New York: Wiley. 2007. | ||||
| 4.Greinacher A, Pötzsch B, Amiral J, Dummel V, Eichner A., Mueller-Eckhardt C. et al. Heparin-associated thrombocytopenia: isolation of the antibody and characterization of a multimolecular PF4-heparin complex as the major antigen, Thrombosis and Haemostasis 2010; 8: 2025-31. | ||||
| 5. Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia and cardiac surgery. Ann Thorac Surg 2003; 76: 638-48. https://doi.org/10.1016/S0003-4975(03)00756-2 PMid:12902132 |
||||
| 6. Price EA, Hayward CP, Moffat KA, Moore JC, Warkentin TE, Zehnder JL. Laboratory testing for heparin-induced thrombocytopenia is inconsistent in North America: a survey of North American specialized coagulation laboratories. Thromb Haemost 2007; 98: 1357-61. https://doi.org/10.1160/TH07-06-0401 PMid:18064336 |
||||
| 7. Messerli, Adrian W, Askari, Arman T. Management strategies in antithrombotic therapy. New York: Wiley, 2007. | ||||
| 8. Warkentin TE, Heddle NM. Laboratory diagnosis of immune heparin-induced thrombocytopenia. Cur Hematol Rep 2003; 2: 148-57. https://doi.org/10.1201/9780824758844 |
||||
| 9. Warkentin TE, Sheppard JA, Moore JC, Moore KM, Isgouin CS, Kelton JG. Laboratory testing for the antibodies that cause heparin-induced thrombocytopenia: how much class do we need? J Lab Clin Med 2005; 146: 341-6. https://doi.org/10.1016/j.lab.2005.08.003 PMid:16310517 |
||||
| 10. Grouzi E. Update on argatroban for the prophylaxis and treatment of heparin-induced thrombocytopenia type II. J Blood Med 2014; 5: 131-41. https://doi.org/10.2147/JBM.S38762 PMid:25152637 PMCid:PMC4140228 |
||||
| 11.Tardy-Poncet B, Nguyen P, Thiranos JC, Morange PE, Biron-Andréani C, Gruel Y, et al.. Argatroban in the management of heparin-induced thrombocytopenia: a multicenter clinical trial. Crit Care 2015; 19: 396. doi: 10.1186/s13054-015-1109-0 https://doi.org/10.1186/s13054-015-1109-0 PMid:26556106 PMCid:PMC4641392 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

